Published online Jan 26, 2019. doi: 10.12998/wjcc.v7.i2.221
Peer-review started: October 13, 2018
First decision: November 15, 2018
Revised: December 9, 2018
Accepted: December 12, 2018
Article in press: December 12, 2018
Published online: January 26, 2019
Processing time: 107 Days and 1.1 Hours
Metastatic low-grade endometrial stromal sarcoma (LG-ESS) with sex cord-like and smooth muscle-like differentiation is rare. This article reports such a case with multiple recurrences and with extensive pelvic and abdominal metastasis.
A 47-year-old female patient was diagnosed with multiple cystic masses in the pelvic cavity by magnetic resonance imaging examination. Based on the postoperative pathological and immunohistochemical analyses of the surgical specimen, she was diagnosed with a metastatic low-grade endometrial stromal sarcoma with sex cord and smooth muscle differentiation.
LG-ESS is a low-grade malignant tumor with a high recurrence rate and metastasis probability. It is easily misdiagnosed initially. It is essential to distinguish LG-ESS with sex cord-like differentiation from uterine tumour resembling ovarian sex cord tumour.
Core tip: Endometrial stromal sarcomas are common tumours. Metastatic low-grade endometrial stromal sarcoma (LG-ESS) with sex cord and smooth muscle differentiation, however, is an extremely rare diagnosis. To date, only two cases have been reported in the literature. Histopathologic examination revealed a uterine leiomyoma within a pelvic mass. LG-ESS with sex cord-like differentiation should be distinguished from uterine tumor resembling ovarian sex cord tumor.
- Citation: Zhu Q, Sun YQ, Di XQ, Huang B, Huang J. Metastatic low-grade endometrial stromal sarcoma with sex cord and smooth muscle differentiation: A case report. World J Clin Cases 2019; 7(2): 221-227
- URL: https://www.wjgnet.com/2307-8960/full/v7/i2/221.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i2.221
Endometrial stromal sarcoma (ESS) is a mesenchymal tumor, including low-grade ESS (LG-ESS), high-grade ESS (HG-ESS), and undifferentiated ESS (UESS). Uterine stromal sarcoma accounts for about 1% of all uterine malignant tumors and 10% of uterine sarcomas[1]. However, LG-ESS with sex cord-like and smooth muscle-like tissue differentiation as well as extensive metastasis is rarely reported. Here, we report such a case for the first time and review the relevant literature.
Abdominal distension without obvious cause, frequent urination, and urinary incontinence for 10 d.
On January 14, 2018, a 47-year-old female patient came to our hospital due to "abdominal distension for 10 d and swelling of both lower limbs for 3 d". The ultrasound examination showed a huge hyperechoic mass in the liver and multiple peritoneal solid space-occupying lesions. Then the patient was admitted as having “primary liver cancer”.
A laparoscopic partial hysterectomy was performed in 2014 for uterine fibroids, with a pathological diagnosis of uterine leiomyoma. In 2011, a laparoscopic myomectomy was performed for uterine fibroids, with a pathological diagnosis of endometrial stroma and smooth muscle mixed tumor with sex cord-like differentiation.
Regular menstrual cycle but more menstrual flow. The patient denied any family history of genetic disease or history of cancer.
The patient's mental state, appetite, food intake, and sleeping status were good, and her body weight was 5 kg higher than it was before onset. The pelvic mass was clinically investigated, and the patient was admitted to the hospital for a malignant ovarian tumor.
Laboratory findings included CA125, 189.5 U/mL; albumin, 29.5 g/L; alanine aminotransferase, 7.8 U/L; creatinine, 50.0 μmol/L; indirect bilirubin, 3.3 μmol/L; direct bilirubin, 2.1 μmol/L; total bilirubin, 5.4 μmol/L; prothrombin time, 12.6 s; and platelet volume distribution width, 9.6%.
Ultrasound examination showed a large hyperechoic mass in the liver, multiple solid space-occupying lesions in the abdominal cavity, as well as a large amount of fluid in the abdominal cavity. Magnetic resonance imaging examination revealed multiple cystic masses in the pelvic cavity of the middle and lower abdomen, with local fusion. A tumor of adnexal origin was strongly considered, as the tumor was pushing the adjacent organs and blood vessels, with a clear boundary. The uterus could not be seen, and the bilateral adnexa were unclear. Plain and enhanced computed tomography scanning revealed multiple masses of different sizes in the middle and lower abdominal-pelvic cavity, strongly suggesting malignant tumors originating from reproductive organs. Intravenous pyelography showed no visualization of the right ureter and the lower segment of the left ureter, no dilatation of the bilateral pelvis and calyx, and poor bladder filling.
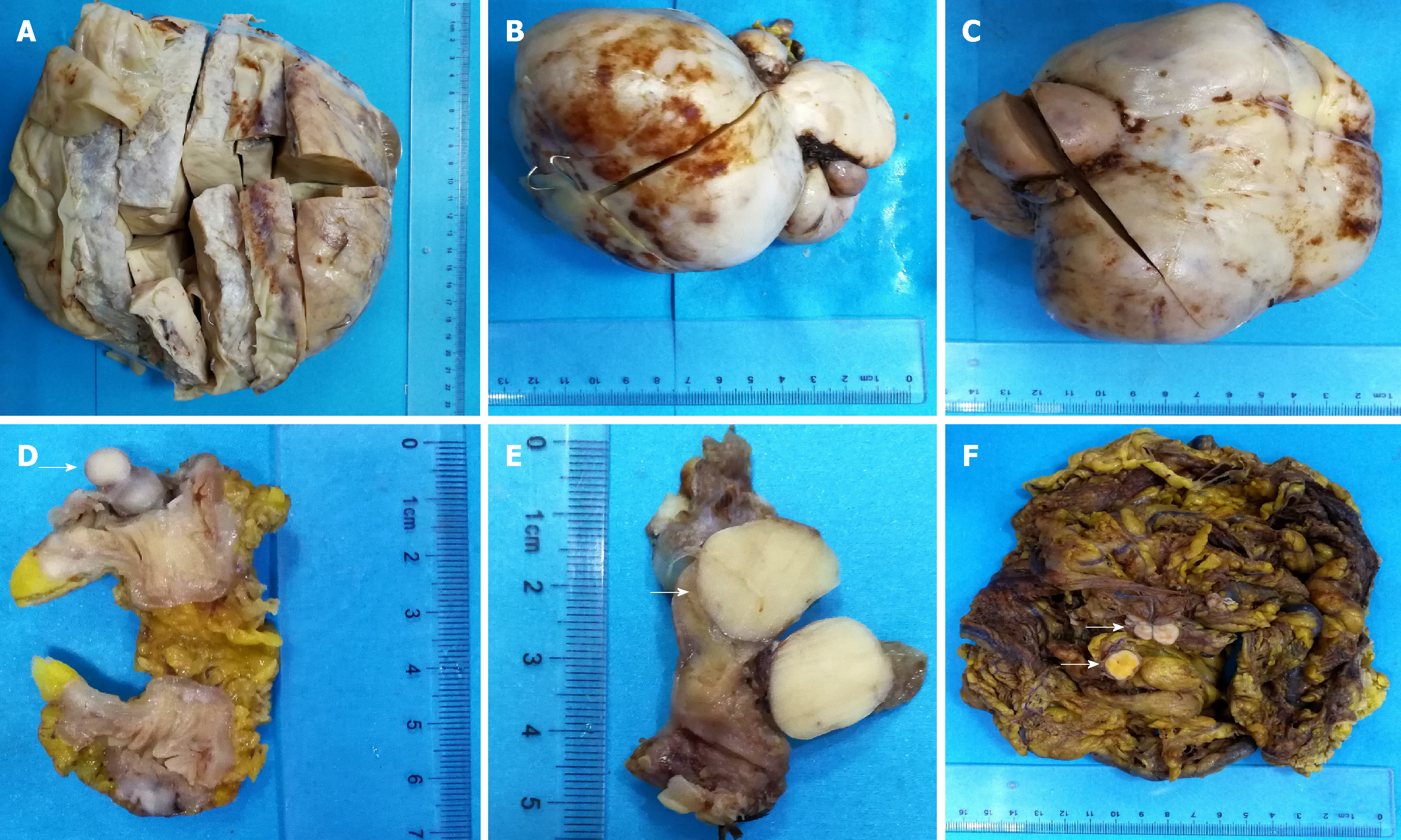
The specimen contained three solitary masses of 13-25 cm in diameter. The cut surface was grayish-white-yellow, with a tough texture and intact capsule, showing hemorrhage and cystic changes (Figure 1A-C). A 3.5 cm segment of the small intestine contained a nodule on the cut surface between the muscle and serosal layers, with a 1.5 cm diameter, grayish-white color, and a tough texture (Figure 1D). A segment of the oviduct, 4 cm long and 0.5 cm in diameter, contained a nodule under the serosa of 1.5 cm in diameter, and the cut surface was gray with a tough texture (Figure 1E). A large mass of omentum tissue, measuring 26 cm × 23 cm × 22 cm, showed several scattered nodules, with diameters of 1.5-2.5 cm, a grayish-white or -yellow cut surface, and a tough texture (Figure 1F).
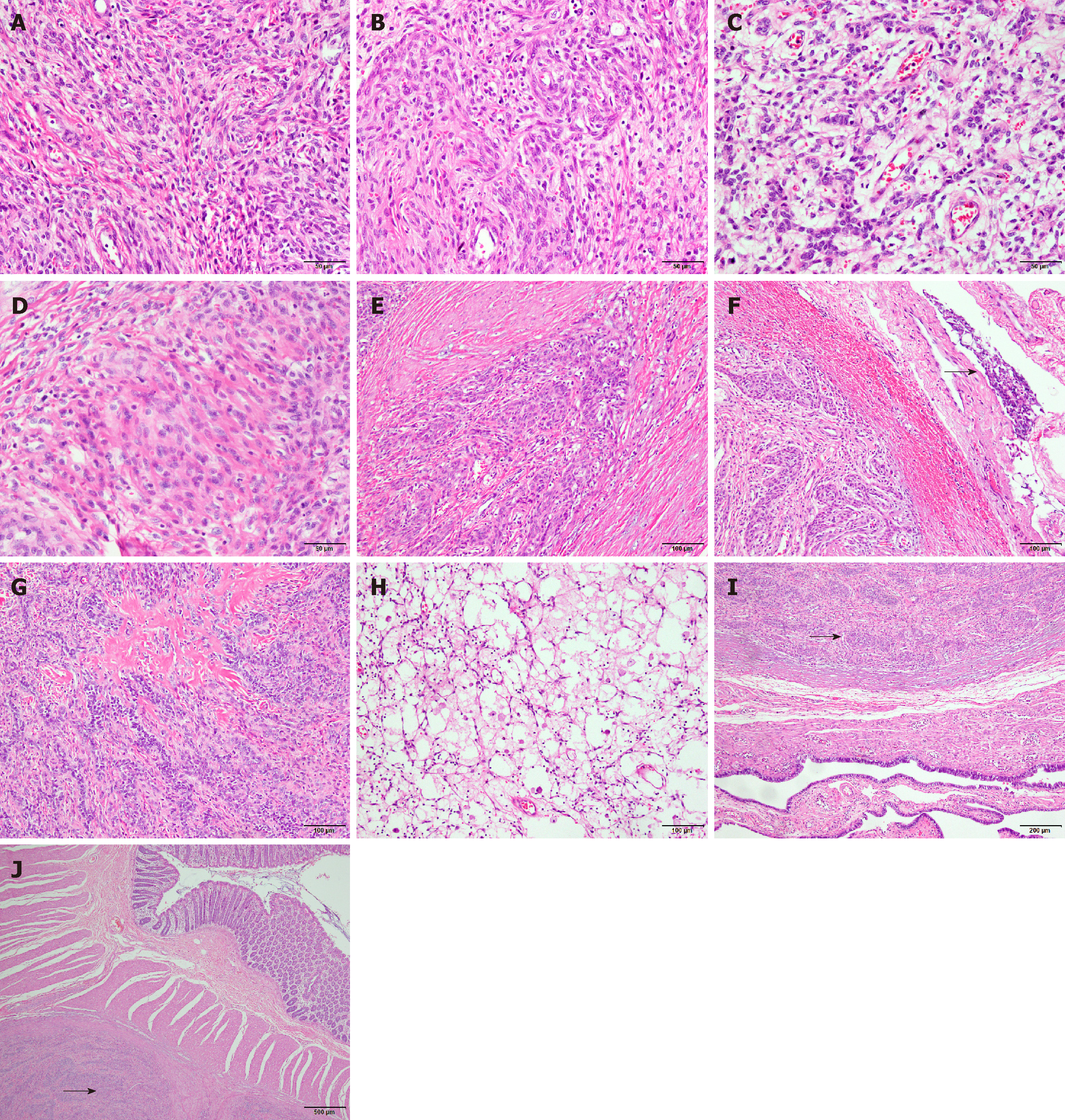
The specimens were fixed in 10% neutral formalin, processed, embedded in paraffin, routinely sectioned at a thickness of 4 μm, stained with hematoxylin and eosin, and observed and photographed under a light microscope. Microscopic observation of the (pelvic/abdominal) mass showed diffusely distributed tumor cells, in the shapes of cords or small islands in some areas, which were connected in a network. The tumor cells were uniformly sized and shaped like short spindles. The cells were benign, and mitotic figures were rare (< 3/10 high power fields) (Figure 2A). The tumor tissue was rich in blood vessels, and some tumor cells were distributed in a vortex-like appearance around the blood vessels (Figure 2B). Many sex cord-like differentiated tumor cells were observed (Figure 2C), and some tumor cells were differentiated into smooth muscle cells (Figure 2D). The tumor cells showed "tongue-like" invasive growth (Figure 2E), and vascular invasion in the encapsulated portion was observed in some areas (Figure 2F). Part of the stroma showed obvious hyaline degeneration, forming a starburst-like structure (Figure 2G), while foam cells and inflammatory cells were also observed (Figure 2H). Combined with the immunohistochemical analysis, the results were in line with a low-grade endometrial stromal sarcoma with sex cord-like and smooth muscle-like differentiation (pelvic/abdominal cavity, small mesentery, and right oviduct). The microscopic morphologies of the small intestine, oviduct, and omental nodules were consistent with that of the pelvic/abdominal tumor (Figure 2I-J). The pathological sections from 2011 and 2014 were reviewed, and their pathological morphologies were consistent with those of the new one. The final pathological diagnosis was low-grade endometrial stromal sarcoma with sex cord-like and smooth muscle differentiation, with extensive metastasis in the sigmoid colon, oviduct, and omentum.
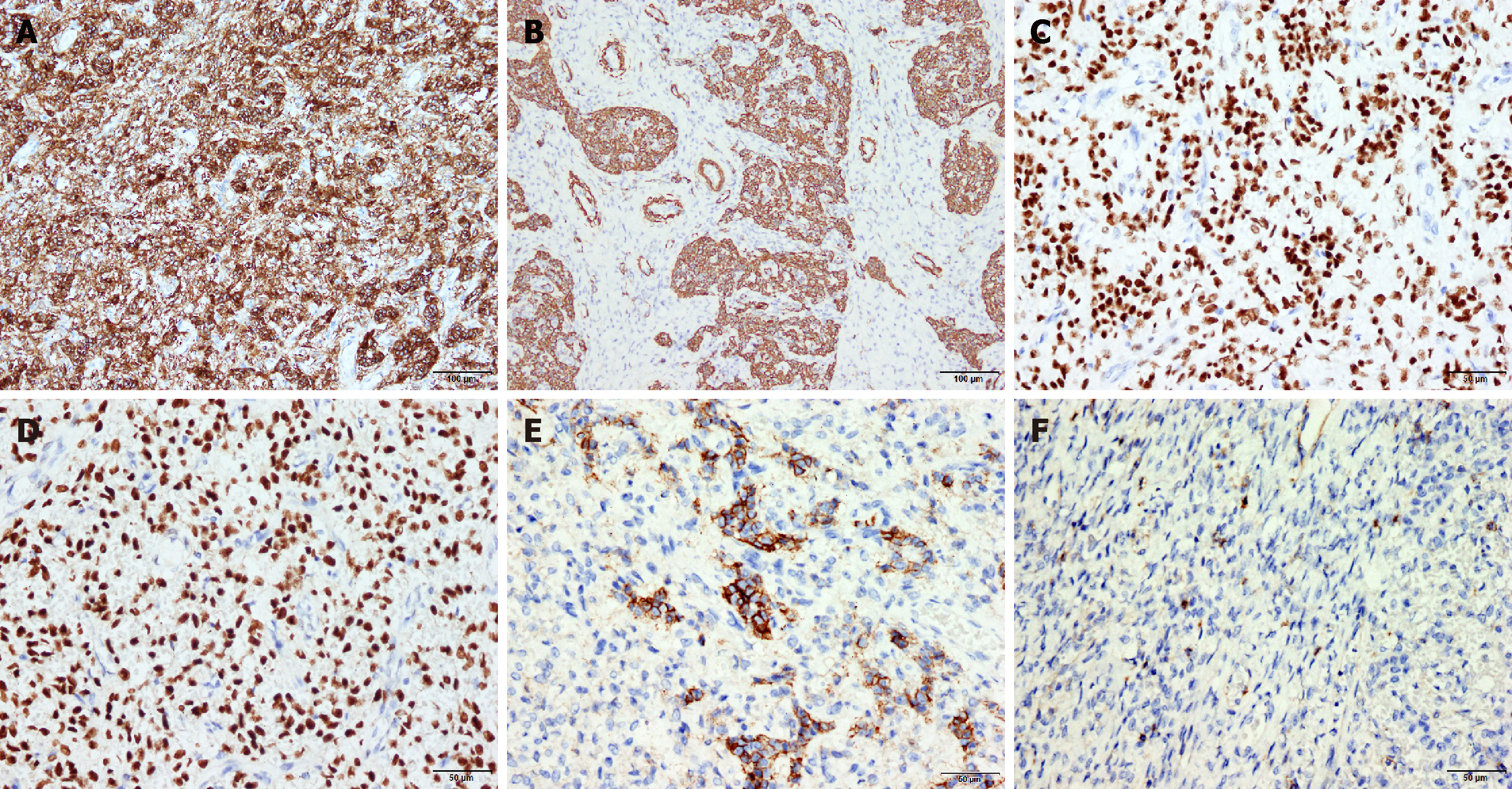
The tumor cells were CD10 diffuse (+) (Figure 3A), Vimentin (+), SMA (partial +) (Figure 3B), cyclinD1 (weak focal +), DOG-1 (partial +), CD34 (vessel +), ER (+++, 90%) (Figure 3C), and PR (+++, 90%) (Figure3D, CD56 (partial +) (Figure 3E), CD99 (focal +) (Figure 3F),and the ki-67 index was approximately 3%. The results of the remaining immunohistochemistry tests were negative.
Metastatic low-grade endometrial stromal sarcoma with sex cord and smooth muscle differentiation.
On January 30, 2018, the patient underwent a surgery involving small mesenteric tumor resection, partial sigmoid omentectomy, and left salpingectomy, with intraoperative observation of 400 mL intra-abdominal pale yellow ascites, the absence of a uterus, normal appearance of the left adnexa and right ovary, a nodule approximately 1.5 cm in diameter on the isthmus of the right oviduct, and multiple tumors in the small intestinal mesentery. The largest tumor was approximately 30 cm × 25 cm × 20 cm, the diameters of the other two tumors were 15 and 13 cm, and the nodule in the sigmoid colon was 3 cm × 2 cm × 2 cm. Different sized nodules were scattered throughout the omentum, pelvic cavity, and abdominal cavity, and the lesion diameters were 0.5-3 cm. Then, the patient was given anti-infection treatment with antibiotics, potassium chloride rehydration, as well as infusion of albumin to correct hypoproteinemia. The postoperative recovery was acceptable, but the patient was followed without further treatment.
A recent regular follow-up was carried by telephone, and the patient recuperated at home without any abnormality.
Per the new World Health Organization classification for female genital tumors (4th edition, 2014), endometrial stroma and related tumors are divided into five types: endometrial stromal nodules, LG-ESS, HG-ESS, UESS, and uterine tumors resembling ovarian sex cord tumors (UTROSCTs)[1]. LG-ESS is a rare uterine tumor that accounts for 0.2% of gynecologic malignancies.
LG-ESS tumors are usually 1-25 cm in size, with an average value of 8-11 cm. The tumors are mostly nodular, and the cut surface is grayish-white or brownish-yellow, with cystic changes in some cases. Microscopically, the tumor cells are elliptical to short fusiform, with less cytoplasm, similar to the interstitial cells of the proliferative endometrium, and the tumor cells can be arranged around the small spiral arteries. LG-ESS has low cellular atypia, and mitosis is rare. The tumor cells invade the myometrium in a tongue-like pattern, often accompanied by vascular invasion. Tumor stromal cells can differentiate into various tissues, such as ovarian cord, smooth muscle, and glandular tissue. Foam cells can be observed in the lesions, and hyaline degeneration and mucoid changes often occur in the matrix[1]. In addition to the basic pathomorphological characteristics of LG-ESS described above, the case reported here was accompanied by extensive sex cord-like and smooth muscle differentiation in the ovary. Immunohistochemical CD10 was positive in all regions, including the regions with sex cord-like and smooth muscle differentiation, indicating that these cells were derived from the endometrial stromal cells, which is consistent with LG-ESS[2]. In the region with ovarian sex cord-like tumor cell differentiation, CD56 was positive, and CD99 was focal positive, while all other antibodies, including inhibin, calretinin, and Melan-A, were negative, suggesting that the tumor cells in this ovarian sex cord-like differentiated region were immature, and the characteristics of ovarian mature sex cord-like cells were not fully expressed. Although some tumor cells were morphologically characterized as having cord-like differentiation in trabecular, cord, nested, or small tubular structural arrangements, no Leydig cell or Sertoli cell-like tubular sex cord-like structures were observed. Studying cases with LG-ESS-associated sex cord-like differentiation revealed that the available literature included mainly case reports, with no systematic research[3-7]. Regarding how to assess the sex cord-like differentiation in LG-ESS, we believe that in addition to the presence of the sex cord-like differentiated structures, including the trabecular, cord, or small tubular structures in the LG-ESS background, immunohistochemical assays are conducive to making assessments. Inhibin, calretinin, Melan-A, CD99, and CD56 are marker antigens for sex cord-like differentiation, and their positive expression suggests sex cord-like differentiation[1]. However, not all these antigens are positive, and in most cases, only some of these antigens are expressed. CD56 is generally expressed only in the region with sex cord-like differentiation, while CD10 is usually expressed weakly in this region. For the case reported here, CD56 and CD99 were expressed in the sex cord-like differentiated region, while inhibin, calretinin, and Melan-A were negative.
LG-ESS with sex cord-like differentiation should be distinguished from UTROSCTs. The concept of UTROSCTs was first proposed by Clement et al[6] in 1976. When sex cord-like elements are found in LG-ESS, they are also called endometrial stromal tumors with sex cord-like elements (ESTSCLEs). Morphologically, ESTSCLEs and UTROSCTs are greatly overlapped, and distinguishing them is difficult. Generally, for ESTSCLEs, the proportion of sex-like elements is low (no more than 10%), CD10 is strongly positive, and some ovarian sex cord tumor marker antigens are expressed such as inhibin, calretinin, Melan-A, CD99, and CD56. In UTROSCTs, the sex cord-like elements must be greater than 50%, the differentiation should be relatively mature, and the positive marker antigen expression rate in ovarian cord tumors should be high. Molecular detection is also conducive to making this distinction. ESTSCLEs are prone to t (7;17) (p15;q21) JAZF1-SUZ12 (JJAZ1) gene fusion, while UTROSCTs have no JAZF1-SUZ12 gene fusion[1]. Distinguishing ESTSCLEs from UTROSCTs is clinically significant. ESTSCLEs are a concomitant malignancy of LG-ESS, while UTROSCTs have low-grade malignancy potential, and most UTROSCTs are benign in clinical practice. Younger women can undergo hysterectomies with ovarian preservation[8].
In addition to sex cord-like differentiation, LG-ESS may also be associated with differentiation to other tissues such as smooth muscle. Interestingly, in the case reported here, the regions with sex cord-like and smooth muscle-like differentiation were greatly overlapped. Whether this suggests that these two differentiated tissues were derived from the same progenitor cells requires further study. LG-ESS can occur in many areas outside the uterus, such as the ovaries, small intestine, pelvic cavity, abdominal cavity, retroperitoneum, vagina, bladder, and lymph nodes. Their morphologies are the same as those in LG-ESS of uterine origin, which often causes difficulty in the initial pathological diagnosis, and the initial diagnosis is correct in only 50% of cases[9]. LG-ESS occurring outside the uterus may be associated with endometriosis. In the initial diagnosis, a comprehensive assessment should be made carefully by reviewing the morphology, immunohistochemical expression, molecular pathology, and clinical history. Our case is one of recurrence and metastasis, previously diagnosed as endometrial stroma and smooth muscle mixed tumor with sex cord-like differentiation and uterine leiomyoma. The morphologies of the previous two diagnoses were the same as those of the new one. Therefore, when LG-ESS is associated with a heterogeneous component such as sex cord-like and smooth muscle-like differentiation, it must be distinguished from other tumor types in the diagnosis. Most importantly, immunohistochemistry application should be highlighted in such cases in order to abolish misleading diagnosis and subsequent clinical decisions in favor of the patient. Clinically, LG-ESS is generally indolent, and total hysterectomy is currently the main treatment.
LG-ESS is a low-grade malignant tumor with a high recurrence rate and metastasis probability. It is easily misdiagnosed initially. It is essential to distinguish LG-ESS with sex cord-like differentiation from uterine tumour resembling ovarian sex cord tumour.
CARE Checklist (2013) statement: The manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bonanno E, Georgios P S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classilication of Tumours of Female Reproductive Organs. Lyon: International Agency for Research on Cancer 2014; 141-145. |
| 2. | McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 227] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Richmond AM, Rohrer AJ, Davidson SA, Post MD. Low-grade endometrial stromal sarcoma with extensive sex cord differentiation, heterologous elements, and complex atypical hyperplasia: Case report and review of literature. Gynecol Oncol Rep. 2016;19:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Yenicesu O, Tokmak A, Sirvan AL, Danisman N, Güngör T. Low-grade endometrial stromal sarcoma combined with leiomyoma (stromomyoma): A bizarre tumor of the uterus in a premenopausal woman. J Exp Ther Oncol. 2018;12:281-286. [PubMed] |
| 5. | Sato S, Ojima Y, Kanda M, Kizaki T, Ohara N. Endometrial Stromal Sarcoma Arising from Endometrial Polyp: A Case Report. Kobe J Med Sci. 2018;64:E36-E42. [PubMed] |
| 6. | Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol. 1976;66:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 211] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. New York: Springer US 2011; . |
| 8. | Schraag SM, Caduff R, Dedes KJ, Fink D, Schmidt AM. Uterine Tumors Resembling Ovarian Sex Cord Tumors - Treatment, recurrence, pregnancy and brief review. Gynecol Oncol Rep. 2017;19:53-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Mukhopadhyay M, Das C, Parvin T, Basu K. Undifferentiated Uterine Sarcoma: An Uncommon Case Report. J Clin Diagn Res. 2017;11:ED03-ED04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |