Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2794
Peer-review started: June 26, 2019
First decision: August 1, 2019
Revised: August 5, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 26, 2019
Processing time: 98 Days and 1.9 Hours
Donor-origin cancer is a well-recognized but rare complication after liver transplantation (LT). The rise in the use of extended criteria donors due to the current shortage of organs increases the risk. Data on donor-origin neuroendocrine neoplasms (NENs) and the most appropriate treatment are scarce. Here, we report a case of a patient who developed a NEN confined to the liver after LT and was treated with liver re-transplantation (re-LT).
A 49-year-old man with no other medical co-morbidities underwent LT in 2013 for alcoholic liver cirrhosis. The donor was a 73-year-old female with no known malignancies. Early after LT, a hypoechogenic (15 mm) lesion was detected in the left hepatic lobe on abdominal ultrasound. The lesion was stable for next 11 mo, when abdominal magnetic resonance identified two hypovascular lesions (20 and 11 mm) with atypical enhancement pattern. Follow-up abdominal ultrasound revealed no new lesions for the next 2.5 years, when magnetic resonance showed a progression in size and number of lesions, also confirmed by abdominal computed tomography. Liver biopsy proved a well-differentiated NEN. Genetic analysis of the NEN confirmed donor origin of the neoplasm. As NEN was confined to liver graft only, in 2018, the patient underwent his second LT. At 12 mo after re-LT the patient is well with no signs of NEN dissemination.
The benefits of graft explantation should be weighed against the risks of re-LT and the likelihood of NEN dissemination beyond the graft.
Core tip: Donor-origin neuroendocrine neoplasm is a rare but well-recognized complication after liver transplantation. The management is individualized and liver re-transplantation may be considered a long-term treatment option. However, the benefits of graft explantation should be weighed against the risks of liver re-transplantation and the likelihood of neuroendocrine neoplasm dissemination beyond the graft.
- Citation: Mrzljak A, Kocman B, Skrtic A, Furac I, Popic J, Franusic L, Zunec R, Mayer D, Mikulic D. Liver re-transplantation for donor-derived neuroendocrine tumor: A case report. World J Clin Cases 2019; 7(18): 2794-2801
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2794.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2794
Donor origin cancer is a well-recognized complication after liver transplantation (LT). Different cases of donor origin malignancies after LT have been published including various central nervous system tumors, choriocarcinoma, adenocarcinoma of the lung, colon, pancreas, prostate, ovarian cancer, melanoma, sarcoma, lymphoma, and neuroendocrine neoplasms (NENs).
NENs are a rare heterogeneous group of neoplasms arising from the diffuse neuroendocrine system commonly located in the gastroenteropancreatic or bronchial tract[1]. NENs confined to the liver are a rare entity. With the aging of the population, the incidence of NEN has increased[2-5]. Notably, NENs are difficult to diagnose and at the time of diagnosis usually have metastasized in about 50% of cases[6]. In selected cases, non-resectable liver metastases are an acceptable indication for LT[7,8].
Donor-origin NENs are extremely rare and data on the most appropriate treatment are scarce, ranging between different non-surgical and surgical options that include conversion or reduction of immunosuppression, chemotherapy, locoregional therapy, or LT with modest results[9-12].
Given the scarcity of data regarding the management of this entity after LT, we believe it is important to share single experiences and collect as much data as possible to form future evidence-based recommendations. Here, we present a case of a donor-origin NEN confined to the liver and treated with liver re-transplantation (re-LT).
The patient reported no complaints.
A 49-year-old Caucasian man with no medical comorbidities underwent LT in 2013 due to alcoholic cirrhosis. His Model of End Stage Liver disease score was 17. The deceased donor was a 73-year-old woman with no known malignancies, who died of intracerebral hemorrhage. Two other recipients received kidneys from this donor. The patient’s postoperative period was uneventful and his immunosuppression consisted of tacrolimus, mycophenolate mofetil, and a prednisone taper.
At 11 d after LT, abdominal ultrasound revealed an oval hypoechogenic 15 mm lesion in the left hepatic lobe morphologically characterized as hemangioma.
His physical examination was unremarkable.
His graft function was stable and his kidney function was normal. His blood count and tumor markers (carcinoembryonic antigen, carbohydrate antigen 19-9, alpha-fetoprotein, and prostate-specific antigen) were within the normal limits. At year 3 after LT, his liver function tests worsened slightly, with a hepatocellular pattern (aspartate aminotransferase, 151 U/L; alanine aminotransferase, 107 U/L; γ-glutamyl transferase, 325 U/L; alkaline phosphatase, 114 U/L). His bilirubin level was normal (20 µmol/L) and serum chromogranin level was elevated (279.9 ng/mL).
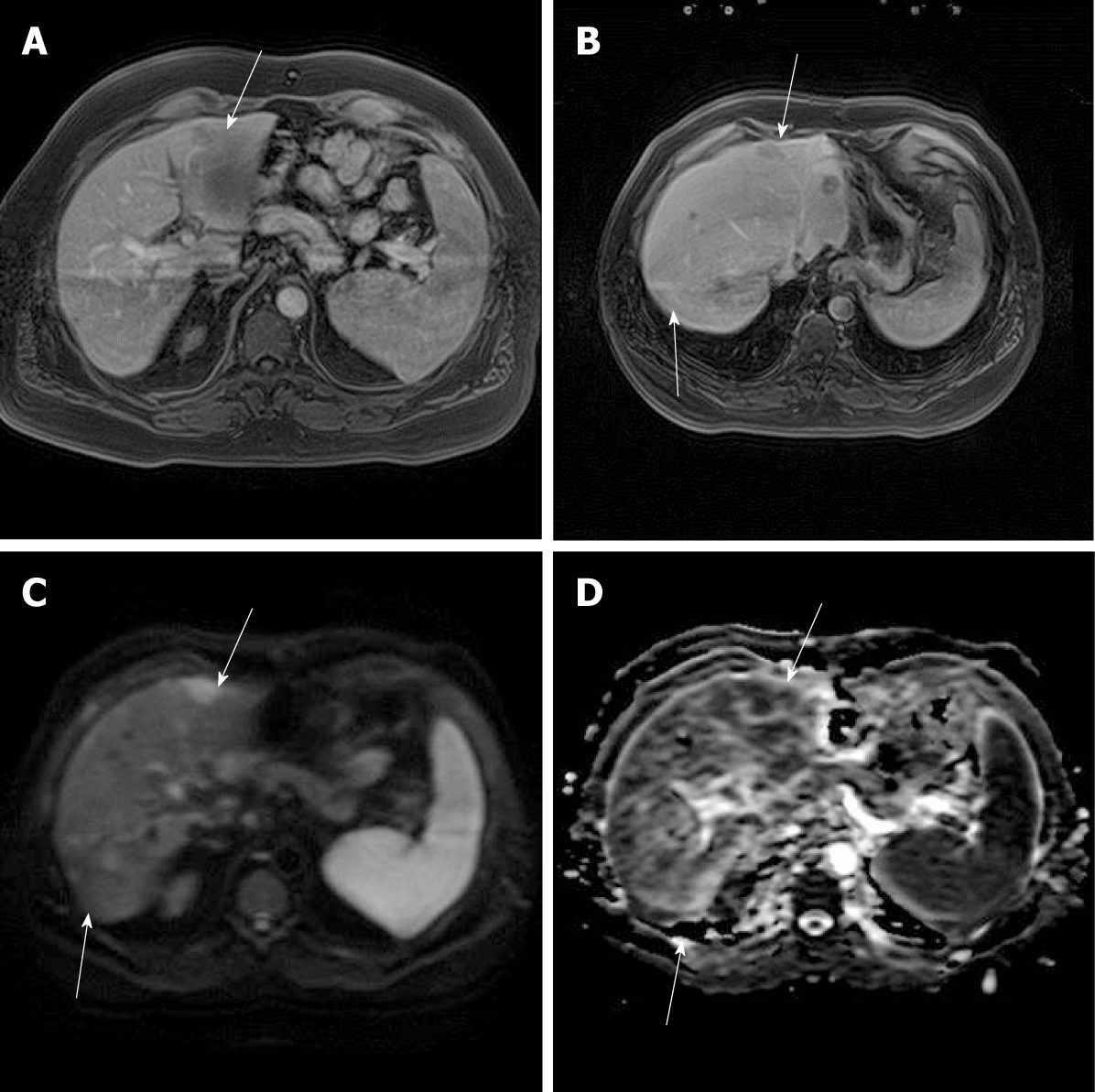
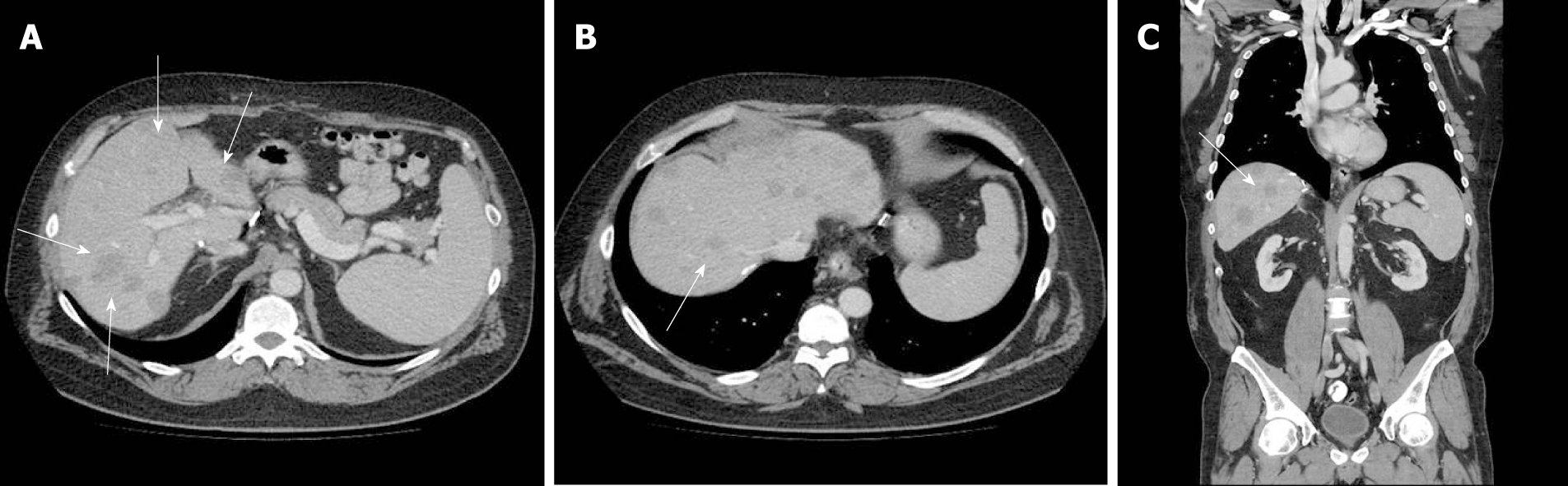
Imaging follow-up continued and 11 mo after LT, abdominal magnetic resonance (MR) identified two hypovascular lesions (20 and 11 mm) with an atypical enhancement pattern: Peripheral enhancement in the arterial phase and hypovascularity in the portal venous and hepatobiliary phases (Figure 1A and B). The lesions showed restriction on diffusion-weighted images (Figure 1C and D). Abdominal US follow-up revealed no new lesions for the next 2.5 years. At year 3 after LT, MR showed multiple lesions throughout the liver, confirmed also by abdominal computed tomography (CT) and described as secondary lesions (Figure 2A-C).
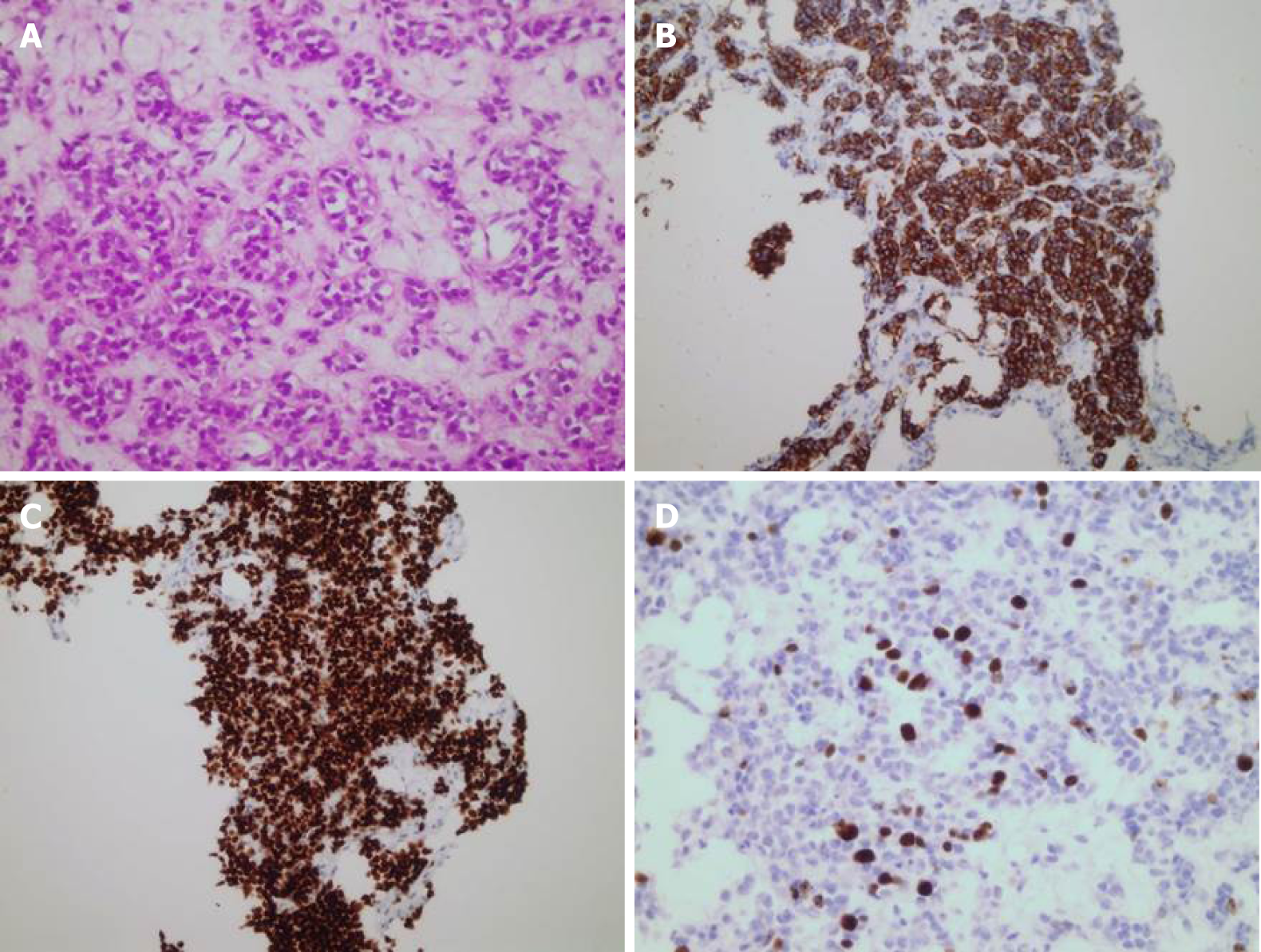
Liver biopsy revealed closely packed nests of well-differentiated neuroendocrine tumor cells with four mitoses/2 mm2. Necrosis was not found. Immuno-histochemically, tumor cells were positive for cytokeratin AE1/AE3, cytokeratin 7, synaptophysin, chromogranin A, and thyroid transcription factor 1 (TTF-1) and were negative for cytokeratin 20, hepatocyte, caudal type homeobox 2, and carcinoembryonic antigen. The Ki67 index was 18% (Figure 3).
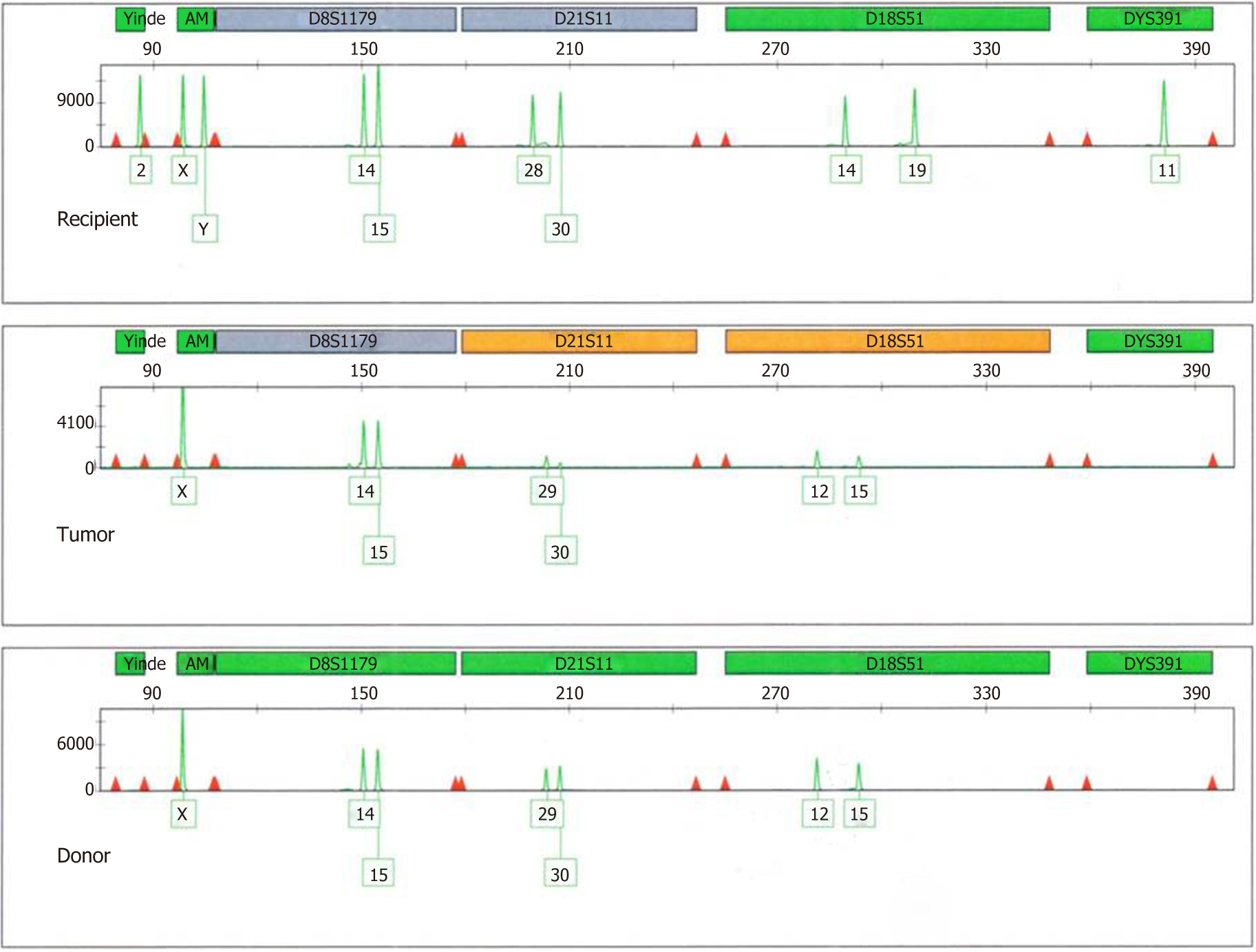
Positron emission tomography (PET)-CT, octreotide scan, chest CT, upper gastrointestinal endoscopy, and colonoscopy did not reveal a primary extrahepatic source. DNA fingerprinting was done to determine if the tumor was of donor or recipient origin. Samples analyzed included the explanted tumor and reference samples of recipient blood and donor DNA extract. DNA extraction from formalin-fixed, paraffin-embedded tissue blocks was performed in duplicate using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). DNA from recipient blood was extracted using InstaGene Matrix (Bio-Rad, Hercules, CA, United States). DNA was quantified with the Quantifiler Trio DNA Quantification Kit (Applied Biosystems, Framingham, MA, United States) according to the manufacturer’s instructions using the 7500 Real Time PCR System (Applied Biosystems). Genotyping was performed using the GlobalFiler PCR Amplification Kit and ProFlex PCR System, and analyses were conducted with the 3500 Genetic Analyzer using GeneMapper ID-X 1.5 software (Applied Biosystems). This kit amplifies 21 autosomal short tandem repeat loci, 1 Y-short tandem repeat, and 1 Y-indel and amelogenin (sex-determining marker). All samples were successfully amplified. The results of the tumor sample were consistent with the predominant donor genotype. There was a complete match between the tumor specimen and reference donor sample (Figure 4). The likelihood ratio of 4 × E25 strongly indicated that the tumor cells were of donor origin.
The final diagnosis of the case presented is a well-differentiated donor-origin NEN, classified as lung-NEN, atypical carcinoid (AC).
The multidisciplinary team decided that re-LT was the best treatment option for the patient, and he was re-listed on the liver waitlist. In 2018, 5 years after his first LT, the patient received a second whole liver graft from a deceased donor. No other NEN treatments were performed before the second LT. Multiple whitish, partially hemorrhagic, soft nodules up to 0.5 cm diffusely occupied 60% of the explanted liver graft. Morphology and immunohistochemistry of tumors were identical to the biopsied liver tumor.
At 12 mo after re-LT, the patient is well and on maintenance immunosuppression consisting of tacrolimus and mycophenolate mofetil, with no signs of NEN dissemination as confirmed by PET-CT.
The risk of donor-transmitted (present at the time of LT) or donor-derived (developed within the graft after LT) cancer is rather small, ranging from 0.017% to 0.03%[13,14]. However, given the current shortage of donors in the face of increasing organ demand, this risk could increase in the future. The use of extended criteria donor organs has risen, especially the use of older donors and donors with comorbidities. In the Eurotransplant region in 2013, 454 liver donors (22.9%) were 65 years of age or older[15] and the percentage has slowly risen to 26.6% in 2017[16].
The risk of donor-transmitted cancer should be reduced by careful screening of the donor; however it is impossible to abolish it. Even though our donor pool is aging and shifting to margins of “reliable and safe,” the standardized donor work-up in many cases still does not include thoracic and abdominal CT scans.
In our case, in 2013 the use of an organ from a 73-year-old donor was considered a good opportunity for an end-stage alcoholic patient, whose clinical deterioration was not reflected by a high laboratory Model of End Stage Liver disease score. The donor had no comorbidities other than hypertension and acute pyelonephritis, and was screened by both thoracic and abdominal CT scans, where the only detected abnormality was a left adrenal adenoma.
Detection of a small lesion in the graft early after LT had initially aroused suspicion of a donor-transmitted tumor, but given the size stability as well as atypical morphological characteristics mimicking hemangioma during the follow-up, it was monitored routinely. Three years later, the multiple liver lesions indicated liver biopsy, which revealed a well-differentiated NEN.
Gastro-enteropancreatic NENs are classified based on the proliferative index Ki-67, whereas lung NEN (Lu-NENs) classification is based on mitotic counts and assessment of necrosis[17,18]. TTF-1 is a useful marker of pulmonary origin; however, only a minority of well-differentiated Lu-NETs are TTF-1-positive[19]. Immuno-histochemical staining in our case suggested a tumor of pulmonary origin, and fitted a histologic variant of AC-an intermediate-grade malignant tumor. Lu-NENs are rare tumors with an incidence rate ranging from 0.2 to 2/100000 population/year. AC is the most uncommon of the Lu-NENs[20,21]. Lu-NENs demonstrate a heterogeneous clinical behavior, but many of the well-differentiated tumors are indolent and slow growing with a 5-year survival that can be up to 50-70%[22].
The presented donor Lu-NEN in an immunocompromised patient had a favorable biology, with a slow growing course in the graft and no dissemination beyond the liver. This strongly favored the argument for LT as the best long-term treatment option for the patient.
Donor-origin NENs are extremely rare, and only a handful of cases have been reported in the literature to date[9-12]. Two separate cases of undifferentiated neuroendocrine small-cell carcinoma demonstrated an aggressive course after LT and a poor outcome. Both were detected early, at 4 mo after LT. The first case was considered for re-LT, but as peritoneal spread was noted during the exploration this option was rejected. The patient’s immunosuppression was switched to rapamycin and chemotherapy was initiated but the patient died 6 mo after LT[10]. In the second case, the patient was also converted to rapamune-based immunosuppression, with addition of locoregional therapy (yttrium-90 spheres), followed by systemic chemotherapy with etoposide and cisplatin and re-LT. However, early after the second LT, tumor detection in the pancreatic tissue led to a near-total distal pancreatectomy and splenectomy, while multiple brain lesions resulted in hydrocephalus requiring ventriculostomy, radiotherapy, and systemic topotecan chemotherapy. The patient died 17 mo after his first LT[11]. An additional two cases of donor-origin NENs management after LT are reported in the literature. The first case was detected 9 mo after LT and was treated with cessation of immunosuppressive therapy and addition of systemic taxol and carboplatin chemotherapy. The duration of the follow-up in this case is unknown[9]. In the second case, a poorly differentiated case of NEN with Ki67 index of 4% was detected 5 years after LT and treated with re-LT and conversion to sirolimus-based immunosuppression. A recurrence was detected 2 years after his second LT. Locoregional therapy (yttrium-90 spheres) with systemic subitinib and octreotid therapy was initiated. The patient died 3 years after his re-LT[12].
A recent report of two cases of NENs that were incidentally detected during living donor hepatectomy substantiates the unavoidable risk of donor-transmitted tumors, also in the living donor pool. Both living donors were young (26 and 29 years) and underwent preoperative examinations. In both cases, intestinal NENs were detected intraoperatively after right hepatectomy in the small intestine and appendix. Both donors and recipients were closely followed up, and neither developed any tumor suspected lesions[23].
The risk of donor-transmitted or donor-derived tumor is small, but unavoidable. It is possible that it will increase in the future due to the use of extended criteria donors, which are becoming a substantial part of our donor pool. The risk should be minimized by careful screening of the donor; however, at the time of listing, it is important that potential recipients are informed about it. In the context of donor-derived neuroendocrine tumors after LT, the management is individualized and the benefits of graft explantation should be weighed against the risks of re-LT and the likelihood of NEN dissemination beyond the graft.
The authors thank Marijana Mašić, BSc for her assistance and Ivona Paurović for valuable technical help regarding genetic analysis.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdel-Salam OME S-Editor: Ma RY L-Editor: Filipodia E-Editor: Liu JH
| 1. | Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 3. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2490] [Article Influence: 311.3] [Reference Citation Analysis (4)] |
| 4. | Boyar Cetinkaya R, Aagnes B, Thiis-Evensen E, Tretli S, Bergestuen DS, Hansen S. Trends in Incidence of Neuroendocrine Neoplasms in Norway: A Report of 16,075 Cases from 1993 through 2010. Neuroendocrinology. 2017;104:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Guo LJ, Wang CH, Tang CW. Epidemiological features of gastroenteropancreatic neuroendocrine tumors in Chengdu city with a population of 14 million based on data from a single institution. Asia Pac J Clin Oncol. 2016;12:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, Nieveen van Dijkum EJM, Pape UF, Pascher A, Ramage J, Reed N, Ruszniewski P, Scoazec JY, Toumpanakis C, Kianmanesh R, Falconi M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2017;105:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 7. | Clift AK, Frilling A. Liver transplantation and multivisceral transplantation in the management of patients with advanced neuroendocrine tumours. World J Gastroenterol. 2018;24:2152-2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Lim C, Lahat E, Osseis M, Sotirov D, Salloum C, Azoulay D. Liver Transplantation for Neuroendocrine Tumors: What Have We Learned? Semin Liver Dis. 2018;38:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Baehner R, Magrane G, Balassanian R, Chang C, Millward C, Wakil AE, Osorio RW, Waldman FM. Donor origin of neuroendocrine carcinoma in 2 transplant patients determined by molecular cytogenetics. Hum Pathol. 2000;31:1425-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Foltys D, Linkermann A, Heumann A, Hoppe-Lotichius M, Heise M, Schad A, Schneider J, Bender K, Schmid M, Mauer D, Peixoto N, Otto G. Organ recipients suffering from undifferentiated neuroendocrine small-cell carcinoma of donor origin: a case report. Transplant Proc. 2009;41:2639-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Begum R, Harnois D, Satyanarayana R, Krishna M, Halling KC, Kim GP, Nguyen JH, Keaveny AP. Retransplantation for donor-derived neuroendocrine tumor. Liver Transpl. 2011;17:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Al-Azzawi Y, Stein LL, Shrestha R, Bhasin D, Citron SJ, Rubin RA. Donor-Derived Hepatic Neuroendocrine Tumor: Pause Before Proceeding With Liver Retransplantation. Transplant Direct. 2016;2:e88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Myron Kauffman H, McBride MA, Cherikh WS, Spain PC, Marks WH, Roza AM. Transplant tumor registry: donor related malignancies. Transplantation. 2002;74:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Desai R, Collett D, Watson CJ, Johnson P, Evans T, Neuberger J. Cancer transmission from organ donors-unavoidable but low risk. Transplantation. 2012;94:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Eurotransplant. Available from: https://members.eurotransplant.org/cms/mediaobject.php?file=AR20134.pdf. |
| 16. | Eurotransplant. Available from: https://members.eurotransplant.org/cms/mediaobject.php?file=Annual+Report+2017+HR9.pdf. |
| 17. | Lloyd RV, Osamura RY, Kloppel G. WHO classification of tumours of endocrine organs, chapter 6. Lyon: International Agency for Research on Cancer 2017; 210-239. |
| 18. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3126] [Article Influence: 347.3] [Reference Citation Analysis (0)] |
| 19. | Brambilla E, Beasley MB, Austin JHM, Capelozzi VL, Chirieac LR, Devesa SS, Frank GA, Gazdar A, Ishikawa Y, Jen J, Jett J, Marchevsky AM, Nicholson S, Pelosi G, Powell CA, Rami-Porta R, Scagliotti G, Thunnissen E, Travis WD, van Schil P, Yang P, Travis W, Brambilla E, Burke A, et al. Neuroendocrine tumours. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth Ed. ed. World Health Organization Classification of Tumours. Travis W, Brambilla E, Burke A, et al. Lyon: IARC Press. 2015;63-77. |
| 20. | Beasley MB, Thunnissen FB, Hasleton PhS, Barbareschi M, Pugatch B, Geisinger K, Brambilla E, Gazdar A, Travis WD. Carcinoid tumour. In: Travis W, Brambilla E, Muller-Hermelink H, et al. Tumours of the lung, pleura, thymus and heart.. Lyon: IARC Press; 2004; 59-62. |
| 21. | Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Tsoli M, Chatzellis E, Koumarianou A, Kolomodi D, Kaltsas G. Current best practice in the management of neuroendocrine tumors. Ther Adv Endocrinol Metab. 2018;10:2042018818804698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Akbulut S, Isik B, Cicek E, Samdanci E, Yilmaz S. Neuroendocrine tumor incidentally detected during living donor hepatectomy: A case report and review of literature. World J Hepatol. 2018;10:780-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |