Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3104
Peer-review started: June 19, 2019
First decision: August 1, 2019
Revised: September 8, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 6, 2019
Processing time: 109 Days and 17.3 Hours
Neurofibromatosis type I (NF1) is the most frequent subtype of neurofibromatosis. Its related tumor-suppressor syndromes are characterized by a predisposition to multiple tumor types and other disorder presentations. In addition, the incidence of tumors is much higher in patients with neurofibromatosis type I. However, there are very few reports at home and abroad on this topic. Here, we present a case of NF1 with spindle cell sarcoma.
A 50-year-old male was found to have a right axillary mass for 20 years. Specialist examination found cafe-au-lait spots on many parts of the skin, rounded nodules in the skin, a bulge in the right armpit, touching a lump (10 cm × 6 cm, hard, unclear boundary, poor mobility, local tenderness). The anterior side of the thigh felt weakened on the opposite side; in the right groin a swollen lymph node (hard, clear border, good mobility, local tenderness). According to the results of positron emission tomography/computed tomography, puncture pathology and immunohistochemistry, genetic testing, a diagnosis of NF1 with spindle cell sarcoma was confirmed. According to the genetic testing result, the patient was given a targeted treatment with crizotinib.
Surgery, chemotherapy and radiotherapy are the main treatment methods of NF1. However, with the continuous progress of molecular biology research, molecular targeted therapy may bring benefits for patients.
Core tip: Neurofibromatosis type I with malignant tumor is very rare. Because of its rarity in clinical practice, there are many uncertainties in the selection of treatment regimens. Therefore, it is necessary to study the effective and reasonable standard treatment regimens. We present a case of neurofibromatosis type I with soft tissue sarcoma. Since there is limited evidence of targeted therapy for this disease, the selection of treatment strategies should be based on rich clinical experience and research.
- Citation: Zhang Y, Chao JJ, Liu XF, Qin SK. Type I neurofibromatosis with spindle cell sarcoma: A case report. World J Clin Cases 2019; 7(19): 3104-3110
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3104.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3104
Neurofibromatosis type I (NF1), also known as von Recklinghausen disease, is an autosomal dominant neurocutaneous disorder caused by a mutation in the NF1 gene, and it usually presents during the first decade of life. NF1 is the most frequent subtype of neurofibromatosis (approximately 97% of NF patients), with a family history of approximately 50% and an incidence of 1/3000[1-3]. Clinical manifestations include abnormal skin pigmentation (cafe-au-lait patches and freckles), multiple discrete dermal neurofibromas, iris hamartomas known as Lisch nodules, bone dysplasia, mental retardation, and affected tissue and organ damage (neural, skeletal, visceral). In addition, approximately 10%-25% of patients may experience involvement of the digestive system (e.g., bleeding, perforation, intestinal obstruction, etc)[4,5].
The exact pathogenesis of NF1 is unclear, and mutations in the NF1 gene are important causes of NF1. Moreover, NF1 is also associated with the development of tumor, disease efficacy, prognosis, and even poor resistance to certain tumor types[6]. In recent years, it has been found that malignancies are up to four to six times more common in patients with NF1 than in the general population, and the probability of malignant connective tissue tumors or other soft tissue tumors is 34 times than that of unaffected people[7]. The expression of NF1 is highly variable in patients with the same mutation and within the same family. Large complete gene deletions (5%–10%) have a severe clinical phenotype with intellectual disability, higher benign tumor burden, and higher incidence of malignant nerve sheath tumors[3]. NF1 fibromatosis is usually benign, and cases of malignant transformation are rare. There are few reports on NF1 with undifferentiated pleomorphic sarcoma at home and abroad. A case of NF1 with spindle cell sarcoma is reported as follows.
A 50-year-old male with a mass and pain at the right fossa iliaca for 3 mo.
Half a year ago, the patient again palpated a mass at the right fossa iliaca. About 3 mo ago, the patient felt tingling from the right fossa iliaca mass, and this was accompanied by radiation pain in the anterior side of the right thigh. No nausea and vomiting, abdominal pain, bloating, obvious weight loss, and other discomforts were reported.
There was no history of chronic diseases such as coronary heart disease, hypertension, and diabetes. About 20 years ago, the right fossa iliaca mass was found in the health examination (about 4 cm × 4 cm), and a resection operation in the local hospital was performed (specifically unknown).
The patient presented with cafe-au-lait spots on the body and subcutaneous nodules; at the right fossa iliaca a bulge was seen, touching a lump (10 cm × 6 cm, hard, unclear boundary, poor mobility, local tenderness). The anterior side of the right thigh felt weaker than the contralateral side; in the right groin there was a swollen lymph node (hard, clear border, good mobility, local tenderness.
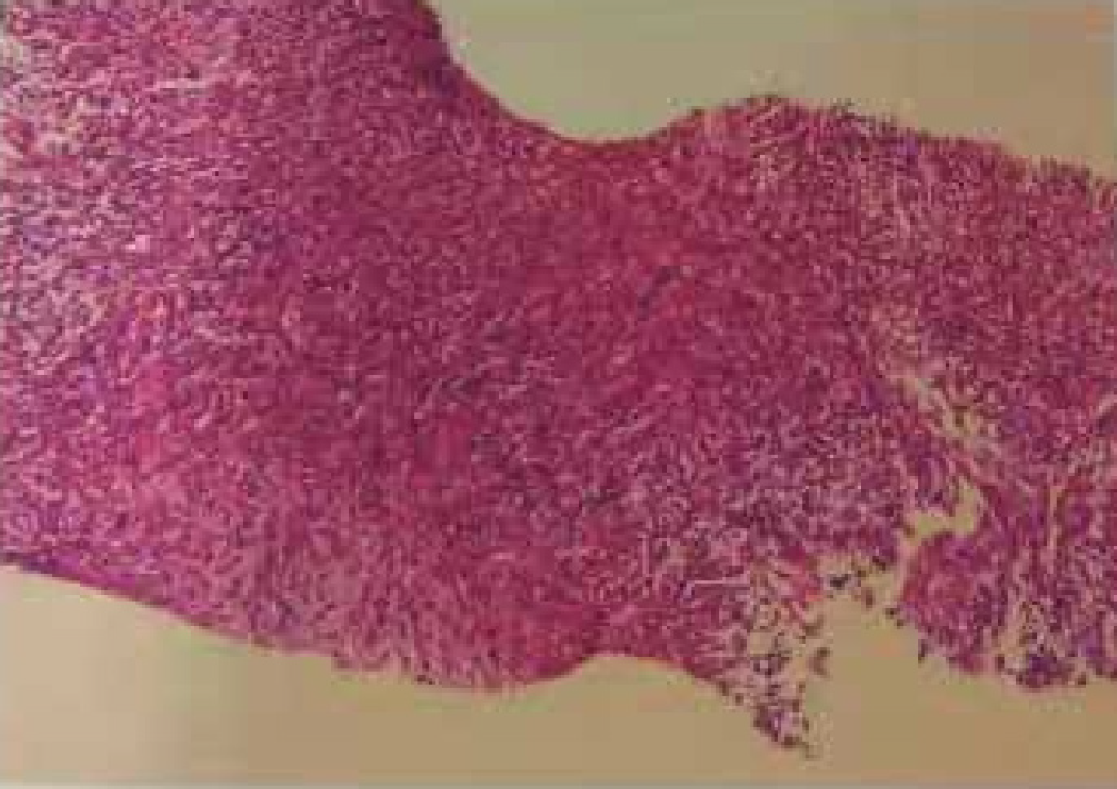
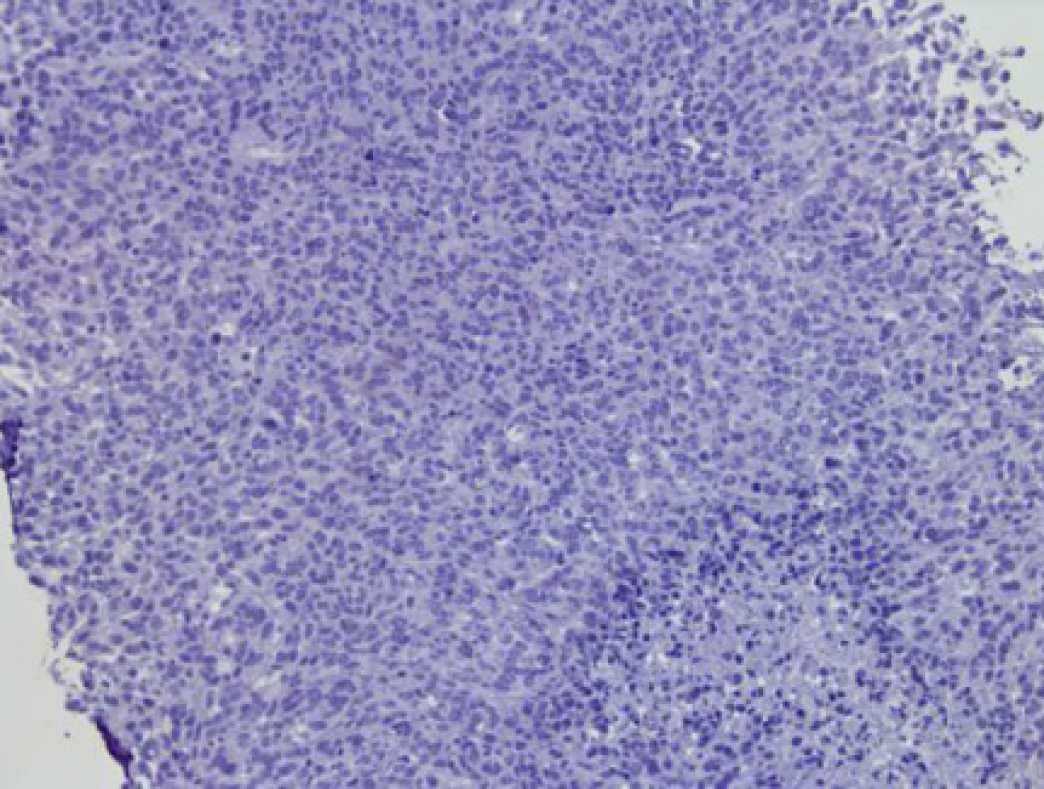
With a suspicion of malignancy, fine-needle-aspiration biopsy was performed. The right abdominal mass aspiration biopsy revealed a spindle cell malignancy (Figure 1). Immunohistochemistry revealed S-100 (small +), CD34 (+++), desmin (individual +), SMA (-), Ki67 (20%+), pan-cytokeratin (-), NF (-), CK-L (-), myogenin (-), and myogenic differentiation 1 (-) and was combined with hematoxylin & eosin staining. This case is a spindle cell sarcoma with limited puncture tissue, and it was recommended after tumor removal to perform pathology for tumor differentiation. In cases of neurological tumors, they tend to be malignant peripheral nerve sheath tumors. Genetic testing and NF1 gene analysis found a pathogenic variant in exon 32 (p. Q1395Hfs). Also, a fusion in the ROS1 gene was detected (p. TJP1 exon8); TMB-L, MSS, and PD-L1 were negative (Figure 2).
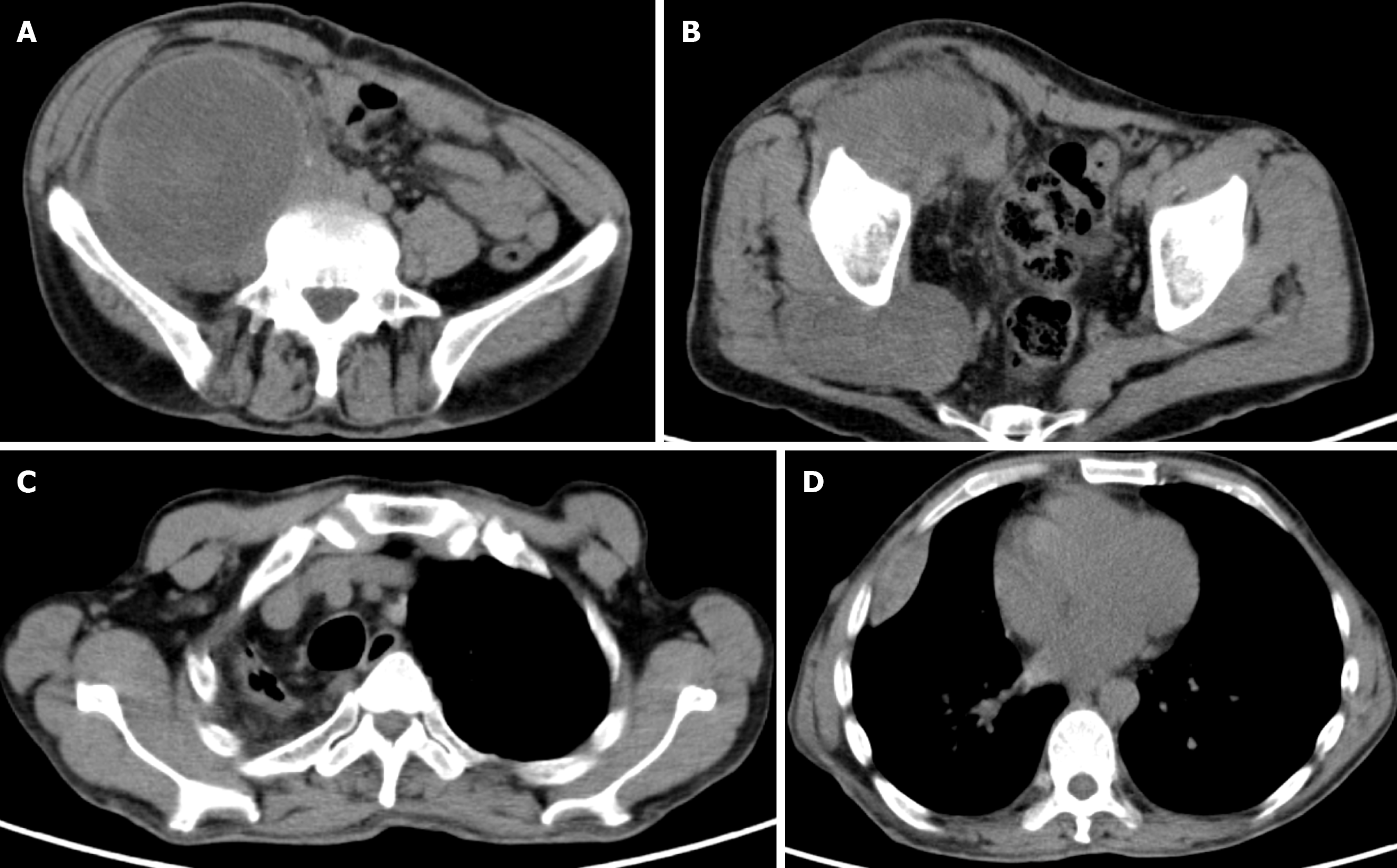
Chest-enhanced computed tomography (CT) revealed soft tissue masses in the subpleural and horizontal fissures of the right middle lobe of the right lung. Multiple soft tissue nodules were seen under the chest wall on both sides, and neurofibromatosis was considered in combination with medical history. The mediastinum and the main bronchus were moved to the right. There was pleural thickening of the right upper lobe with localized atelectasis. Abdominal pelvic magnetic resonance (MR) revealed a solid cystic mass in the right abdomen and right hip, and multiple small nodules in the abdominal wall, considering neurofibromatosis. Positron emission tomography (PET)/CT is shown in Figure 3. In the right fossa iliaca there was a large soft tissue mass shadow (Figure 3A), the size was about 11.1 cm × 9.3 cm × 17.5 cm, and fluorodeoxyglucose (FDG) metabolism increased. The upper part of the mass showed a non-uniform cystic change, and the radioactive uptake of the cystic area was relatively reduced. The right iliacus was involved; the soft tissue mass in front of the right gluteus maximus (Figure 3B) was about 8.9 cm × 5.1 cm in size, and FDG metabolism was increased; the pleura in the right middle lobe showed multiple adjacent fusiforms (Figures 3C and D). FDG metabolism increased at the marginal site; multiple nodules were seen in the skin of whole body, and FDG metabolism was not increased. Combined with medical history, skin neurofibromatosis may be considered, and hypermetabolic lesions may be considered malignant transformation.
NF1, spindle cell sarcoma with mutations in ROS1 genes.
Because the patient refused surgery, and his general condition could not tolerate chemotherapy or radiotherapy, and after considering the patient’s condition, anti-angiogenic therapy was recommended according to the National Comprehensive Cancer Network (NCCN) guidelines. An oncology expert from another hospital recommended that, based on the results of genetic testing, crizotinib can be considered. After communication with the patient and his family, the patient chose crizotinib as the treatment plan. The patient was given crizotinib 250 mg twice a day.
The patient was discharged home after taking medication and continued medication at home. Follow-up continues for monitoring the patient’s progress.
NF1 is an autosomal dominant genetic disease caused by abnormal development of neural crest cells with no significant gender or racial propensities[8], and it is one of the diseases with the highest mutation rate. Studies have found that mutations in the NF1 gene located in the periphery of the 17q11.2 center are important causes of NF1[9]. This NF1 gene encodes a protein called neurofibromin, which consists of 2818 amino acids. It is a tumor suppressor that negatively regulates the Ras signaling pathway[10]. Mutation in the NF1 gene (such as heterozygous, loss-of-function mutations, etc) results in the loss of function of neurofibromin with increased RAS activity, ultimately leading to increased proliferation and tumorigenesis, especially in the cutaneous and nervous tissues[3,11]. The clinical manifestations of NF1 are diverse, and most of them meet the diagnostic criteria of the National Institutes of Health (Table 1).
| Two or more of the criteria should be met for diagnostic confirmation |
| Cafe-au-lait spots (6 or more) |
| > 5 mm extent—pre-pubertal patients |
| > 15 mm in patients after puberty neurofibromas of any type (2 or more) Or 1 plexiformneurofibroma |
| Axillary and inguinal freckling optic glioma |
| Lisch nodules (2 or more) |
| Bone lesion with sphenoid bone dysplasia or thinning of the cortex of the long bones withor without pseudoarthrosis |
| First-degree relative (parent, sibling, or offspring) that meets National Institute of Health criteria |
The NF1 type is benign but has a certain rate of malignant transformation. The risk of malignant transformation of NF1 in life is estimated to be 2% (in those over 21 years old, this increases to 4.2%)[12]. Most of the NF1 type malignant transformation is converted to malignant peripheral nerve sheath tumors, but some cases may be associated with heterologous differentiation components[13]. Common heterologous components are rhabdomyosarcoma, chondrosarcoma, osteosarcoma, and angiosarcoma, and there are very few adenoid and squamous epithelial components. Undifferentiated pleomorphic sarcoma is a rare tumor that occurs more commonly in the extremities of patients during the sixth and seventh decades of life and is more common than malignant peripheral nerve sheath tumor that happens in young adults[14]. Differentiating between benign and malignant tumors is important for prognosis and treatment planning of NF1 patients. Meanwhile, characterization of malignant tumors is important for pre-operative treatment planning and selecting the best approach for resection as well. As a result, correlation inspection is necessary (including CT scan, MR imaging, pathological examination, gene detection, etc)[15,16]. The final diagnosis of malignant transformation of NF1 depends on pathological examination. When there is clinical manifestation of NF1 type, pathological examination of tumor cells for atypical, mitotic, and neoplastic necrosis should be diagnosed as sarcoma, whether the sarcoma variable component is a single sarcoma. On the other hand, gene detection is a new path to distinguish NF type, and it can guide doctors to choose better treatment plans for patients.
Clinical manifestations of this patient are a light brown spot on the skin of the whole body, and there are multiple, uneven pyknosis under the skin, distributed in the face, neck, trunk, and limbs, which is in line with the diagnostic criteria for clinical diagnosis. In the past 6 mo, the patient’s right humerus mass increased rapidly, accompanied by tingling of the mass and radiation pain in the front of the right thigh, which was suspected of malignant transformation. According to the biopsy pathology, immunohistochemistry and positron emission tomography/CT examination results, NF1 with spindle cell sarcoma was diagnosed. Because the patient has not undergone surgical pathology, the origin of the malignant soft tissue sarcoma was not clear.
At present, the main treatment of NF1 is still surgery. The application range of chemotherapy, radiotherapy, and laser treatment is relatively small with no obvious effect, and there is no systematic molecular targeting or immunotherapy. For advanced soft tissue sarcoma with recurrence or metastasis, chemotherapy is the standard treatment, and the traditional disease control rate of traditional chemotherapy drugs such as doxorubicin, ifosfamide, cisplatin, and other three drugs is only 30%-50%. The median survival time is about 12 mo[17-19]. With the development of molecular biology and deeper understanding of the biological behavior of soft tissue sarcoma, molecular targeted therapy has been continuously applied to the treatment of soft tissue sarcoma, especially anti-angiogenic drugs. Like most other tumors, soft tissue sarcoma is an angiogenesis-dependent tumor[20-22]. Therefore, anti-angiogenic therapy for soft tissue sarcoma will be a promising new treatment strategy. In recent years, a variety of targeted anti-angiogenic drugs have emerged in an endless stream, and they are continuously applied to the treatment of soft tissue sarcoma. These include bevacizumab, pazotinib, sorafenib, and sunitinib, and they have achieved certain effects. The latest NCCN guidelines also recommend these targeted drugs for the treatment of advanced or metastatic soft tissue sarcoma[23]. Because the patient refused surgery, and his general condition could not tolerate chemotherapy or radiotherapy, and after considering the patient’s condition, anti-angiogenic therapy was recommended based on the NCCN guidelines. An oncology expert from another hospital recommended that, according to the results from genetic testing, crizotinib can be considered. After communication with the patient and his family, they choose crizotinib as the treatment plan. The patient was given crizotinib 250 mg twice a day. Follow-up will be continued for monitoring the patient’s progress.
Although surgery, chemotherapy, and radiotherapy are the main treatment methods of NF1, continuous progress of molecular biology research in molecular targeted therapy opens the possibility of new methods for treatment. Given the limited evidence for the best choice of treatment, more case reports are needed to determine optimal treatment strategy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd El-Razek A S-Editor: Zhang L L-Editor: Filipodia E-Editor: Qi LL
| 1. | Brosius S. A history of von Recklinghausen's NF1. J Hist Neurosci. 2010;19:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Razek AAKA. MR imaging of neoplastic and non-neoplastic lesions of the brain and spine in neurofibromatosis type I. Neurol Sci. 2018;39:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Cavallaro G, Basile U, Polistena A, Giustini S, Arena R, Scorsi A, Zinnamosca L, Letizia C, Calvieri S, De Toma G. Surgical management of abdominal manifestations of type 1 neurofibromatosis: experience of a single center. Am Surg. 2010;76:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Yap YS, McPherson JR, Ong CK, Rozen SG, Teh BT, Lee AS, Callen DF. The NF1 gene revisited - from bench to bedside. Oncotarget. 2014;5:5873-5892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Rasmussen SA, Overman J, Thomson SA, Colman SD, Abernathy CR, Trimpert RE, Moose R, Virdi G, Roux K, Bauer M, Rojiani AM, Maria BL, Muir D, Wallace MR. Chromosome 17 loss-of-heterozygosity studies in benign and malignant tumors in neurofibromatosis type 1. Genes Chromosomes Cancer. 2000;28:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zhang L, Sun F, Li H, Du J, Zhao L. Neurofibromatosis type I with malignant peripheral nerve sheath tumors in the upper arm: A case report. Medicine (Baltimore). 2019;98:e15017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | O'Connell P, Leach RJ, Ledbetter DH, Cawthon RM, Culver M, Eldridge JR, Frej AK, Holm TR, Wolff E, Thayer MJ. Fine structure DNA mapping studies of the chromosomal region harboring the genetic defect in neurofibromatosis type I. Am J Hum Genet. 1989;44:51-57. [PubMed] |
| 10. | Jouhilahti EM, Peltonen S, Heape AM, Peltonen J. The pathoetiology of neurofibromatosis 1. Am J Pathol. 2011;178:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | King AA, Debaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392. [PubMed] |
| 12. | Wu J, Patmore DM, Jousma E, Eaves DW, Breving K, Patel AV, Schwartz EB, Fuchs JR, Cripe TP, Stemmer-Rachamimov AO, Ratner N. EGFR-STAT3 signaling promotes formation of malignant peripheral nerve sheath tumors. Oncogene. 2014;33:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | de Oliveira MG, Pozatti Moure S, Sérgio Batista P, Moraes Chaves AC, Rados PV, Sant'Ana Filho M. NF1 diagnosis criteria and associated sarcomatous tumor review of the literature and case report. Oral Maxillofac Surg. 2008;12:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Li H, Velasco-Miguel S, Vass WC, Parada LF, DeClue JE. Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Res. 2002;62:4507-4513. [PubMed] |
| 15. | Razek AA, Huang BY. Soft tissue tumors of the head and neck: imaging-based review of the WHO classification. Radiographics. 2011;31:1923-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Razek AAKA, Ashmalla GA. Assessment of paraspinal neurogenic tumors with diffusion-weighted MR imaging. Eur Spine J. 2018;27:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 17. | In GK, Hu JS, Tseng WW. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations. Ther Adv Med Oncol. 2017;9:533-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Heudel P, Cassier P, Derbel O, Dufresne A, Meeus P, Thiesse P, Ranchère-Vince D, Blay JY, Ray-Coquard I. Pazopanib for the treatment of soft-tissue sarcoma. Clin Pharmacol. 2012;4:65-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016;122:2952-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | DuBois S, Demetri G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer. 2007;109:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Yoon SS, Segal NH, Olshen AB, Brennan MF, Singer S. Circulating angiogenic factor levels correlate with extent of disease and risk of recurrence in patients with soft tissue sarcoma. Ann Oncol. 2004;15:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Feldman AL, Pak H, Yang JC, Alexander HR, Libutti SK. Serum endostatin levels are elevated in patients with soft tissue sarcoma. Cancer. 2001;91:1525-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Conrad EU, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Schuetze S, Schupak KD, Schwartz HS, Tap WD, Wayne JD, Bergman MA, Scavone J. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:758-786. [PubMed] |