Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3622
Peer-review started: April 18, 2019
First decision: June 12, 2019
Revised: June 21, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: November 6, 2019
Processing time: 206 Days and 0.2 Hours
Timely reconstitution of a donor-derived immune system is important for recovery and long-term survival of patients after allogeneic hematopoietic stem cell transplantation (HSCT). We describe a case of Wiskott–Aldrich syndrome (WAS) treated by umbilical cord blood transplantation (UCBT) with atypical immune reconstruction.
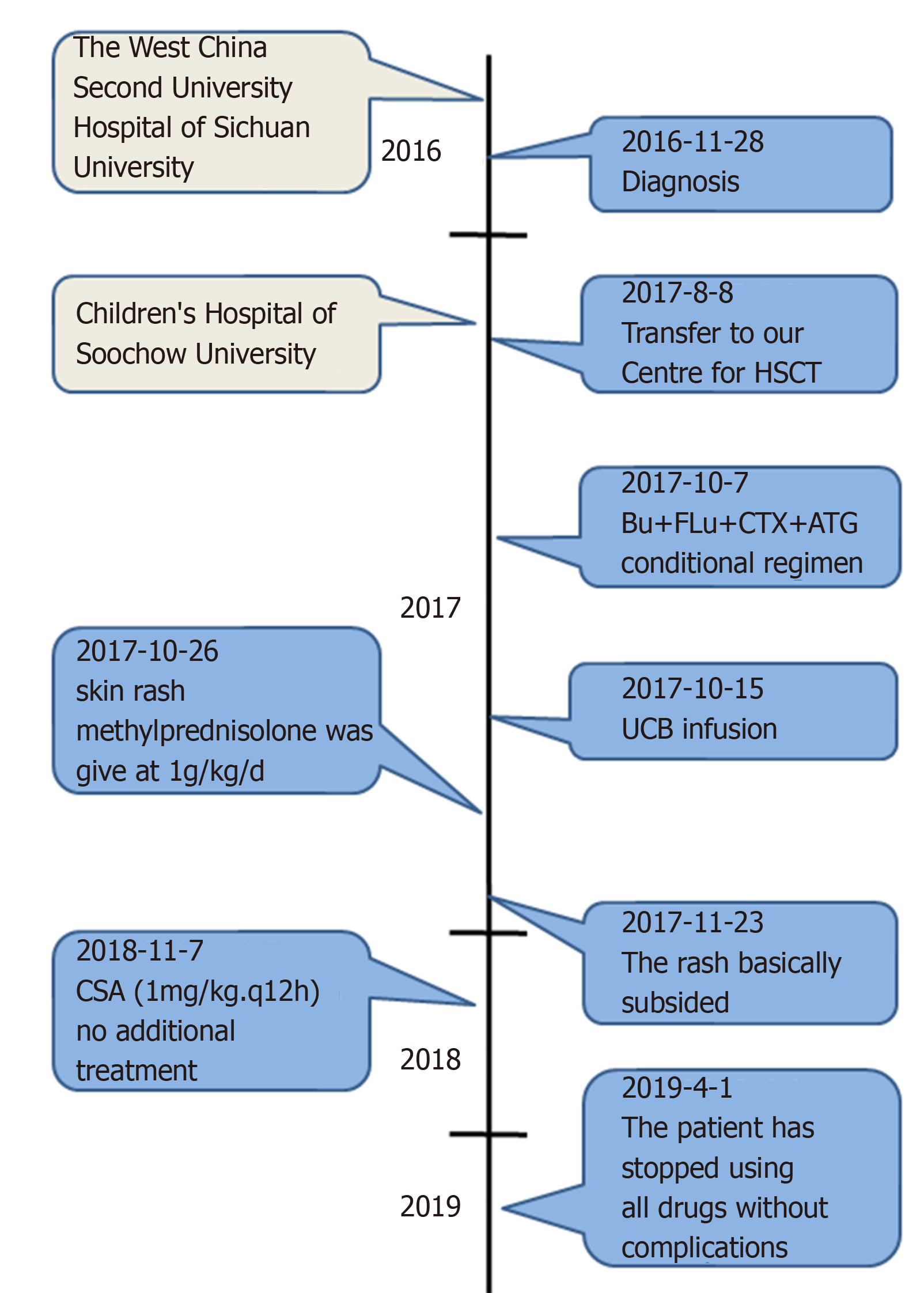
A 1-year-old Chinese male infant was diagnosed with WAS. WAS gene sequencing identified the mutation c.777 + 1G>A (IVS8). On August 8, 2017, he was admitted to our hospital for HSCT. We selected an unrelated Human leukocyte antigen 6/10-matched donor for UCBT. After HSCT, the immune reconstitution process was atypical, the lymphocytes reached 0.5 × 109/L on day 23, and the neutrophils reached 0.5 × 109/L on day 34. The patient’s recovery throughout the year was good.
An increase in lymphocytes (especially T cells) earlier than granulocytes may be a marker of a good prognosis in UCBT.
Core tip: The timely reconstitution of a donor-derived immune system is of utmost importance for the recovery and long-term survival of patients after hematopoietic stem cell transplantation. Here, we describe a case of Wiskott–Aldrich syndrome treated by umbilical cord blood transplantation with the atypical process of immune reconstitution. Our case revealed that an increase in the number of lymphocytes (especially T cells) earlier than granulocytes may be a marker of a good prognosis in patients. This experience will guide clinical scientists, especially hematologists, to deal with similar situations and encourage them to identify more processes that require immune reconstruction.
- Citation: Li BH, Hu SY. Child with Wiskott–Aldrich syndrome underwent atypical immune reconstruction after umbilical cord blood transplantation: A case report. World J Clin Cases 2019; 7(21): 3622-3631
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3622.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3622
Wiskott–Aldrich syndrome (WAS) is a rare X-linked recessive immunodeficiency disorder. The clinical manifestations of WAS are thrombocytopenia and small platelets, eczema, and recurrent infection[1]. The gene responsible for this syndrome is the WAS protein (WASP) gene. Hematopoietic cell transplantation is the only proven cure for WAS. The unrelated donor umbilical cord blood transplantation (UCBT) is a good option for patients that do not have a Human leukocyte antigen (HLA)-matched donor[2]. The advantage of UCBT is that one can search and prepare umbilical cord blood on time without delay, and it has a low incidence and severity of graft versus host disease (GVHD)[3]. However, slow hematopoietic reconstruction and increasing probability of engraftment failure prevent widespread use of UCBT[4]. As a result, the patients who experience slow immune reconstitution are at high risk of infection. Innate immunity usually recovers within several weeks after transplantation. By contrast, adaptive immunity recovers more slowly[5-7]. This delayed engraftment and immune reconstitution result in higher rates of early post-transplantation infection complications in UCBT[8,9]. Therefore, identifying the mechanism that regulates immune reconstitution after transplantation, and developing strategies to enhance immune reconstitution after UCBT, will significantly improve the efficacy of UCBT. Here, we report a case of WAS in a 1-year-old Chinese male infant successfully treated by UCBT with atypical immune reconstruction. The reconstitution of adaptive immunity in this patient was earlier than that of innate immunity. Although the reconstitution of granulocytes was slow, the patient did not show serious infection during engraftment, and remained stable for 1 year after UCBT.
Fecal blood, diarrhea and thrombocytopenia over one year.
A 1-year-old Chinese male patient with WAS was admitted for UCBT. He presented with fecal blood, diarrhea and thrombocytopenia at age 4 d, and was admitted to the West China Second University Hospital of Sichuan University. Admission to our hospital for hematopoietic stem cell transplantation.
None.
Nothing special.
Complete blood count revealed that white blood cell (WBC) count was 13.64 × 109/L, hemoglobin 99 g/L, platelet count 7 × 109/L, and neutrophil count 3.53 × 109/L. Bone marrow (BM) aspiration revealed that the granulocyte/erythrocyte ratio was 0.7:1, thromocytogenic megakaryocyte 15/50, and platelets were deficient. WAS gene sequencing identified the mutation c.777 + 1G>A (IVS8).
We selected an unrelated HLA 6/10-matched donor for UCBT (Table 1). The conditioning regimen was busulfan 1.0 mg/kg day-9 to day-6; fludarabine 40 mg/m2, day-5 to day-2; cyclophosphamide 60 mg/kg, day-5 to day-4; antithymocyte globulin (ATG) 2.5 mg/kg, qd, day-8 to day-5. Prophylaxis for GVHD was cyclosporin A (CSA, 6–8 mg/kg daily) and mycophenolate mofetil (MMF, 20 mg/kg/d). Heparin and alprostadil were used for preventing hepatic venous occlusive disease (VOD). Ganciclovir, acyclovir, micafungin and meropenem were used for preventing viral, fungal and bacterial infection, respectively. The details of drug use is shown in Table 2. The number of CD34+ cells was 4.7 × 105/kg, and total nucleated cells (TNCs) 9.17 × 107/kg. On day 6 after UCBT, granulocyte colony-stimulating factor was given to promote granulocyte recovery, and γ-globulin and composition blood transfusion (irradiated platelets) were used for supportive therapy.
| Patient 0 | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| HLA-A(patient/donor) | 02:07 11:02 | 02:01 33:03 | 02:07 30:01 | 24:20 30:01 | 32:01 11:01 | 03:01 01:01 |
| 02:07 24:02 | 02:06 33:03 | 02:07 30:01 | 24:20 31:01 | 32:01 02:06 | 03:01 32:01 | |
| HLA-B(patient/donor) | 54:01 40:02 | 40:06 58:01 | 13:02 46:01 | 13:02 40:01 | 44:03 48:01 | 27:05 57:01 |
| 54:01 56:01 | 40:06 58:01 | 13:02 46:01 | 13:02 40:01 | 44:03 48:01 | 27:05 57:01 | |
| HLA-C(patient/donor) | 01:02 07:02 | 03:02 08:01 | 01:02 06:02 | 06:02 07:02 | 04:01 08:01 | 02:02 06:02 |
| 01:02 07:02 | 03:02 08:01 | 01:02 06:02 | 06:02 07:02 | 04:01 08:01 | 02:02 06:02 | |
| HLA-DRB1(patient/donor) | 14:54 12:02 | 03:01 09:01 | 07:01 09:01 | 07:01 08:03 | 07:01 15:01 | 15:01 11:04 |
| 14:55 12:02 | 03:01 09:01 | 07:01 09:01 | 07:01 08:03 | 07:01 15:01 | 15:01 10:01 | |
| HLA-DQB1(patient/donor) | 05:03 03:02 | 02:01 03:03 | 02:02 03:03 | 02:02 06:01 | 02:02 06:02 | 03:01 03:01 |
| 05:03 03:01 | 02:01 03:03 | 02:02 03:03 | 02:02 06:01 | 02:02 05:03 | 05:01 06:02 | |
| Matches | 6/10 | 9/10 | 10/10 | 9/10 | 8/10 | 6/10 |
| The conditioning regimen | Bu + FLu + CTX + ATG | BU 1.0 mg/kg, day-9 to day-6; FLu, 40 mg/m2, day-5 to day-2; CTX, 60 mg/kg, day-5 to day-4; ATG 2.5 mg/kg, qd, day-8 to day-5 |
| Prophylaxis for GVHD | CSA + MMF | CSA (6-8 mg/kg/d) and MMF (20 mg/kg/d) |
| Preventing hepatic VOD | Heparin and alprostadil | |
| Preventing infection | Ganciclovir, acyclovir, micafungin and meropenem |
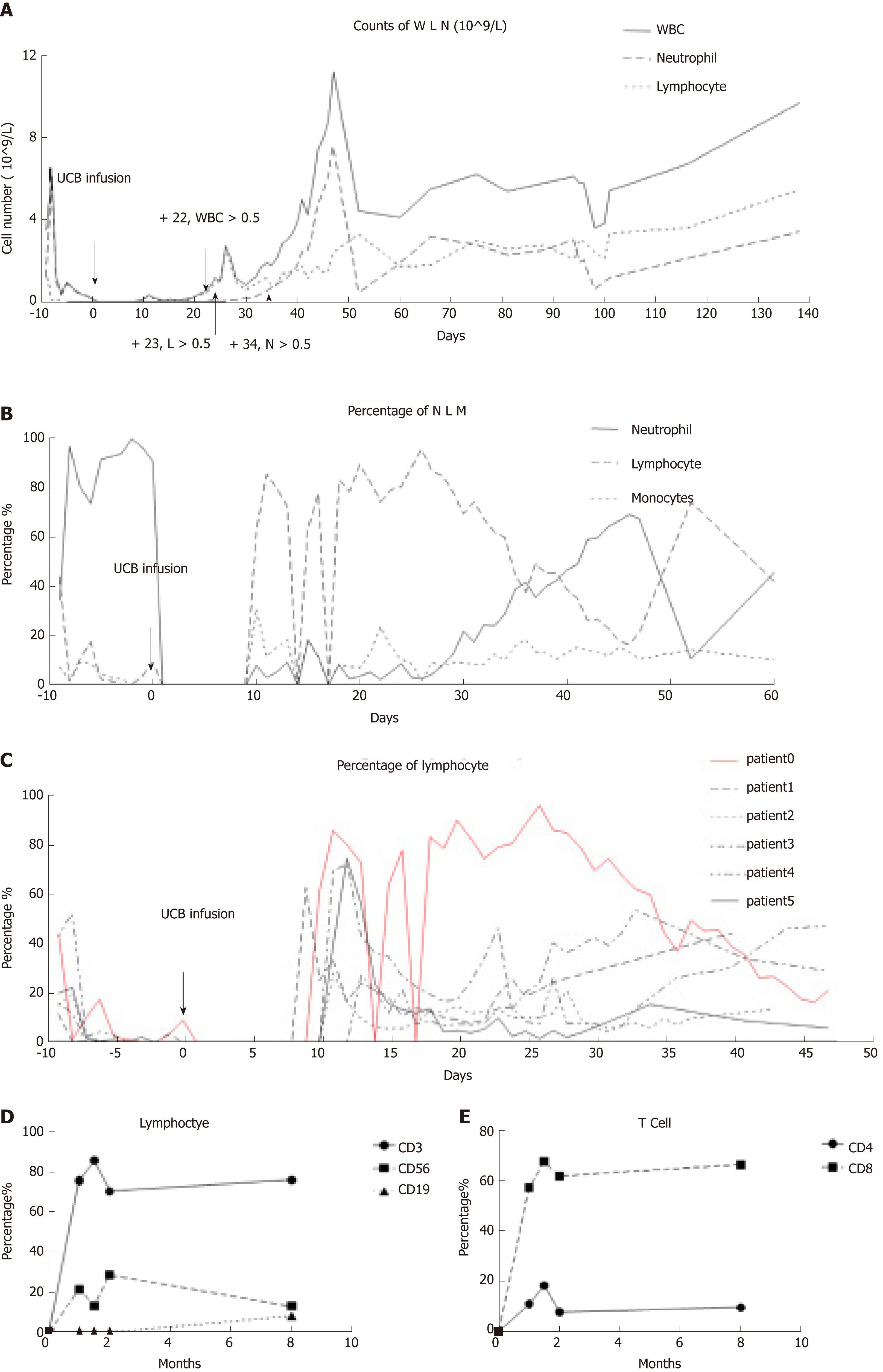
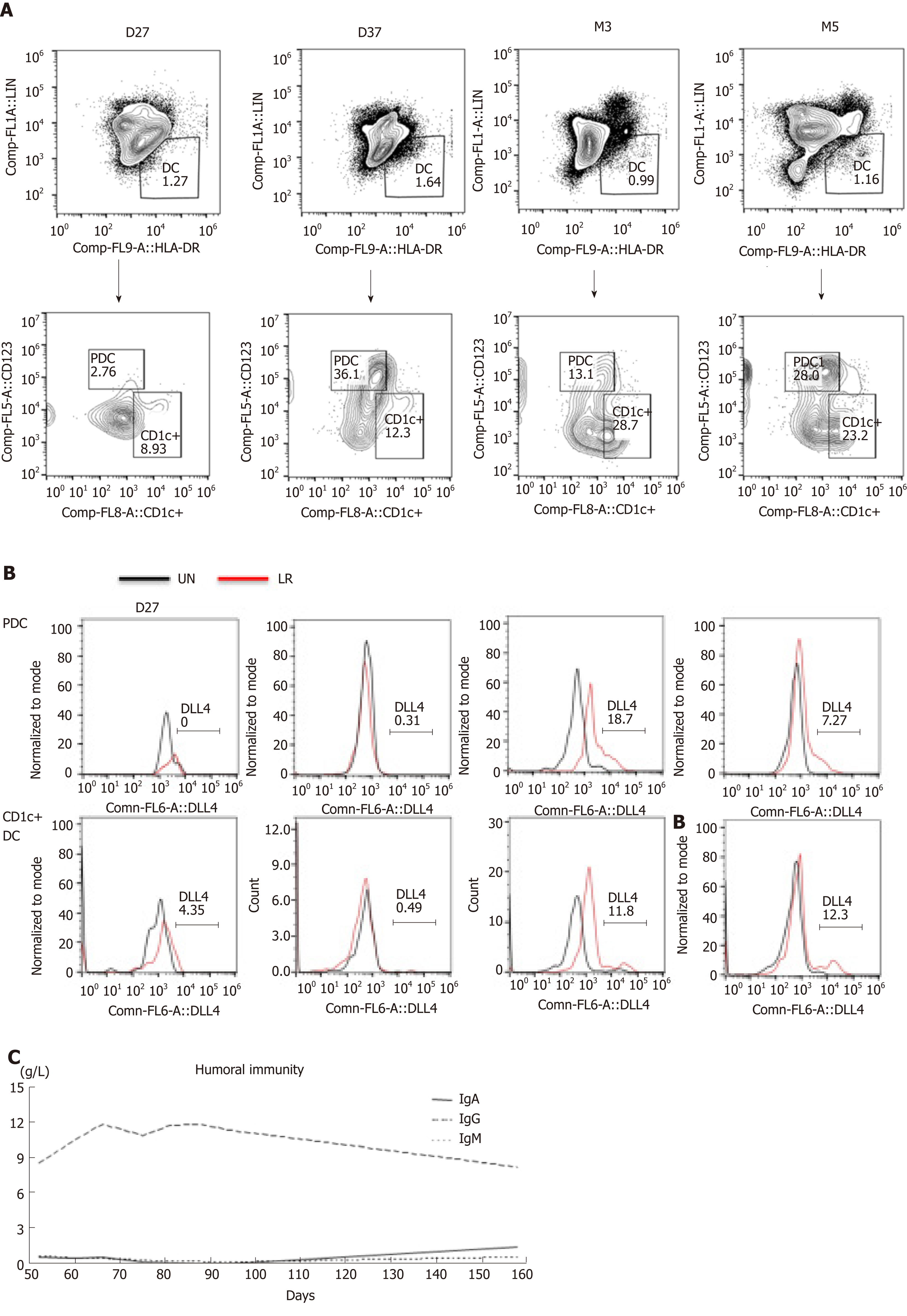
On day 10, a new rash occurred on both earlobes. On day 11, the rash spread throughout the body, and methylprednisolone was given at 1g/kg daily. Complete blood count was monitored daily: WBC count reached 0.5 × 109/L on day 22, lymphocytes reached 0.5 × 109/L on day 23, and neutrophils reached 0.5 × 109/L on day 34 (Figure 1A). Therefore, this patient presented with immune reconstitution, with lymphocytes first and neutrophils later. We analyzed another five patients who received UCBT, who all showed immune reconstitution with neutrophils first and lymphocytes later (Figure 1C and Table 3). T cells, especially CD8+ T cells, were the major lineage in the reconstituted lymphocytes (Figure 1D and 1E). Previous studies showed that dendritic cells (DCs) expressing Notch ligand DLL4 are critical for eliciting alloreactive T cell responses and inducing GVHD in mice[10-12]. We also analyzed the reconstruction of DLL4+ DCs. Compared to healthy people, the percentage of DCs, ratio of plasmacytoid DCs (pDCs) and CD1c+ DCs, and expression of ligand DLL4 were similar (Figure 2). The levels of IgG, IgA and IgM returned to normal within 50 d after UCBT, suggesting recovery of humoral immunity (Figure 2C). The patient did not develop obvious GVHD or infection 12 mo after UCBT. The patient is currently under monthly monitoring. Except for CSA (1 mg/kg q12h), no additional treatment was used to prevent GVHD. The timeline is shown in Figure 3.
| Patient 0 (case) | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Age in yr | 1 | 4 | < 1 (8 m) | 1 | 5 | 2 |
| Sex | Male | Male | Male | Male | Male | Male |
| Time for UCBT | 2017/10/15 | 2017/6/3 | 2018/6/8 | 2018/3/24 | 2017/7/7 | 2017/12/15 |
| CD34 dose | 4.7 × 105/kg | 2 × 105/kg | 4.92 × 105/kg | 2.4 × 1055/kg | 4.55 × 105/kg | 3.0 × 105/kg |
| TNC | 9.17 × 107/kg | 6.73 × 107/kg | 15.3 × 107/kg | 11.1 × 107/kg | 6.26 × 107/kg | 9.23 × 107/kg |
| Conditional regimen | Bu + Flu + CTX + ATG | BU + Flu + CTX + ATG | BU + Flu + CTX + ATG | BU + Flu + CTX + ATG | BU + Flu + CTX + ATG | BU + Flu + CTX + ATG |
| HLA (/6) | 4/6 | 6/6 | 6/6 | 5/6 | 5/6 | 4/6 |
| HLA (/10) | 6/10 | 9/10 | 10/10 | 9/10 | 8/10 | 6/10 |
| Blood type (patient/donor) | B/B | A/O | B/B | O/O | O/O | A/AB |
| Neutrophil grafted time | D34 | D16 | D13 | D15 | D17 | D14 |
| Lymphocytes>0.5 × 109/L | D23 | D28 | D42 | D17 | D30 | D65 |
| PLT grafted time | D66 | D28 | D29 | D30 | D33 | D27 |
| Main complication | No | Hemorrhagic cystitis | Infection by Pneumocystis carinii | No | Intestinal infection | Leak syndrome, hemorrhagic cystitis |
Delayed immune reconstitution limits the success of hematopoietic stem cell transplantation (HSCT). Here, we report a case showing an unusual pattern of immune reconstitution after UCBT, which was characterized by an increase in the number of lymphocytes earlier than granulocytes. The lymphocyte count peaked at day 23 after UCBT; however, granulocytes were engrafted (for clinical engraftment, the number of granulocytes is > 0.5 × 109/L on the first day, and remains so for at least 3 consecutive days) at day 34. This patient did not develop infection or severe GVHD. We propose that there should be another definition of engraftment for lymphocytes, and ponder whether or not WBC engraftment should occur after HSCT.
UCBT has many advantages for treating WAS, as compared to other types of HSCT. For example, the acquisition of CB is easy, collection of CB is harmless, and the CB handling process, such as thawing, does not induce loss of cell proliferation[4,13-16]. In addition, CB transplantation showed a lower risk of acute and chronic GVHD than other transplantation strategies using a different cell source[17,18]. However, delayed immune reconstitution results in higher rates of early post-transplant infectious complications in UCBT[8,9].
The recovery of the immune system after transplantation in humans is an orderly and dynamic process[19]. In general, innate immunity, namely phagocytes and natural killer lymphocytes, recovers in the first few weeks after transplantation. Then, the adaptive immune system, namely B and T lymphocytes, recovers, which may take months to years[7,9,16,20]. Reconstitution of CD4+ T cells is later than that of CD8+ T cells, which in most cases also results in a long period of CD4+/CD8+ inversion after transplantation. Previous studies showed that de novo thymic generation of CD4+CD45RA+ primitive T cells is needed for the recovery of CD4+ T cells[21,22]. Pang et al[23] reported that in a non-acute GVHD group, CD3+ T lymphocytes gradually returned to normal after 2 mo, and the CD4/CD8 ratio was reversed. Here, we report that this patient showed an atypical immune reconstitution. His process of immune reconstitution was lymphocytes (mainly T cells) engrafted first, with granulocytes engrafted later. His lymphocyte ratio was at a high level at 35 d after UCBT, which was different from other WAS patients who underwent UCBT in our Center. The subsets of T cells in the present patient were mainly CD8+, and there was an inverse proportion. Previous studies showed an increased CD4+ ratio in some cases of increased immune abnormalities, such as autoimmune diseases and acute GVHD. An increase in the proportion of CD8+ T cells does not increase the risk of acute GVHD. We hypothesize that this patient with a high percentage of CD8+ T cells protected him from serious GVHD. The patient’s short tandem repeat (STR) in BM and PB was detected on a regular basis in our center. The STR of PB was 76.8% on day 14. The STR of BM was 62.3% on day 17. At this time, the number of cells was still low, and the donor cells had not yet been engrafted. This state may have helped the engraftment of donor lymphocytes. At 20–40 d, when lymphocytes suddenly increased, the STR in PB was 98.6% on day 24 and 98.1% in BM on day 27. The details of STR is shown in Table 4. This also means that the rapid growth of lymphocytes comes from donors. This rapid early growth of donor lymphocytes may not lead to severe GVHD, but can play a role against infection. However, its mechanism needs to be further studied.
| Day | PB or BM | STR |
| 14 | PB | 76.80% |
| 24 | PB | 98.60% |
| 31 | PB | 99.10% |
| 38 | PB | 98.80% |
| 45 | PB | 99.30% |
| 52 | PB | 98.80% |
| 60 | PB | 99.30% |
| 66 | PB | 98.60% |
| 75 | PB | 98.70% |
| 102 | PB | 98.70% |
| 17 | BM | 62.30% |
| 26 | BM | 98.10% |
| 43 | BM | 98.70% |
The reconstitution of DCs after transplantation is significantly correlated with GVHD. Delayed DC recovery and lower DC numbers, especially of pDCs, are found in patients with acute and chronic GVHD[24]. pDCs play an important role in HSCT immunomodulation[25]. In the present case, we found that DCs recovered rapidly, suggesting that it is a favorable prognostic factor in predicting GVHD and infection. Recently, a new population of DLL4+ DCs has been identified. DLL4 is an important ligand of the Notch family[26]. DLL4+ DCs can promote differentiation of Th1 and Th17 cells to a greater degree than DLL4− DCs. A mouse model that received allogeneic DLL4+ DC-induced CD4+ T cells developed only minimal GVHD[10,12]. In humans, less research has been performed on DLL4+ DCs. Human immature CD1c+ DCs and pDCs express low levels of DLL4, and they rapidly upregulate expression of DLL4 upon activation with the TLR7/8 agonist R848 (resiquimod) and/or the TLR4 agonist lipopolysaccharide (LPS). However, allogeneic HSCT recipients had 16-fold more DLL4+CD1c+ DCs than healthy donors[11,27]. In this patient, we found rapid DC reconstitution, and each DC subset recovered to normal within 2 mo. In addition, we measured the population of DLL4+ DCs at day 27 and 37, day 90, and day 150 upon stimulation. We found that the expression of DLL4 on DCs was upregulated after resiquimod and LPS stimulation, and reached the same level as healthy people at 3 mo after transplantation. Therefore, successful DC reconstitution also indicates good prognosis.
Previous studies reported that humoral immunity usually takes 1 year to reach normal levels after HSCT, especially for IgG and IgA[21]. In our case, we found that the levels of IgA, IgG and IgM recovered to normal within 2 mo after UCBT. The recovery of humoral immunity was faster than that reported in other studies. This special immune reconstitution may be a good prognostic indicator for UCBT.
This new way of immune reconstitution can provide a novel means to study immune reconstitution after UCBT. However, the mechanism of immune reconstitution, and whether it can be used as a marker of good prognosis, requires further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xavier-Elsas P S-Editor: Cui LJ L-Editor: Filipodia E-Editor: Xing YX
| 1. | Kaneko R, Yamamoto S, Okamoto N, Akiyama K, Matsuno R, Toyama D, Hoshino A, Imai K, Isoyama K. Wiskott-Aldrich syndrome that was initially diagnosed as immune thrombocytopenic purpura secondary to a cytomegalovirus infection. SAGE Open Med Case Rep. 2018;6:2050313X17753788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Shekhovtsova Z, Bonfim C, Ruggeri A, Nichele S, Page K, AlSeraihy A, Barriga F, de Toledo Codina JS, Veys P, Boelens JJ, Mellgren K, Bittencourt H, O'Brien T, Shaw PJ, Chybicka A, Volt F, Giannotti F, Gluckman E, Kurtzberg J, Gennery AR, Rocha V; Eurocord, Cord Blood Committee of Cellular Therapy and Immunobiology Working Party of the EBMT, Federal University of Parana, Duke University Medical Center and Inborn Errors Working Party of the EBMT. A risk factor analysis of outcomes after unrelated cord blood transplantation for children with Wiskott-Aldrich syndrome. Haematologica. 2017;102:1112-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, Liu D, Liu Q, Liu T, Jiang M, Ren H, Song Y, Sun Z, Wang J, Wu D, Zhou D, Zou P, Liu K, Huang X. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 4. | Luan C, Chen R, Chen B, Ding J, Ni M. Umbilical cord blood transplantation supplemented with the infusion of mesenchymal stem cell for an adolescent patient with severe aplastic anemia: a case report and review of literature. Patient Prefer Adherence. 2015;9:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, Passweg J, Roosnek E. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Mehta RS, Rezvani K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence. 2016;7:901-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Servais S, Hannon M, Peffault de Latour R, Socie G, Beguin Y. Reconstitution of adaptive immunity after umbilical cord blood transplantation: impact on infectious complications. Stem Cell Investig. 2017;4:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Danby R, Rocha V. Improving engraftment and immune reconstitution in umbilical cord blood transplantation. Front Immunol. 2014;5:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | de Koning C, Admiraal R, Nierkens S, Boelens JJ. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig. 2017;4:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Luo X, Xu L, Li Y, Tan H. Notch pathway plays a novel and critical role in regulating responses of T and antigen-presenting cells in aGVHD. Cell Biol Toxicol. 2017;33:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Meng L, Hu S, Wang J, He S, Zhang Y. DLL4+ dendritic cells: Key regulators of Notch Signaling in effector T cell responses. Pharmacol Res. 2016;113:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Mochizuki K, Meng L, Mochizuki I, Tong Q, He S, Liu Y, Purushe J, Fung H, Zaidi MR, Zhang Y, Reshef R, Blazar BR, Yagita H, Mineishi S, Zhang Y. Programming of donor T cells using allogeneic δ-like ligand 4-positive dendritic cells to reduce GVHD in mice. Blood. 2016;127:3270-3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Roura S, Pujal JM, Gálvez-Montón C, Bayes-Genis A. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Res Ther. 2015;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A. 2003;100:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Mitchell R, Wagner JE, Brunstein CG, Cao Q, McKenna DH, Lund TC, Verneris MR. Impact of long-term cryopreservation on single umbilical cord blood transplantation outcomes. Biol Blood Marrow Transplant. 2015;21:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Xie LN, Zhou F. Unexpected unrelated umbilical cord blood stem cell engraft in two patients with severe aplastic anemia that received immunosuppressive treatment: A case report and literature review. Exp Ther Med. 2015;10:1563-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Munoz J, Shah N, Rezvani K, Hosing C, Bollard CM, Oran B, Olson A, Popat U, Molldrem J, McNiece IK, Shpall EJ. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med. 2014;3:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Solh M. Haploidentical vs cord blood transplantation for adults with acute myelogenous leukemia. World J Stem Cells. 2014;6:371-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012;19:324-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Szabolcs P, Cairo MS. Unrelated umbilical cord blood transplantation and immune reconstitution. Semin Hematol. 2010;47:22-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, Weissinger E. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2016;7:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 22. | Chang YJ, Zhao XY, Huang XJ. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Pang N, Duan X, Jiang M, Qu J, Yuan H, Xu J, Cao H, Chen G. Reconstitution and clinical significance of T cell subsets in the early stage after related HLA-mismatched peripheral blood hematopoietic SCT without T-cell depletion in vitro. Int J Clin Exp Pathol. 2015;8:8892-8901. [PubMed] |
| 24. | Nachbaur D, Kircher B. Dendritic cells in allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2005;46:1387-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Auletta JJ, Devine SM, Waller EK. Plasmacytoid dendritic cells in allogeneic hematopoietic cell transplantation: benefit or burden? Bone Marrow Transplant. 2016;51:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 429] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 27. | Meng L, Bai Z, He S, Mochizuki K, Liu Y, Purushe J, Sun H, Wang J, Yagita H, Mineishi S, Fung H, Yanik GA, Caricchio R, Fan X, Crisalli LM, Hexner EO, Reshef R, Zhang Y, Zhang Y. The Notch Ligand DLL4 Defines a Capability of Human Dendritic Cells in Regulating Th1 and Th17 Differentiation. J Immunol. 2016;196:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |