Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4044
Peer-review started: June 19, 2019
First decision: September 23, 2019
Revised: September 30, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: December 6, 2019
Processing time: 179 Days and 8.2 Hours
Uveal melanoma is the most common primary intraocular malignancy in adults, but its incidence is low in Asian populations. Spontaneous corneal perforation and intratumoral calcification are rare presentations of choroidal melanoma (CM) , and reports regarding these presentations have been limited. Even after complete surgical treatment, the prognosis of CM patients is usually poor if distant metastasis is present. We here present a case of CM with unique presentations and early distant metastasis to the liver.
A 63-year-old Asian woman presented to our hospital with complaint of pain and brownish discharge from her left eye for 3 d. Imaging studies revealed intratumoral calcification within the left eye with eyeball rupture. Enucleation of the left eye was performed and pathological examination confirmed the diagnosis of CM. Systemic surveillance revealed no metastatic diseases. However, the patient was lost to follow-up 3 mo after surgery. At 1.5 years after the operation, she presented to our emergency department with complaint of dull epigastric pain that radiated to the back for 1 d. Imaging studies revealed a large mass at the upper abdomen abutting the pancreatic neck and body as well as several nodular lesions in the liver. Fine needle biopsy was performed and findings confirmed liver and pancreatic metastases.
This case highlights the importance of continued follow-up of patients with CM.
Core tip: Although early metastasis of choroidal melanoma is rare, large primary tumor size, advanced stage and extraocular extension of choroidal melanoma increased the risk of metastasis. At initial diagnosis of choroidal melanoma, systemic surveillance can allow early detection of metastatic disease. Even after complete surgical excision, patients should be advised about the importance of continued follow-up monitoring, including physical examination, liver function testing, liver ultrasonography, and positron emission tomography-computed tomography scan or magnetic resonance imaging of the abdomen. Unfortunately, current treatment for metastatic disease is limited and the prognosis is usually poor.
- Citation: Wang TW, Liu HW, Bee YS. Distant metastasis in choroidal melanoma with spontaneous corneal perforation and intratumoral calcification: A case report. World J Clin Cases 2019; 7(23): 4044-4051
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4044.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4044
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults. Most UMs originate in the choroid (90%), followed by the ciliary body (7%), and the iris (2%)[1]. According to a study of 1352 UM cases conducted by Hu et al[2], the incidence of UM was 0.38 per million per year in Asians, which is significantly lower than that reported in Caucasians (6.02 per million per year)[2]. Since the clinical presentation of UM varies from being asymptomatic to vision loss depending on the size and location of the tumor, it may be difficult for affected patients to detect the disease and seek early medical attention. Currently, treatments of UM include surgical excision and radiotherapy. However, distant metastasis, especially to the liver, is common in these cases and is associated with poor prognosis. Therefore, systemic surveillance initiated as soon as UM is diagnosed is important.
In the present report, we describe rare clinical presentations and management of a case of choroidal melanoma (CM). In addition, to highlight the importance of continued follow-up of patients with CM, we report the unfavorable outcome of this case with early liver metastasis.
A 63-year-old Asian woman presented to our emergency department with complaint of pain and brownish discharge from her left eye for 3 d.
The patient had been blind in the left eye for approximately 20 years, but never sought medical attention for her eye. She denied surgical history or experiencing trauma to the eye prior to visiting our emergency department.
The patient had a medical history of type 2 diabetes mellitus which was controlled with oral hypoglycemic agents.
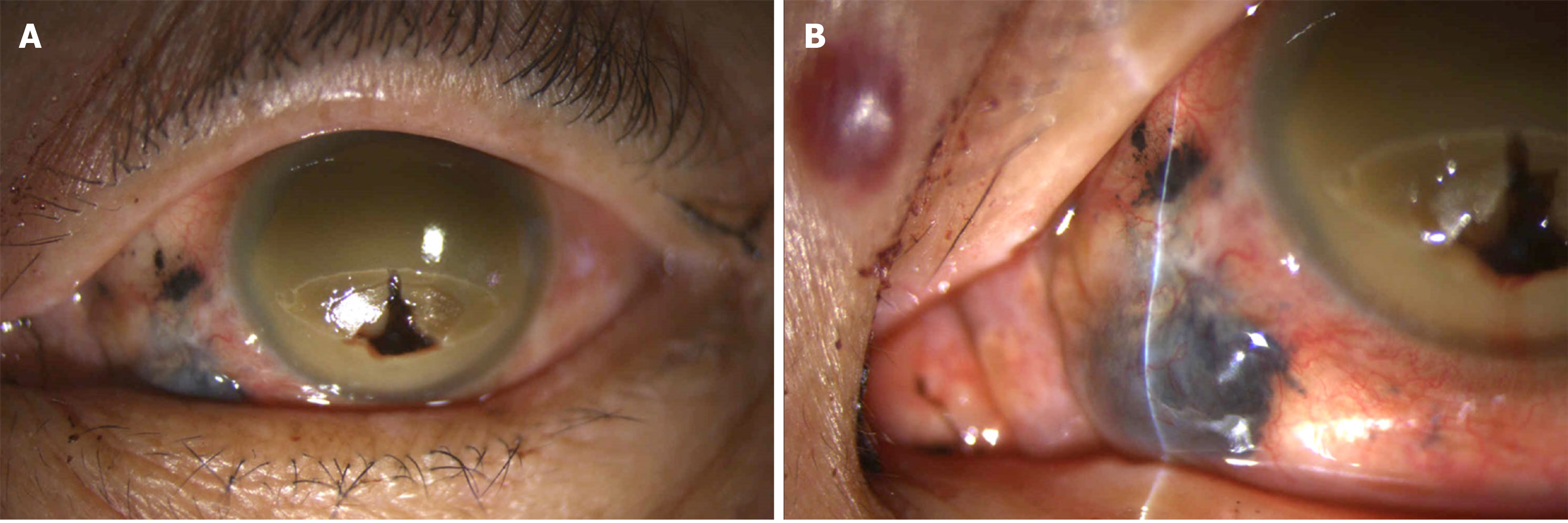
Visual acuity of no light perception was noted in the left eye. Ophthalmologic examination of the left eye revealed a 4 mm x 4 mm fixed dark brown-black subconjunctival mass with dilated and tortuous sentinel vessels at the nasal lower region. In addition, dark brown-black material with uveal tissue protruding from the perforated cornea and flattened anterior chamber was observed (Figure 1).
Serum laboratory testing showed no abnormal results. Electrocardiogram and chest X-ray were normal.
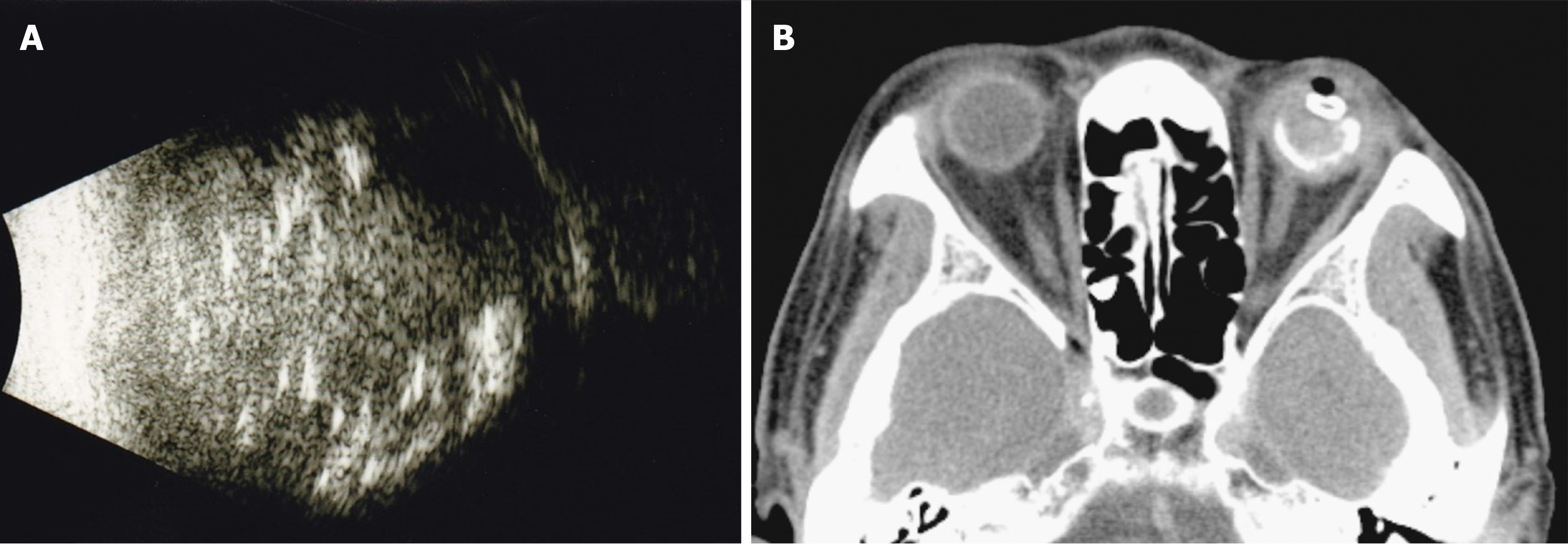
B-scan ultrasonography showed heterogeneous echogenicity throughout the left eyeball (Figure 2A). Computed tomography (CT) scan of the orbits showed a high-density lesion in the left eye representing calcification in the vitreous cavity, choroid, and uveal tissue (Figure 2B). The lesion was confined to the left eyeball without obvious orbital extension or brain involvement. In addition, there was a gas bubble in the left eye, indicating eyeball rupture.
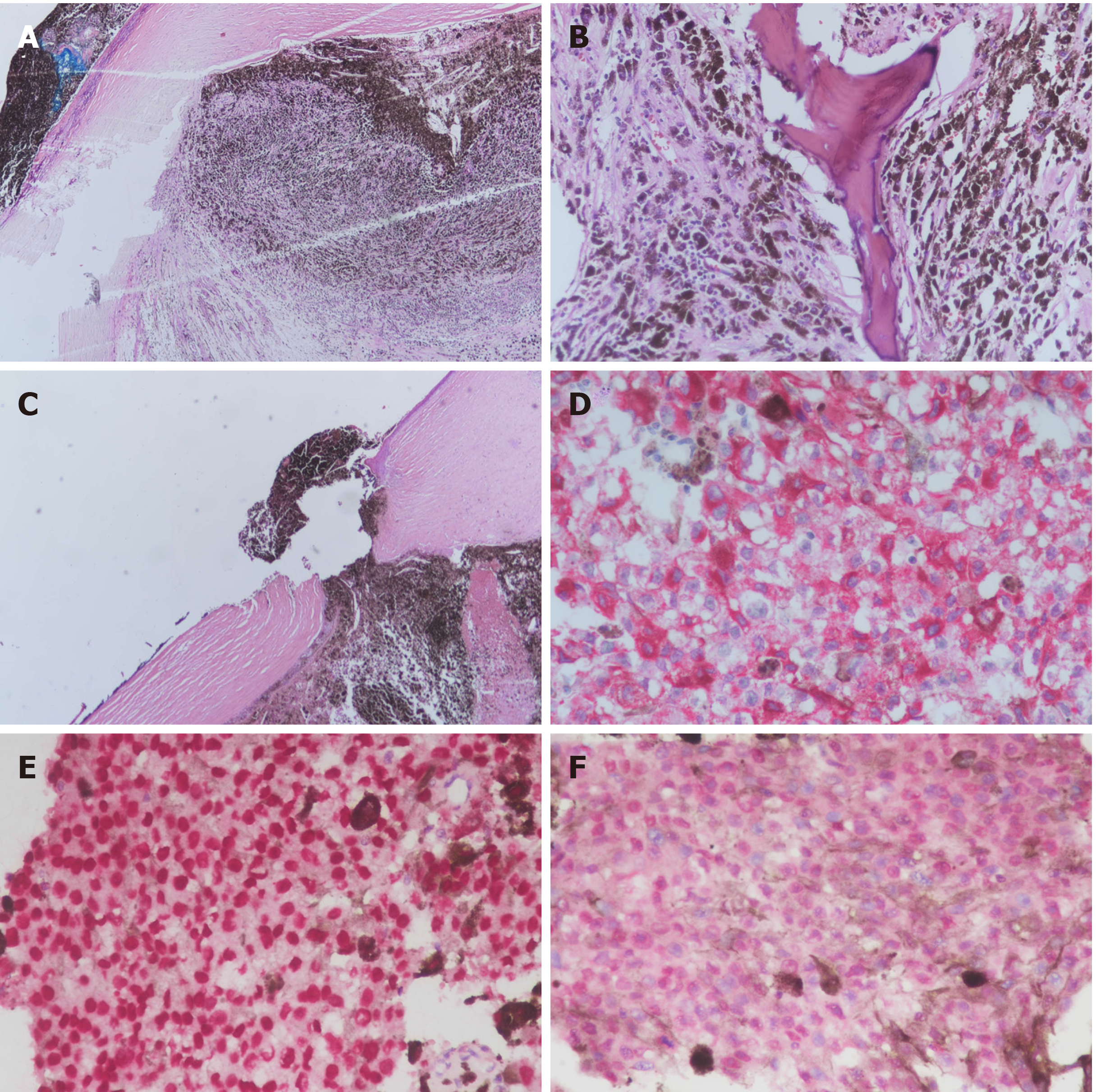
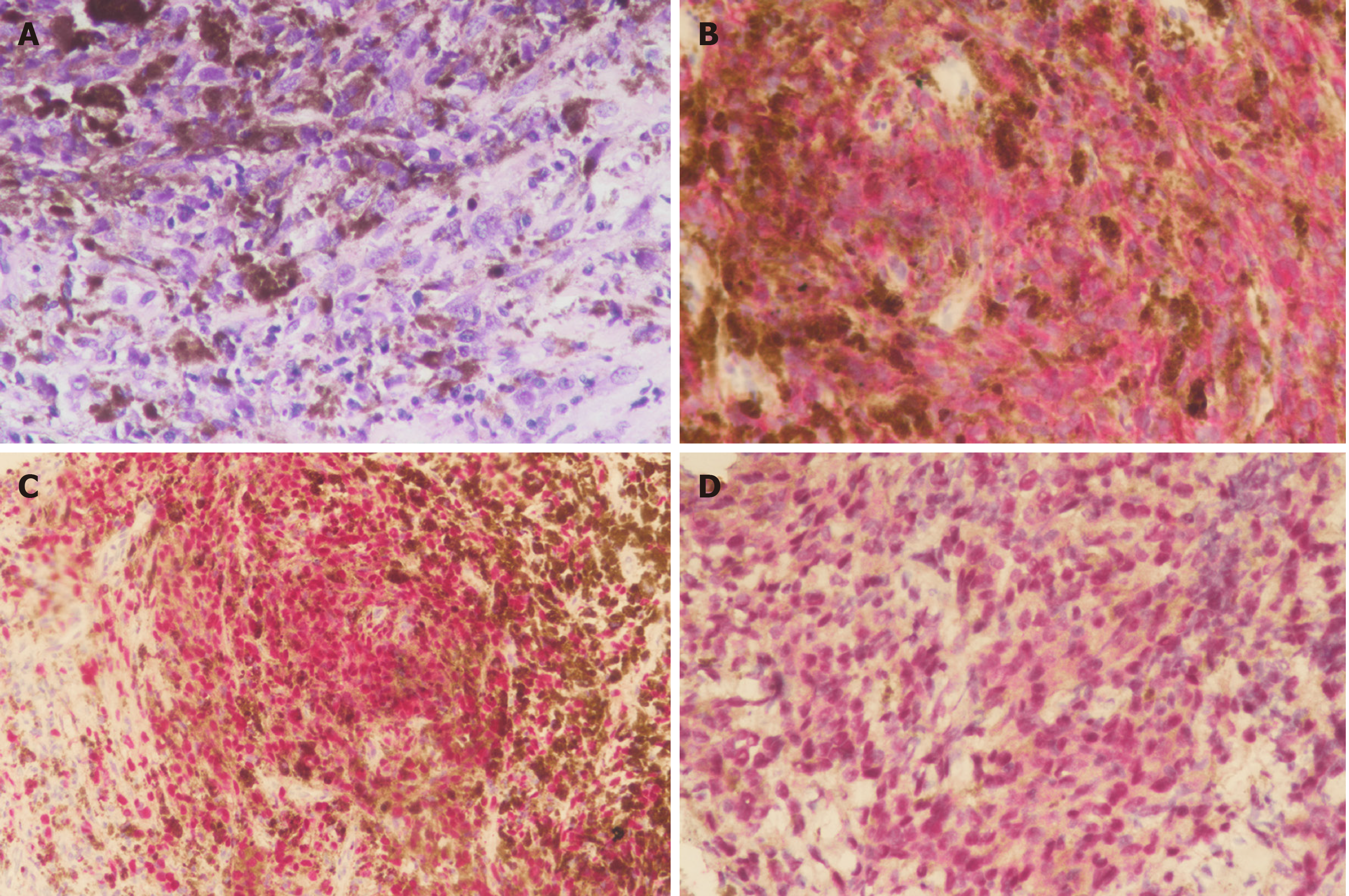
The patient underwent enucleation of the left eyeball with silicone implant (20 mm) placement and removal of pigmented conjunctival tissue on the following day. Histopathological examination revealed that the enucleated eyeball was occupied by a 17 mm × 16 mm-sized tumor. Involvement of ciliary body, iris, anterior chamber, and cornea was present. The cut end of the optic nerve was free from malignancy but an additional 4 mm x 4 mm-sized extrascleral tumor adhering to the nasal lower part of the left eyeball was found. Therefore, pT4d stage was determined according to the American Joint Committee on Cancer (AJCC) Staging Manual, 8th edition[3]. Histological examination revealed total loss of retinal tissue with tumor adjacent to the sclera, uveal tissue in the region of the perforated cornea, and an area of fibrosis-containing ossification in the tumor. Markedly atypical proliferation of epithelioid-shaped cells was found in the tumor. The cells were heavily pigmented and contained abundant cytoplasm and prominent nucleoli. Immunohistochemistry (IHC) showed that the neoplastic cells were positive for melanin-A and Sry-related HMg-Box gene 10 (SOX-10) immunostaining with no aberrant loss of BRCA1-associated protein 1 (BAP1) expression (Figure 3). Pathological diagnosis of epithelioid cell type of CM of the left eye was confirmed.
A hematology oncologist and a radiation oncologist were consulted for further systemic surveillance and treatment plan. Positron Emission Tomography-CT (PET-CT) scan with F-18 Fluorodeoxyglucose (FDG) from head to pelvis was performed and showed FDG-avid lymph nodes in the carotid, submandibular, and posterior cervical spaces of the neck bilaterally. Since the hilum of the FDG-avid lymph nodes was intact, reactive inflammation rather than metastasis in bilateral lymph nodes in the neck was considered (N0), and no definite evidence of FDG-avid tumor was noted elsewhere (M0) (Figure 4A). Adjuvant radiation therapy was not recommended by the radiation oncologist because the surgical margin was tumor free and there was no evidence of other extraocular extension.
Unfortunately, the patient was lost to follow-up 3 mo after surgery. After 1.5 years following surgery, she presented to our emergency department with complaint of dull epigastric pain that radiated to the back for 1 d and poor appetite for 2 - 3 wk. CT scan of the abdomen at a district hospital showed suspected pancreatic cancer with liver metastasis, and she was referred to our hospital for further treatment. Tests for liver function and tumor markers including alpha-fetoprotein, carcinoembryonic antigen and CA 19-9 were within normal limits.
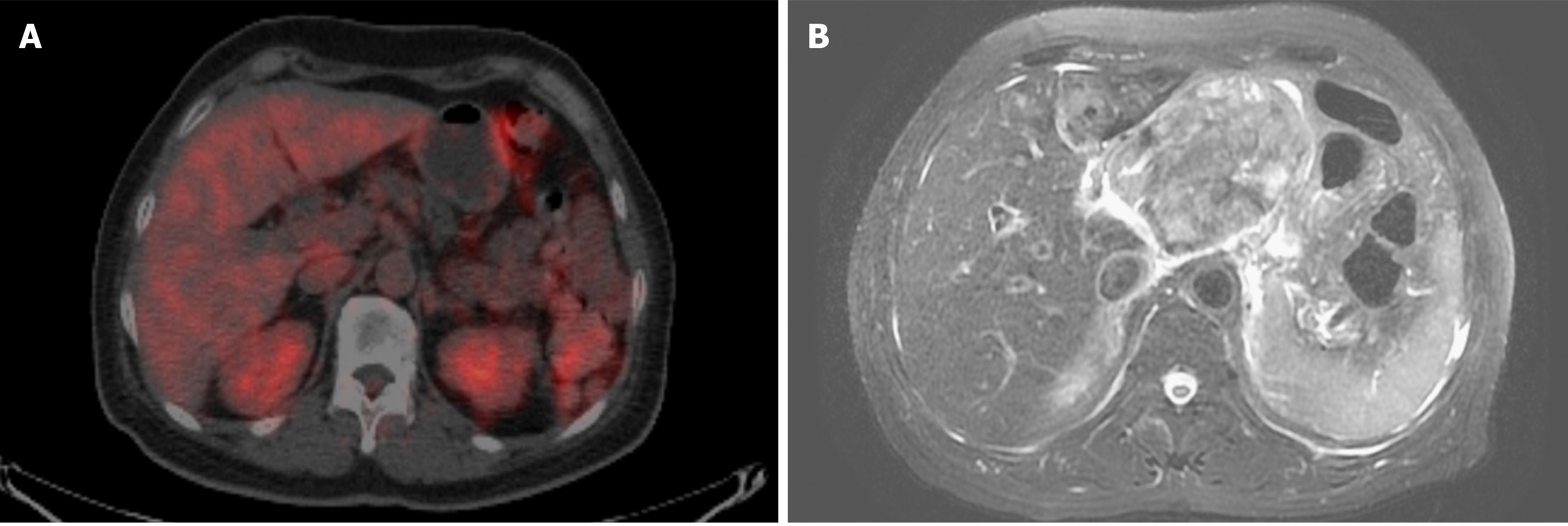
Magnetic resonance imaging (MRI) of the abdomen showed a large mass-like lesion at the upper abdomen abutting the pancreatic neck and body with a maximal diameter of at least 9.5 cm. Several nodular lesions in the liver with left portal vein involvement were also found (Figure 4B). Endoscopic ultrasonography with fine needle aspiration was performed. Histological examination showed sheets of epithelioid and spindle neoplastic cells arranged in a solid pattern with occasional prominent nucleoli and eosinophilic cytoplasm that exhibited stromal reaction and abundant melanin pigment. IHC showed that neoplastic cells were positive for melanin-A and SOX-10 immunostaining and no aberrant loss of BAP1 expression (Figure 5) was found. Based on both the morphology and immunophenotype, a diagnosis of metastatic melanoma with liver and pancreatic involvement was confirmed.
To the best of our knowledge, this is the first case report that discusses CM with presentation of spontaneous corneal perforation. Histopathological examination showed tumor cells protruding through the corneal perforation without invasion to adjacent cornea layers. Perry et al[4] reported a case of CM that protruded through the perforating corneal ulcer wound because of spontaneous expulsive choroidal hemorrhage. However, in our case, there was no evidence of corneal thinning, corneal ulcer, or choroidal hemorrhage. The patient denied experiencing trauma or any surgical history before presenting to our hospital. Therefore, the definite cause of spontaneous corneal perforation remained undetermined.
Intratumoral calcification is a rare presentation of CM, although it is commonly seen in retinoblastomas and choroidal osteomas. Previous case reports[5-7] indicated that destructive therapeutic interventions, such as fractional transpupillary thermotherapy or brachytherapy, might cause intratumoral calcification. Chan et al[8] and Csakany et al[9] reported cases of CM with spontaneous calcification. The former case had calcification and the presence of osteocytes but the latter case had pure calcification. Both cases showed no evidence of tumor regression or necrosis, making the definite mechanism of spontaneous calcification unknown. In our case, however, tumor necrosis was noted. Tumor necrosis in CM is not uncommon. In a potential mechanism proposed by Thareja et al[10], when the tumor outgrows its blood supply, it leaves a watershed area in the center and causes hypoxia and later ischemic tumor necrosis. Afterward, cytokines are released, causing tumor swelling with further necrosis. Consequently, dystrophic calcification might occur in the necrotic tissue after an extended period of time. Although currently no study has discussed the interval between tumor necrosis and calcification, we believe that there was enough time for pathological change in our patient, who had been blind for approximately 20 years but had not sought medical attention. Therefore, we report this case to highlight the possibility of calcification due to tumor necrosis in CM.
Extrascleral extension is also rare in CM. Routes of extrascleral extension previously described include via aqueous outflow vessels or emissary canals carrying posterior ciliary nerves, arteries, and vortex veins[11,12]. However, it may be difficult for pathologists to precisely cut the eyeball specimen at the point where tumor cells exit the eye, especially when the extrascleral extension is large. In addition, a solitary metastatic tumor was noted in the orbital area but did not adhere to eyeball, creating difficulty for pathologists to differentiate these two types of tumors and determine the correct pathological stage. In the AJCC Cancer Staging Manual, 8th edition, stage N1b was added for distinguishing cases of CM with extrascleral extension, typically adherent to the eye, with those in whom the tumor has spread regionally to the orbital area but was not contiguous with the eye with the primary tumor[3]. In our case, an additional 4 mm x 4 mm-sized extrascleral tumor adhering to the lower nasal part of the left eyeball was found. Although we did not find the exiting route for the tumor cell, we highly suspected that the extrascleral extension of the tumor extended via the vortex vein based on its location. Therefore, the pathological stage was determined to be T4d based on the clinical evidence.
It is worth mentioning that our case had an early distant metastasis (within 1.5 years of completion of enucleation). Early metastasis of CM is rare. Usually the median time to detection of distant metastasis from the diagnosis was approximate 2 to 3 years according to several previous studies[13,14]. Currently, there is only a case report of early liver metastasis from CM (tumor size of 10 mm × 8.0 mm, spindle cell type, pT2cNx, within 8 mo of completion of enucleation and 3-dimensional conformal radiation therapy) by Mandal et al[15] It has been shown that increasing original tumor size and stage and extraocular extension added the risk of metastasis. In the study of 8033 patients by Shields et al[16], each increasing millimeter of thickness added approximately 5% increase in risk for metastasis at 10 years [from 6% (0-1.0 mm thickness) to 51% (> 10.0 mm thickness)]. For large melanoma (> 8.0 mm), the estimated risk for metastasis at 1, 3, 5, and 10 years was 5.3%, 22.3%, 35.0%, and 49.2%, respectively. Shields et al[17] furthermore focused on 1311 patients with large melanoma (> 10.0 mm thickness) and analyzed the estimated the risk for metastasis at 1, 3, 5, and 7 years. In the groups of > 16.0 mm thickness, which was the maximal range in all groups, the risk was 20%, 57%, 66%, and 66%, respectively. In addition, histologic factors including epithelioid type of melanoma, high mitotic activity, inflammatory infiltration, increased HLA expression, and loss of nuclear immunostaining for BAP1 protein predicted unfavorable prognosis[18,19]. In the present case, epithelioid type of melanoma with a size of 17 mm × 16 mm suggested high risk of metastasis and unfavorable prognosis.
With PET-CT imaging, early detection of metastasis is possible. However, there is no test that can identify microscopic metastatic tumors, and up to 50% of patients eventually develop metastases, typically involving the liver[20]. In our case, no obvious sign of metastasis was found on initial whole-body PET-CT imaging, and unfortunately the patient missed follow-up examinations that would have included physical examination, liver function tests, liver ultrasonography, and PET-CT scan or MRI of the abdomen[21]. These examinations are strongly suggested for early detection of distant metastasis because prognosis is poor once metastasis occurs. One-year survival rate was reported to be 15%, and median survival varied from 4 to 15 mo[22]. Current treatment options for metastatic disease included immunotherapy, such as antibodies targeting the cytotoxic T-lymphocyte antigen-4, antibodies targeting the programmed cell death 1, and MEK inhibitor. However, all of the above-mentioned treatments have shown relatively low response rates. In liver metastasis, although evidence was limited, hepatic resection, regional chemotherapy such as hepatic intra-arterial chemotherapy, hepatic arterial chemoembolization, and localized radioembolization using yttrium-90-labelled microspheres might improve outcome.
Even though early metastasis of CM is rare[15], large tumor size increases the risk for metastasis[16]. Spontaneous tumor rupture or tumor involvement outside the globe are likely to increase the likelihood of future distant metastasis. Regular follow-up with an ophthalmologist and oncologist is needed for each case. Current treatment for metastatic disease is limited and the prognosis is usually poor. Therefore, it is important for ophthalmologists to ensure that the patient receives sufficient information about the prognosis, the benefits and risks of treatment, and further follow-up plan at the time of diagnosis of CM.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inan U S-Editor: Zhang L L-Editor: Ma JY E-Editor: Ma YJ
| 1. | Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012;32:1363-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005;140:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Kivelä T, Simpson E, Grossniklaus H, Jager M, D, Amin MB, Edge S, Greene F, Byrd DR, Brookland RK. Singh A, M. Caminal J, C. Pavlick A, Kujala E, Coupland S, Finger P. Uveal Melanoma. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK. AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer 2017; 805-817. |
| 4. | Perry HD, Hsieh RC, Evans RM. Malignant melanoma of the choroid associated with spontaneous expulsive choroidal hemorrhage. Am J Ophthalmol. 1977;84:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Lambert SR, Char DH, Howes E, Crawford JB, Wells J. Spontaneous regression of a choroidal melanoma. Arch Ophthalmol. 1986;104:732-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Kellner U, Foerster MH, Bornfeld N. Calcification-like echographic patterns in uveal melanomas treated with brachytherapy. Br J Ophthalmol. 1993;77:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Kiratli H, Bilgiç S. Calcification in choroidal melanoma after transpupillary thermotherapy. Am J Ophthalmol. 2001;132:939-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Chan TK, Atta HR, Scott GB. Ossification in choroidal melanoma. Br J Ophthalmol. 1995;79:705-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Csákány B, Tóth J. Spontaneous calcification of a choroidal melanoma. Acta Ophthalmol. 2009;87:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Thareja S, Rashid A, Grossniklaus HE. Spontaneous Necrosis of Choroidal Melanoma. Ocul Oncol Pathol. 2014;1:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Coupland SE, Campbell I, Damato B. Routes of extraocular extension of uveal melanoma: risk factors and influence on survival probability. Ophthalmology. 2008;115:1778-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Burris CKH, Papastefanou VP, Thaung C, Restori M, Arora AK, Sagoo MS, Cohen VML. Detection of extrascleral extension in uveal melanoma with histopathological correlation. Orbit. 2018;37:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Gragoudas ES, Seddon JM, Egan KM, Glynn RJ, Goitein M, Munzenrider J, Verhey L, Urie M, Koehler A. Metastasis from uveal melanoma after proton beam irradiation. Ophthalmology. 1988;95:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Koutsandrea C, Moschos MM, Dimissianos M, Georgopoulos G, Ladas I, Apostolopoulos M. Metastasis rates and sites after treatment for choroidal melanoma by proton beam irradiation or by enucleation. Clin Ophthalmol. 2008;2:989-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Mandal S, Chaudhuri T, Devleena M, Sil S. Choroidal melanoma of left eye with very early liver metastasis. J Cancer Res Ther. 2015;11:957-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade OA, Mehta S, Forte L, Long A, Dellacava EF, Kaplan B, Shields JA. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Shields CL, Sioufi K, Robbins JS, Barna LE, Harley MR, Lally SE, Say EAT, Mashayekhi A, Shields JA. LARGE UVEAL MELANOMA (≥10 MM THICKNESS): Clinical Features and Millimeter-by-Millimeter Risk of Metastasis in 1311 Cases. The 2018 Albert E. Finley Lecture. Retina. 2018;38:2010-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Berus T, Halon A, Markiewicz A, Orlowska-Heitzman J, Romanowska-Dixon B, Donizy P. Clinical, Histopathological and Cytogenetic Prognosticators in Uveal Melanoma - A Comprehensive Review. Anticancer Res. 2017;37:6541-6549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Dogrusöz M, Jager MJ, Damato B. Uveal Melanoma Treatment and Prognostication. Asia Pac J Ophthalmol (Phila). 2017;6:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 704] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Weis E, Salopek TG, McKinnon JG, Larocque MP, Temple-Oberle C, Cheng T, McWhae J, Sloboda R, Shea-Budgell M. Management of uveal melanoma: a consensus-based provincial clinical practice guideline. Curr Oncol. 2016;23:e57-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017;101:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |