Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4029
Peer-review started: September 8, 2019
First decision: October 24, 2019
Revised: October 31, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: December 6, 2019
Processing time: 88 Days and 15.6 Hours
Laron syndrome (LS) is an autosomal recessive hereditary condition affecting only 1/1000000 births. The cause is associated with mutations in the growth hormone (GH) receptor (GHR), leading to GH insensitivity. LS patients typically present with severe growth retardation, obesity, and abnormal sexual maturation. Currently, LS diagnosis is performed post-delivery. Therefore, we assessed the efficiency of Pre-implantation Genetic Testing (PGT) coupled with monoplex-polymerase chain reaction (PCR) technology for detecting this monogenic disease in embryos from a couple confirmed as LS heterozygous carriers
The couple LS-carriers were confirmed by the presence of a first child born with LS. The couple underwent a standard in vitro fertilization (IVF) protocol. DNA was collected from trophectoderm cells from day 5 embryos. Whole genome amplification (WGA) was performed using a Sureplex DNA Amplification System and analyzed by PCR, targeting the deletion of the exons 5 and 6 in the GHR gene as well as PGT by Next-generation Sequencing (Illumina). Eleven embryos were collected and analyzed. 27.3% were the wild type for GHR, 45.5% were heterozygotes, and 18.2% homozygous mutants. One embryo yielded no results. Three 2-embryos transfers were performed; 2 normal homozygous and four heterozygous carriers were selected for transfer. The first two transfers were unsuccessful, whereas the final transfer with two heterozygous embryos resulted in clinical pregnancy. The genomic composition of the fetus was verified, applying the same techniques using amniocytes, extracted after 21 wk of the ongoing pregnancy. The fetus was confirmed as GHR deletion in exon 5-6, carrier. A non-affected baby was born.
Here, we present a case demonstrating that using WGA as a template in addition to PCR targeting specific gene regions, exons 5 and 6 on the GHR gene, could identify LS carrier embryos. This provides evidence that WGA and PCR serve as an excellent tool to detect this specific monogenic disease in IVF embryos, thus allowing selection of candidate embryos for transfer successfully when a specific inherited genetic mutation/disease is suspected.
Core tip: Laron syndrome (LS) is a low prevalent, autosomal recessive hereditary disorder affecting the Jewish population; however, when LS is expected, genetic testing is required. This case study demonstrates that by using monoplex-polymerase chain reaction (PCR) during Pre-implantation Genetic Diagnosis, we were able to accurately identify mutations in the growth hormone receptor (GHR). Here, we show that, in Mexico, the cause of LS was the deletion of the exons 5 and 6 in the GHR gene; moreover, we were able to select an embryo, which produced an LS negative fetus. This study provides evidence that monoplex-PCR can serve as an excellent tool to detect diseases during in vitro fertilization.
- Citation: Neumann A, Alcántara-Ortigoza MÁ, González-del Ángel A, Camargo-Diaz F, López-Bayghen E. Diagnosis of Laron syndrome using monoplex-polymerase chain reaction technology with a whole-genome amplification template: A case report. World J Clin Cases 2019; 7(23): 4029-4035
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4029.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4029
The prevalence of Laron syndrome (LS), first described in 1966[1] as insensitivity to growth hormone (GH) due to mutations in the extracellular domain of the GH receptor (GHR)[2,3], is not commonly diagnosed in Latin America. Moreover, in Mexico, there are less than 10 documented cases of LS[4-7]. LS is typically found among the Jewish community[8], but with a very low prevalence. For Latin Americans, most cases are found in Ecuador, most likely of Jewish descendants, due to a mass migration during the 15th century[4]. Therefore, LS is typically not assessed in most Pre-implantation Genetic Testing (PGT), neither in Mexico nor in the rest of the world.
LS is an autosomal recessive disorder[9], characterized by dwarfism, abnormal development of the reproductive organs (micropenis or enlarged breasts)[10-12]. Most LS patients have an increased risk for seizures[13] but a decreased risk for cancer[14-16]. LS subjects have elevated GH levels with usually low insulin-like growth factor 1[17]. To date, the diagnosis of LS is performed as a post-clinical presentation. Here, we present a case of a couple that underwent PGT to identify potential LS-free embryos to achieve a normal pregnancy.
A 31-year-old Jewish, Mexican woman [body mass index (BMI) = 21.76 kg/m2] and her husband, a 32-year-old Jewish, Mexican man (BMI = 24.5 kg/m2) decided to attend the INGENES Institute in Mexico City for selection of a healthy embryo for a second pregnancy, as her first pregnancy produced a child with LS.
Both parents were presented as fully developed adults with healthy sexual development. Upon the diagnosis of LS, the borne-child mutation in the GHR descendant as deletion of exons 5 and 6 (del5-6) of GHR. Subsequent clinical and molecular analysis of the parents confirmed that they were carriers (heterozygotes) of the del5-6. We proposed an in vitro fertilization (IVF) protocol, complemented with pre-implantation genetic diagnosis (PGT) using monoplex polymerase chain reaction (PCR) targeting the del5-6 mutation present in GHR, as an intervention for healthy embryo selection.
The only relevant history is five years before attending INGENES, the mother had one previous pregnancy, which resulted in a child with LS. Afterward, both patients underwent genetic testing, not at our facility, and were diagnosed as carriers for mutations in GHR that could develop LS.
The mother had no additional medical complications, nor was not taking any medications. For the father, no causes of male infertility were suspected; furthermore, he was healthy. Both parents are Jewish.
IVF and embryo isolation: IVF, embryo biopsy and PGT were performed according to the standard protocols of the INGENES Institute as previously described (Cedillo 2016; Schaeffer 2017). The mother underwent two standard courses of controlled ovarian stimulation (Depot GnRH antagonist, Cetrotide 0.25 mg daily dose, Merck, Darmstadt, Germany). Stimulation was prolonged until the diameter of leading follicles was > 18 mm. Afterward, recombinant human chorionic gonadotropin (hCG, Choragon 1000 IU, Ferring Laboratories, Saint-Prex, Switzerland) was administered; then, after 36 h, the oocytes were retrieved with ultrasound guidance. All 14-18 mm follicles were aspirated, and 35 ova were collected (summed total for both stimulations). It was decided to proceed with fertilization and culture.
The ova were fertilized by intracytoplasmatic sperm injection, and 25 embryos were produced. Only morphologically optimal embryos were considered for this study, using the criteria established by the Istanbul consensus Workshop on Embryo Assessment[18]. Eleven embryos presented as good quality (AB/BB) embryos by Embryo day 5 and were biopsied. Using micromanipulation, 10-15 trophectoderm cells per embryo were isolated and placed into a 0.2 μL PCR tube; afterward, the embryos were frozen.
Embryo transfer and pregnancy test: For day 5 biopsied embryos, the resulting blastocysts were cryopreserved using the vitrification technique. The endometrial preparation was carried out with the transdermal application of 17-β-estradiol (Evorel 50; 150 μg/subcutaneous/every 48 h) and the luteal phase support was carried with Utrogestan (300 mg/day/vaginal). Clinical decisions about which and how many embryos to transfer were determined by the Physician and Specialist in Reproductive Medicine with the patient’s approval. Embryo implantation was confirmed on day 14 by β-hCG serum levels >10 mUI/mL or the presence of a fetal heartbeat by ultrasound at 6.5 to 8 wk. All the patient´s demographics, IVF cycle, PGT results, implantation rate, and IVF outcomes (pregnancies and miscarriages) were recorded by the Specialist.
Whole genome amplification and next-generation sequencing: Biopsies were taken from trophectoderm from at-risk LS embryos. For each sample, the whole genome was amplified (WGA) using the SurePlex amplification system (Illumina San Diego, CA, United States) according to the manufacturer’s instructions. WGA products were quantified using Qubit 3.0 Fluorometer (Life Technologies). The library preparation was carried out with the VeriSeq PGS Library Prep Kit (Illumina Inc.). DNA indexing was performed to simultaneously analyze samples from different embryos using the Nextera XT 96-Index Kit (Illumina, Inc.). For library preparation, 5µl (0.2 ng/µL) of each WGA product from each sample were tagmented (tagged and fragmented) by the VeriSeq PGS transposome using the manufacturer's protocol and neutralized by adding 5 µL of neutralization buffer. The tagmented DNA was amplified with Index 1 primers (N701 and N712) and Index 2 primers (S503 and S504) to become the next-generation sequencing (NGS) library via a limited cycle PCR program. Each sample’s NGS library was purified to remove short fragments and primers. Finally, NGS libraries were pooled, denatured with HT1, and loaded to the VeriSeq PGS (Illumina Inc.) sequencing cartridge following the manufacturer's protocol. NGS library was sequenced with a MiSeq apparatus using the MiSeq Reporter Software. Chromosome composition was determined as indicated above.
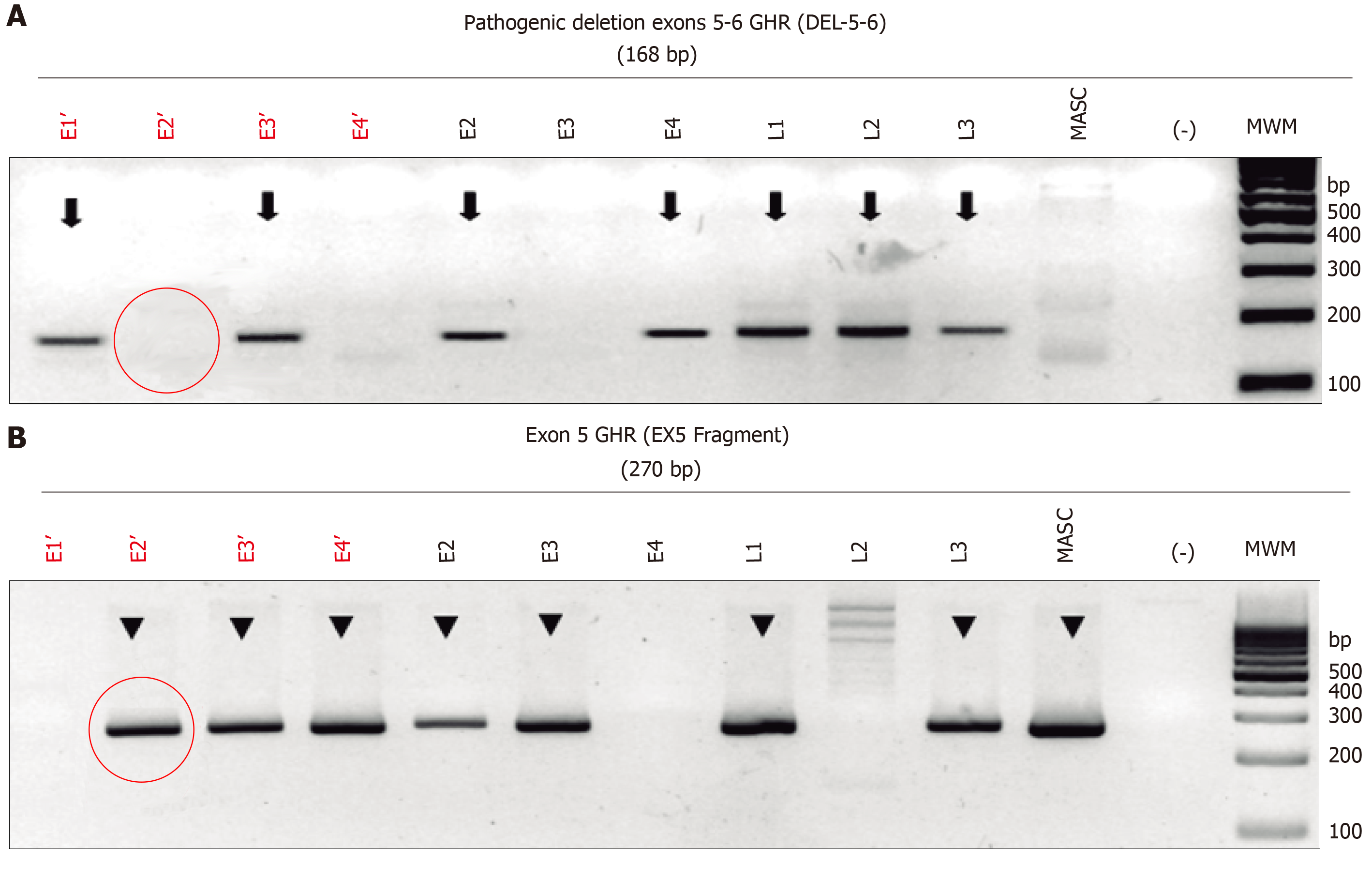
Determination of GHR mutations presence: Successful WGA was verified by agarose electrophoresis, which revealed an optimal DNA concentration to carry-out the PCR amplification of a 186-bp specific fragment of the deletion breakpoint of exons 5 and 6 of the GHR gene (NM_000163.4, 5p13-p12, MIM+600946) using the primers “5606” and “5662” and according to conditions previously published[19]. These primers would amplify a 186-bp fragment in both homozygous and heterozygous del5-6 genotypes, but not in normal wild-type samples. To distinguish the homozygous (affected) from heterozygous (carrier) del5-6 GHR genotype, a second end-point PCR assay was performed simultaneously with the primers “4947” and “5077” (19)to obtain a 269-bp fragment that evaluates the integrity of exon 5, which is present both in heterozygous del5-6 and wild-type GHR genotypes. These PCR assays were previously validated in genomic DNA samples derived from peripheral blood leukocytes from the unaffected obligate del5-6 heterozygous parents, as well as in their affected homozygous child with LS. All PCR assays were done in duplicate using 60 ng of WGA-derived DNA per reaction from at-risk LS and normal control, embryos (n = 3), the two heterozygous parents and the homozygous LS-affected child (DNA samples were isolated by standard genomic DNA isolation from blood), and water as a control. Amplicons were resolved by agarose gel electrophoresis stained with ethidium bromide and visualized and photographed under ultraviolet light (Figure 1).
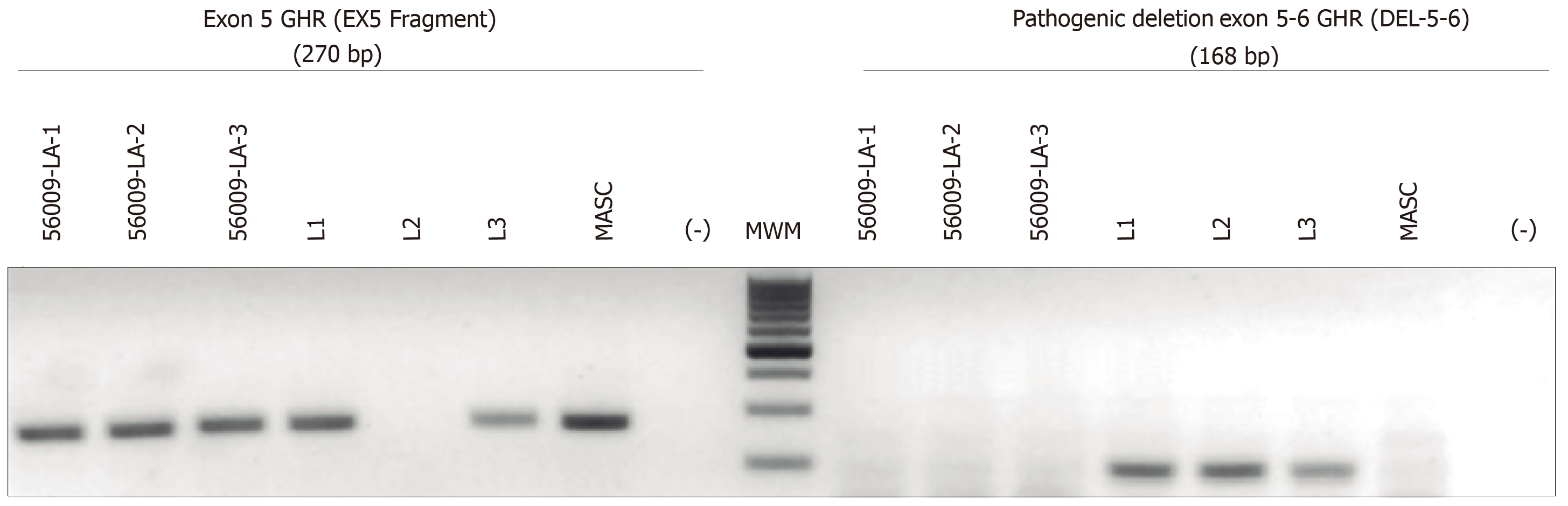
Confirmation of implantation and fetus GHR mutational status: Embryo implantation was confirmed on day 14 by β-hCG serum levels > 10 mUI/mL or the presence of a fetal heartbeat by ultrasound at 6.5 to 8 wk. The genomic composition of the fetus was verified and confirmed by applying the same techniques (WGA obtained from amniotic cells and PCR) using amniocytes extracted after 21 wk of the ongoing pregnancy (Figure 2).
Eleven embryos were collected from 2 rounds of IVF; 27.3% were the wild type for GHR, 45.5% were heterozygotes, and 18.2% homozygous mutants. One embryo yielded no results. Eight embryos were determined acceptable for transference. The patients agreed to have two embryos transferred.
Three 2-embryos transfers were performed (2 normal homozygous and 4 heterozygous carriers) were selected for transfer. The first 2 transfers were unsuccessful, whereas the final transfer, with 2 heterozygous embryos, resulted in a clinical pregnancy (β-hCG serum levels = 252.28 mUI/mL and the presence of one fetal heartbeat sac). The genomic composition of the fetus was verified, applying the same techniques but using DNA from amniocytes, extracted after 21 wk of the ongoing pregnancy. The fetus was confirmed as a heterozygous healthy carrier.
At 36 wk, the mother delivered a healthy baby. No physical deformities were present, suggesting the absence of LS. Moreover, at age 11 mo, the child was clinically confirmed as normal by the family pediatrician.
In the review by Zvi Laron in 2015, the author indicates that the use of PGT can aid LS patients to have healthy children; however, a search of the literature yielded no studies supporting this claim[8]. Here, we present a case study where a couple underwent IVF to select embryos that were wild-type or heterozygous carriers of the GHR mutations previously characterized in the parents. The procedure was successful, as a fetus amnion-analysis result excludes in the fetus in gestation the clinical and molecular diagnosis of LS attributable to a homozygous genotype for the pathogenic deletion that eliminates exons 5 and 6 ("del5-6") of the GHR gene previously characterized in the parents. Likewise, the absence of the "del5-6" fragment excludes the healthy carrier/heterozygous character in the fetus for LS due to the pathogenic deletion that eliminates exons 5 and 6 of the GHR gene. Eventually, the child was born and was physiologically normal.
One key concern for the procedure was the DNA source. With most LS patients, sample DNA comes from blood or buccal samples. With embryos, the DNA is more limited as for actual day 5-trophectoderm biopsies from blastocyst produce 10 to 15 cells for extraction of genomic DNA. To improve the signal, WGA was performed. With our method and using control samples (the parents, their affected child, and normal embryos), we can show the specificity of the monoplex PCR to determine the del5-6 of GHR. Moreover, when we applied the method to the embryo cohort, we determined that a minority of embryos would be unsuitable for implantation. When the selected embryos were implanted, we were able to confirm the genotype of the fetus, demonstrating the applicability of the method.
There are over 70 documented mutations associated with the development of LS[20]. For Latin Americans, the E180 polymorphism is the most common due to a mass migration to Ecuador[21]; nevertheless, the E180 polymorphism has been found in Brazil, Argentina, and Mexican-descendants in the United States[4]. Recently, in Monterrey, Mexico, three patients were identified with LS, and the key mutations that were identified as possible causes were not of GHR[5-7]. Here, we demonstrate that the LS in the parents was associated with the del5-6 of GHR. This is the first report to demonstrate the presence of this mutation in Mexico.
Interestingly, the connection with this family and other locations where the mutation is present is difficult to discern. Other than the family being Jewish, there is not another connection. This suggests that, for the Jewish population, members may want to undergo genetic analysis of common diseases.
In conclusion, to detect the del5-6 of GHR using WGA embryonic DNA and monoplex-PCR can identify LS at-risk embryos during PGT. Moreover, we provide evidence that WGA is useful and can serve as a template to diagnose other monogenic diseases pre-implantation in patients undergoing fertility treatments.
We would like to express our gratitude to the participants of the study, to Tania Rojas for their technical assistance in PGT, to the members of the INGENES-IVF Laboratory, and to Dr. Leonardo M. Porchia for his contributions in preparing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Settin A S-Editor: Yan JP L-Editor: A E-Editor: Wu YXJ
| 1. | Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone--a new inborn error of metabolism? Isr J Med Sci. 1966;2:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Godowski PJ, Leung DW, Meacham LR, Galgani JP, Hellmiss R, Keret R, Rotwein PS, Parks JS, Laron Z, Wood WI. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci U S A. 1989;86:8083-8087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 354] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Amselem S, Duquesnoy P, Attree O, Novelli G, Bousnina S, Postel-Vinay MC, Goossens M. Laron dwarfism and mutations of the growth hormone-receptor gene. N Engl J Med. 1989;321:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 191] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Gonçalves FT, Fridman C, Pinto EM, Guevara-Aguirre J, Shevah O, Rosembloom AL, Hwa V, Cassorla F, Rosenfeld RG, Lins TS, Damiani D, Arnhold IJ, Laron Z, Jorge AA. The E180splice mutation in the GHR gene causing Laron syndrome: witness of a Sephardic Jewish exodus from the Iberian Peninsula to the New World? Am J Med Genet A. 2014;164A:1204-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Castilla-Cortazar I, Femat-Roldán G, Rodríguez-Rivera J, Aguirre GA, García-Magariño M, Martín-Estal I, Espinosa L, Díaz-Olachea C. Mexican case report of a never-treated Laron syndrome patient evolving to metabolic syndrome, type 2 diabetes, and stroke. Clin Case Rep. 2017;5:1852-1855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Castilla-Cortazar I, De Ita JR, Aguirre GA, García-Magariño M, Martín-Estal I, Lara-Diaz VJ, Elizondo MI. Growth hormone insensitivity: Mexican case report. Endocrinol Diabetes Metab Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Castilla-Cortazar I, De Ita JR, Aguirre GA, Castorena-Torres F, Ortiz-Urbina J, García-Magariño M, de la Garza RG, Diaz Olachea C, Elizondo Leal MI. Fanconi Anemia and Laron Syndrome. Am J Med Sci. 2017;353:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Laron Z. Lessons from 50 years of study of laron syndrome. Endocr Pract. 2015;21:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Hamosh A. 262500 Laron Syndrome in Online Mendelian Inheritance in Man. Available from: https://www.omim.org/entry/262500?search=Laron%20syndromehighlight=%28syndrome%7Csyndromic%29%20laron. |
| 10. | Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N. Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity). Clin Endocrinol (Oxf). 2006;65:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Phanse-Gupte SR, Khadilkar VV, Khadilkar AV. Clinical features and endocrine profile of Laron syndrome in Indian children. Indian J Endocrinol Metab. 2014;18:863-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Laron Z, Mimouni F, Pertzelan A. Effect of human growth hormone therapy on penile and testicular size in boys with isolated growth hormone deficiency: first year of treatment. Isr J Med Sci. 1983;19:338-344. [PubMed] |
| 13. | Bissonnette B, Luginbuehl I, Marciniak B, Dalens BJ. Syndromes: Rapid recognition and perioperative implications. 1st ed. McGraw-Hill New York, NY, 2006. . |
| 14. | Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 530] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 17. | Koren D, Palladino A. Hypoglycemia. Genetic Diagnosis of Endocrine Disorders. 2nd ed. Elsevier. 2016;31-75. [DOI] [Full Text] |
| 18. | Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 1285] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 19. | Gastier JM, Berg MA, Vesterhus P, Reiter EO, Francke U. Diverse deletions in the growth hormone receptor gene cause growth hormone insensitivity syndrome. Hum Mutat. 2000;16:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Janecka A, Kołodziej-Rzepa M, Biesaga B. Clinical and Molecular Features of Laron Syndrome, A Genetic Disorder Protecting from Cancer. In Vivo. 2016;30:375-381. [PubMed] |
| 21. | Berg MA, Guevara-Aguirre J, Rosenbloom AL, Rosenfeld RG, Francke U. Mutation creating a new splice site in the growth hormone receptor genes of 37 Ecuadorean patients with Laron syndrome. Hum Mutat. 1992;1:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |