Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4052
Peer-review started: October 5, 2019
First decision: October 23, 2019
Revised: October 26, 2019
Accepted: October 30, 2019
Article in press: October 30, 2019
Published online: December 6, 2019
Processing time: 62 Days and 10.3 Hours
Paraneoplastic neurological syndrome manifesting as secondary Parkinson disease caused by breast cancer is extremely rare.
We report a 39-year-old primipara of 31 gestational weeks, who presented with worsening tremors, facial stiffness and speech disfluencies, and decreased limb strength. Thorough physical examinations and auxiliary tests suggested secondary Parkinson’s disease, but the pathogenesis was unknown. During the cesarean section at the 31 weeks plus 6 d, an exploration and liver biopsy revealed a metastatic, poorly differentiated adenocarcinoma. The positron emission tomography and immunohistochemical analysis confirmed a breast ductal carcinoma of stage IV. To our knowledge, only two reports have documented the association between the breast cancer and the Parkinson disease, and neither occurred in pregnant women.
Our case alerts the secondary Parkinson disease as the possible presentation of breast cancer, the most common malignancy during pregnancy.
Core tip: We described a rare case of severe secondary Parkinson disease which deteriorated during pregnancy. The underlying pathogenesis was confirmed to be breast cancer, which resulted in paraneoplastic neurological syndrome (PNS). Recognition of the variable manifestations of PNS is as important as diagnosis at earlier stages of the underlying malignancy and facilitates prompt treatment to improve prognosis.
- Citation: Li L. Secondary Parkinson disease caused by breast cancer during pregnancy: A case report. World J Clin Cases 2019; 7(23): 4052-4056
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4052.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4052
Paraneoplastic neurological syndrome (PNS) is an infrequent complication of systemic malignant tumors, characterized by subacute to profound progression of neurological disability[1]. Initial neurological symptoms of PNS may occur more than two years prior to the diagnosis of the underlying neoplasm. One of the important syndromes associated with PNS is the secondary Parkinson disease, which is defined as conditions with clinical manifestations resembling primary Parkinson disease. Clinical features may include bradykinesia, rigidity, parkinsonian gait, and masked facies. In general, tremor is less prominent in secondary Parkinsonism than in the primary form. Possible causes include vascular injury, drugs, trauma, toxin exposure, neoplasms, infections and degenerative or hereditary conditions. PNS occurs in less than 1.0% of patients with breast cancer[2]. In English literature there were only two published cases of secondary Parkinson disease caused by breast cancer. Here we report a case of secondary Parkinson disease attributed to the breast cancer diagnosed during the pregnancy. A review of literature was also conducted.
In May 8, 2012, a 39-year-old primipara of 31 gestational weeks was admitted, who presented with a history of dysgraphia for one year, slurred speech and decreased limb strength for four months, and incapableness of voluntary urinate and defecate for 20 d.
She presented with tremors in one hand and smaller hand writing from May 2011. On October 6, 2011 she conceived by in vitro fertilisation. Her tremors became increasingly obvious and spread to the upper limbs after January 2012. At the same time, facial stiffness and speech disfluencies appeared. The patient complained of decreased limb strength from March 2012 and ceased voluntary urination and defecation after April 19, 2012 (27 + 6 gestational weeks). Persistent catheterization and intermittent enema were given.
She had no special medical history except for primary infertility due to her husband’s azoospermia.
No family history of oncology or any hereditary disease was found.
Physical examination on admission revealed decreased muscle strength in the limbs, hyperreflexia of the left achilles tendon, positive bilateral Rossolimo signs and bilateral Babinski signs.
All tumor biomarkers [ carcinoembryonic antigen (CEA), carbohydrate antigen 153 (CA153), CA19-9, CA125, neuron specific enolase (NSE)] were within the reference ranges. Tests for anti-Hu, Yo, Ri, CV2/CRMP5, Ma2 and amphiphysin antibodies were negative.
Cerebral magnetic resonance imaging on February 20, 2012 exhibited an abnormal signal on the posterior pituitary which revealed no clinical significance later. Results of electroencephalogram on March 6, 2012 were normal. An abdominal ultrasonography on May 7, 2012 revealed multiple hypoechoic masses in the liver, the largest size being 2.0 cm × 1.5 cm.
Pregnancy 31 wk plus 6 d, secondary Parkinson disease due to PNS, and poorly differentiated breast ductal carcinoma of stage IV.
The possible pathogenesis includes medication, infection, intoxication, trauma to the brain and malignancies. Medication is the most common reason. She denied administration of drugs, such neural tranquilizer, metoclopramide and lithium, which could probably cause Parkinsonian features. No evidence of infection, trauma or intoxication was available. Essential tremor and genetic degenerative disease were also excluded. PNS was mostly suspected as underlying pathogenesis because of lesions in the liver.
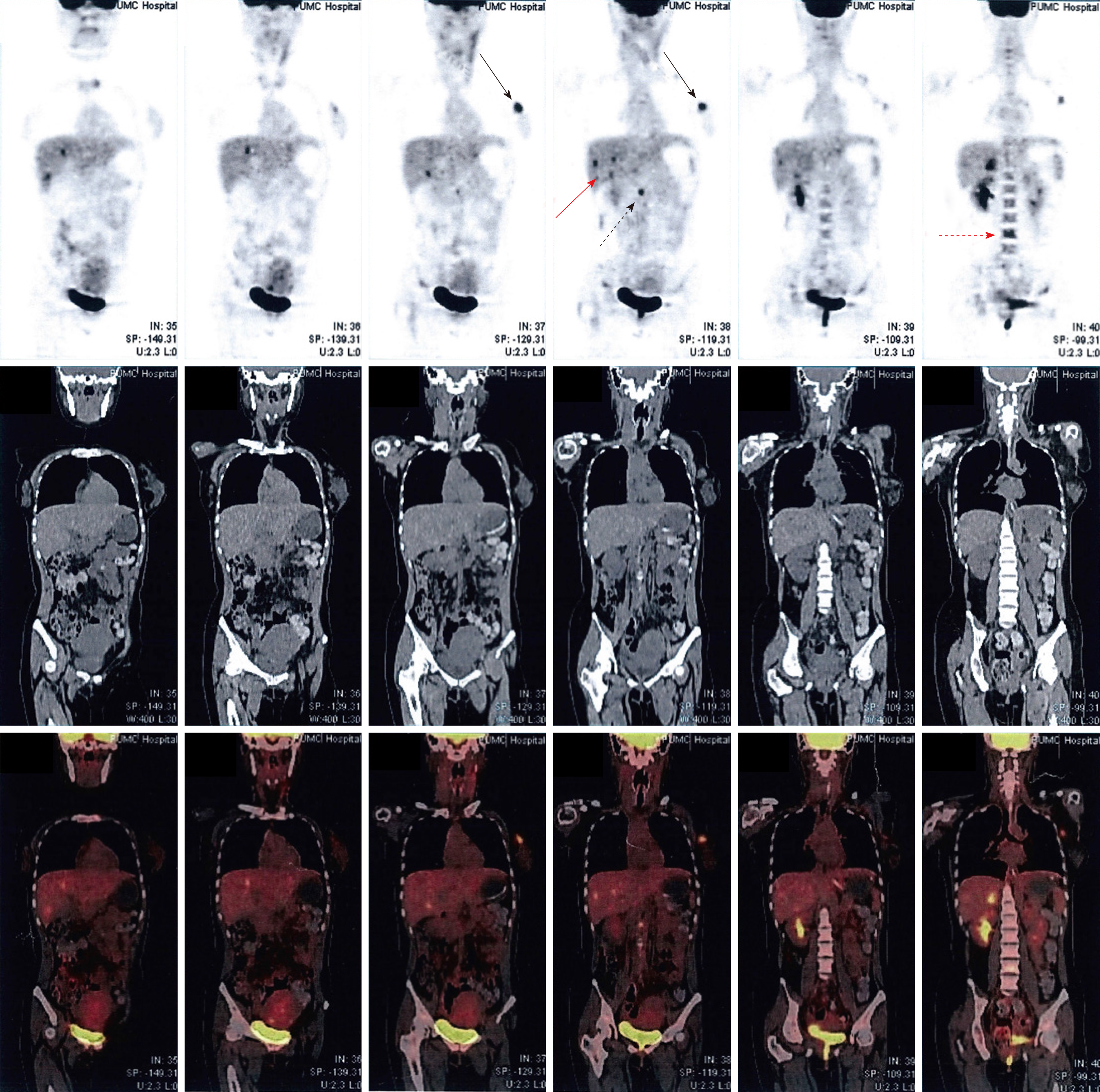
However, considering her deteriorating neurological condition, a cesarean section was performed on May 14, 2012 at the 31 wk plus 6 d, and she delivered a baby girl of 2100 g with Apgar score of 8 at 5 min. During the cesarean section, exploration of the peritoneal cavity revealed multiple hard masses and nodules in the liver, and a biopsy identified a metastatic, poorly differentiated adenocarcinoma. The immunohistochemical analysis showed positive CD34, negative α-fetoprotein, negative hepatocyte, 60% positive estrogen receptor, 10% positive progestin receptor and positive human epidermal receptor-2 (score value of 3+). The positron emission tomography revealed a metabolism-elevated lesion in the upper outer quadrant of the left breast (2.0 cm × 3.3 cm × 2.4 cm), with multiple metastases to axillary lymph nodes, liver parenchyma, bones, and para-aortic lymph nodes (Figure 1). Combining the above results with her clinical conditions, the diagnosis of a poorly differentiated breast ductal carcinoma of stage IV, and secondary Parkinson disease was confirmed.
From September to December 2012, taxotere and carboplatin were given once every three weeks for four months. Meanwhile, Trastuzumab, a humanized monoclonal antibody targeted human epidermal growth factor receptor protein (HER2), was administered with the initial loading dose at 4 mg/kg, with subsequent weekly maintenance dose at 2 mg/kg for four months, and the final dosage at 6 mg/kg every three weeks for one year. Repeated radiofrequency ablations were also applied for her major liver tumors from March 10, 2014 to April 30, 2015. Madopar was given to relieve her neurologic symptoms but no obvious effect was observed.
Despite of a transient improvement of speech and dysmetria, her neurological conditions deteriorated shortly thereafter, and the metastatic liver lesions persisted. The patient refused further follow-up after her last visit on August 30, 2015, with a known progression-free survival of four months. Her mortality was not confirmed and an autopsy was impossible.
Due to the wide-ranging clinicopathologic manifestations of PNS, only two reports had documented the association between breast cancer and Parkinson disease[3,4]. In the report of Mousa et al[3], the paraneoplastic affection in a young female preceded the detection of carcinoma of the breast by nine months, including paraneoplastic subacute cerebellar degeneration, Parkinsonian syndrome, autonomic disturbance, profound depression, myopathy and cardiomyopathy. Block dissection of the carcinoma resulted in alleviation of her muscle weakness and a return of the electrocardiogram to normal. No evaluation of autoantibodies or immunotherapy was performed in this patient. In the report of Golbe et al[4], a 42-year-old woman had unexplained weight loss followed by action tremor and difficulty in initiating gait. The patient rapidly developed Parkinsonian signs and symptoms, and did not respond well to typical medications against Parkinson disease. No autoantibodies were found in the serum of this patient, and the patient ultimately succumbed despite of intensive chemotherapy. The case reported by Golbe et al[4] had the similar characteristics with the case in our report, as they both had negative antibodies and poor response to adjuvant therapy. These findings are different from the neurologic symptoms of cerebella degeneration and opsoclonus caused by gynecologic or breast cancers, in which positive expression of anti-Yo and anti-Ri was found most frequently.
Because of its exceedingly low prevalence rate, no established standard treatment for PNS is available. Eradication of the underlying malignancy is the mainstay of the management of paraneoplastic neuropathy, and successful anti-neoplastic therapy is associated with a favorable prognosis for most paraneoplastic neuropathy patients[5]. Immunosuppressive treatment for neurologic symptoms is also important, which includes corticosteroids, plasma exchange and intravenous immunoglobulins. Immunosuppressive chemotherapeutics and B-cell targeting drugs such as rituximab also may be useful[5]. For breast cancer associated PNS, anti-Ri was related with poor response to immunotherapy[6]. In our case, despite of aggressive therapy, remission of cancer or neuropathy symptoms was not achieved, which was consistent with the previous report.
Breast cancer is the most common malignancy diagnosed in women and during pregnancy. The incidence of pregnancy-associated breast cancer was estimated to be 28/100 000 live births[7]. Due to the negligence of diagnosis and inconvenience of evaluation, a median delay of 2.2 mo to diagnosis in pregnant patients was found compared to 1.2 mo in non-pregnant women[8]. Multidisciplinary management of women with breast cancer in pregnancy is mandatory for its complex entity and ambiguous prognosis. Physiologic pregnancy-associated breast changes, including engorgement, hypertrophy and nipple discharge, obscure detection, make physical examination more difficult as pregnancy advances, until they steadily revert to pre-pregnancy state approximately three months after breastfeeding cessation[9]. On physical examination, breast cancer in pregnancy typically presents as a palpable painless lump. Triple assessment (history/clinical examination, imaging and cytology/histology) and awareness of less common presentations are the fundamental methods of accurately diagnosing symptomatic breast complaints in pregnancy[10]. As illustrated in our reports, acute and subacute neurologic symptoms should warrant consideration of malignancies, especially the most possible chance of breast cancer during pregnancy.
In summary, we describe a rare case of severe secondary Parkinson disease associated with breast cancer diagnosed during pregnancy. Recognition of the variable manifestations of PNS is as important as diagnosis at earlier stages of the underlying malignancy and facilitates prompt treatment to improve prognosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ajmal M, Seeman MV S-Editor: Dou Y L-Editor: Ma JY E-Editor: Qi LL
| 1. | Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 662] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 2. | Gatti G, Simsek S, Kurne A, Zurrida S, Naninato P, Veronesi P, Frasson A, Millen E, Rososchansky J, Luini A. Paraneoplastic neurological disorders in breast cancer. Breast. 2003;12:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Mousa AR, Al-Din AN. Neurological and cardiac complications of carcinoma of the breast. Case report. Acta Neurol Scand. 1985;72:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Golbe LI, Miller DC, Duvoisin RC. Paraneoplastic degeneration of the substantia nigra with dystonia and parkinsonism. Mov Disord. 1989;4:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Rosenfeld MR, Dalmau J. Diagnosis and management of paraneoplastic neurologic disorders. Curr Treat Options Oncol. 2013;14:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Pittock SJ, Parisi JE, McKeon A, Roemer SF, Lucchinetti CF, Tan KM, Keegan BM, Hunter SF, Duncan PR, Baehring JM, Matsumoto JY, Lennon VA. Paraneoplastic jaw dystonia and laryngospasm with antineuronal nuclear autoantibody type 2 (anti-Ri). Arch Neurol. 2010;67:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Andersson TM, Johansson AL, Hsieh CC, Cnattingius S, Lambe M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Bonnier P, Romain S, Dilhuydy JM, Bonichon F, Julien JP, Charpin C, Lejeune C, Martin PM, Piana L. Influence of pregnancy on the outcome of breast cancer: a case-control study. Societe Francaise de Senologie et de Pathologie Mammaire Study Group. Int J Cancer. 1997;72:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Kakoulidis I, Skagias L, Politi E. Pregnancy associated breast cancer (PABC): aspects in diagnosis. Breast Dis. 2015;35:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Amant F, Deckers S, Van Calsteren K, Loibl S, Halaska M, Brepoels L, Beijnen J, Cardoso F, Gentilini O, Lagae L, Mir O, Neven P, Ottevanger N, Pans S, Peccatori F, Rouzier R, Senn HJ, Struikmans H, Christiaens MR, Cameron D, Du Bois A. Breast cancer in pregnancy: recommendations of an international consensus meeting. Eur J Cancer. 2010;46:3158-3168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |