Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2787
Peer-review started: April 4, 2019
First decision: August 1, 2019
Revised: August 17, 2019
Accepted: August 27, 2019
Article in press: August 26, 2019
Published online: September 26, 2019
Processing time: 175 Days and 18.4 Hours
Gallbladder cancer is the most common malignant tumor of the biliary tract. The majority of cases are adenocarcinoma. Squamous cell carcinoma is the histological type present in 12% of all neoplasias accounting for approximately 12% of gallbladder neoplasms. It can occur in its pure form reaching 1%-3% of the tumors. Many patients are at an advanced stage when diagnosed and have bad therapeutic efficacy.
A 45-year-old male patient presented with left flank pain for 1 year and irradiated to the mesogastric region. He denied fever, vomiting, and any other intestinal changes. He reported a weight loss of 10 kg in a period of 7 mo. He denied alcoholism, smoking, drug use, or prior illness. Computed tomography of the abdomen showed in the gallbladder fossa a voluminous mesogastric heterogeneous collection that had a thick and irregular capsule with liquid and gaseous contents. A predominantly hypoattenuating rounded material with partially calcified margins measuring about 2.0 cm related to gallstone was also emphasized. No lymphadenomegalies or free fluid was observed in the abdominal cavity. Patient underwent laparotomy where a huge tumor was observed affecting the transverse colon and gallbladder. This mass was resected en bloc removing gallbladder and transverse colon together with corresponding mesocolon and regional lymphadenectomy. There were no complications in the postoperative period. Although oncological treatment was performed, the patient died 6 mo after surgery.
Squamous cell carcinoma represents a rare disease. Patients often present with large, bulky tumors with involvement of adjacent organs. In spite of progress in surgical techniques and adjuvant chemotherapy, the prognosis remains poor.
Core tip: Pure squamous cell carcinoma represents a very rare condition. Usually the patient will present with a huge mass in the upper right quadrant involving other organs. A high index of suspicion is fundamental for the surgical planning because the surgical removal of the lesion respecting the oncological surgical principles may be the only chance of cure for this aggressive disease. Patients must be submitted to adjuvant chemotherapy in order to increase the survival rates. The prognosis remains poor.
- Citation: Junior MAR, Favaro ML, Santin S, Silva CM, Iamarino APM. Giant squamous cell carcinoma of the gallbladder: A case report. World J Clin Cases 2019; 7(18): 2787-2793
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2787.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2787
Squamous cell carcinoma (SCC) is the histological type present in 12% of neoplasias accounting for approximately 12% of gallbladder neoplasms. This can occur in a pure form reaching 1%-3% of the gallbladder neoplasms[1-3]. Without well-understood etiology, pure SCC can be derived from glandular metaplasia, heterotopic tissue, and mixed types originating from differentiated adenocarcinoma. It is about three times more common in women than in men and has its most frequent appearance after the fifth decade of life[2,4].
The pure SCC of the gallbladder is characterized by invasive growth and less tendency to metastasize compared to adenocarcinoma of the gallbladder. This invasion mainly affects the liver, and its growth laterally to the vesicular fossa invades adjacent organs such as the stomach, pancreas, duodenum, and less frequently the transverse colon[2-5].
The suspicion occurs in elderly patients with pain in the right hypochondrium, and physical examination is a palpable tumor. In the initial cases, the symptoms resemble those of cholelithiasis. Upper abdominal ultrasonography may suggest the presence of thickening of the gallbladder wall and involvement of other organs, but computed tomography and magnetic resonance imaging are more sensitive and specific[6].
There is no consensus regarding treatment, and most reports observe mixed-type SCC arising from adenocarcinomas. However, pure SCC seems to benefit from the initial surgical treatment with aggressive resections independent of the compromised organ and non-anatomical hepatectomies, which are usually not performed in adenocarcinoma. There is no clear role of the importance of locoregional lymph-adenectomy as well as adjuvant treatment with radiotherapy and chemotherapy[2-5].
A 45-year-old male patient presented with left flank pain for 1 year and irradiated to the mesogastric region.
Patient denied fever, vomiting, and any other intestinal changes. He reported a weight loss of 10 kg in a period of 7 mo.
The patient denied alcoholism, smoking, drug use, or prior illness. Any other relevant or additional aspects on his personal and/or familiar history related to tumors were identified during clinical investigation.
At the physical examination, he presented a regular general condition, stained, dehydrated +/4 +, anicteric, and afebrile. Abdomen was flaccid and painful on the right flank surface palpation with palpable hardening bulging. The initial diagnostic suspicions were of complicated cholecystitis or gallbladder neoplasia.
Laboratory examinations included general blood tests, liver function tests and tumors markers (CEA and CA 19-9). All the results were within normal values.
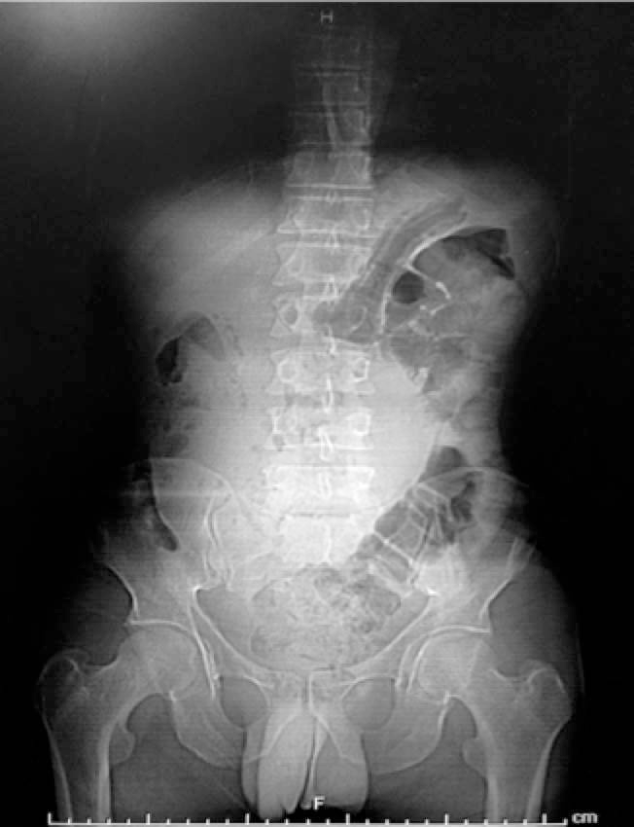
Radiograph of the abdomen: Tumor in the epigastric region, rejecting adjacent loops (Figure 1).
Ultrasonogram: Heterogeneous mass of large volume predominantly hyper-echogenic with areas of central necrosis with infiltrative aspect occupying almost every abdominal cavity.
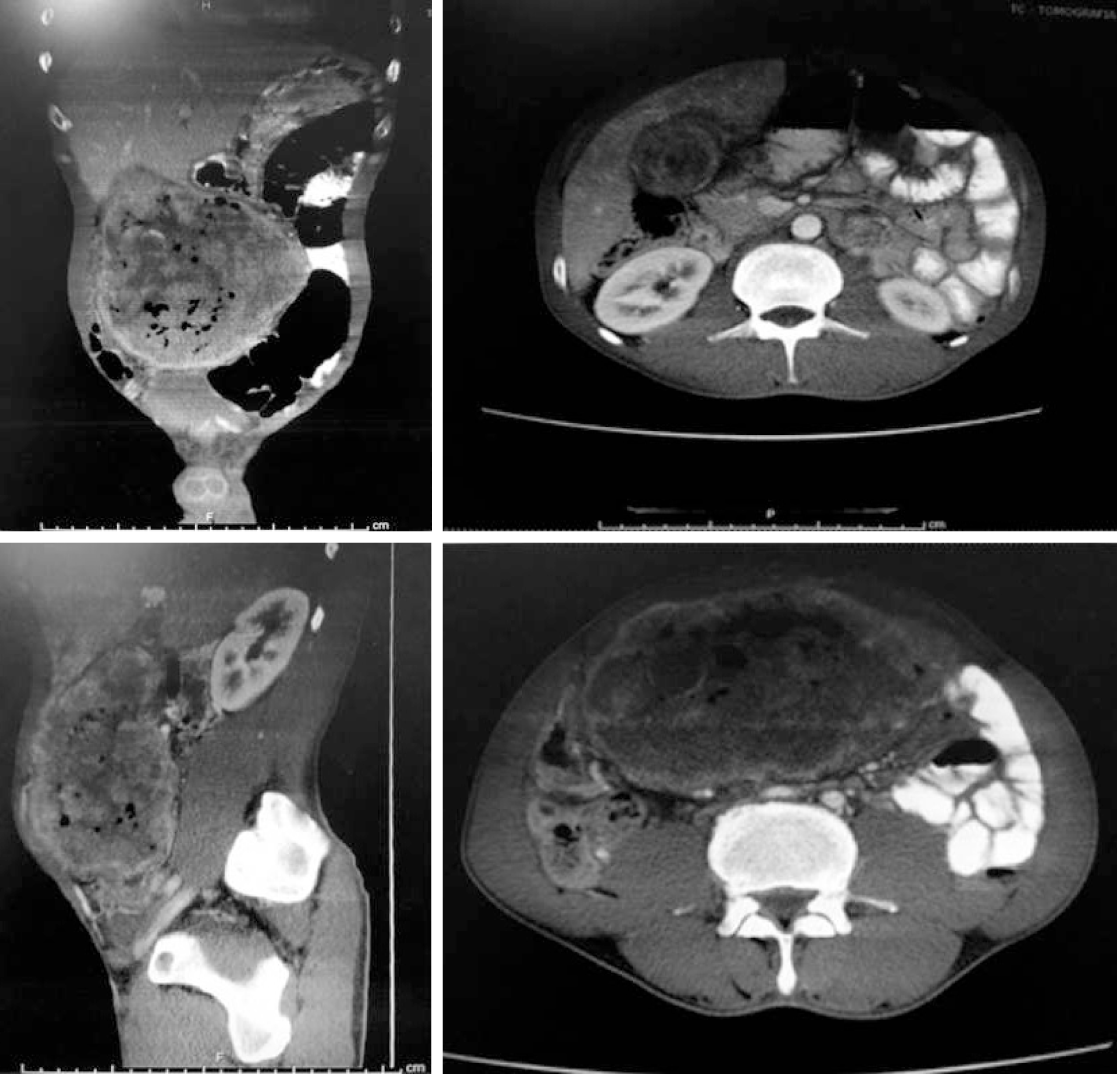
Computed tomography scan: In the location of the gallbladder was evidenced a voluminous mesogastric heterogeneous collection that had a thick and irregular capsule with liquid and gaseous contents. A predominantly hypoattenuating rounded material with partially calcified margins measuring about 2.0 cm related to gallstone was also highlighted. No lymphadenomegalies or free fluid was observed in the abdominal cavity (Figure 2).
Based on the images, the final diagnosis was gallbladder neoplasm.
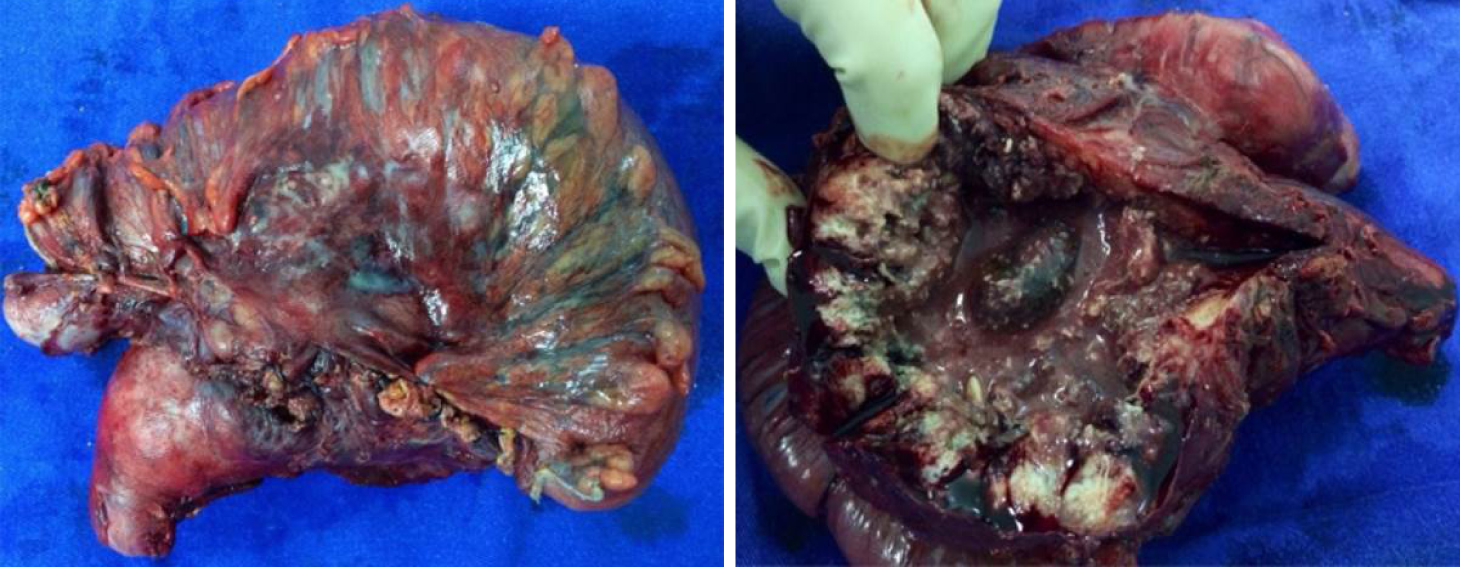
Patient underwent laparotomy where a massive tumor was seen affecting the transverse colon and gallbladder. This mass was resected en bloc removing gallbladder and transverse colon together with corresponding mesocolon and regional lymphadenectomy (Figure 3).
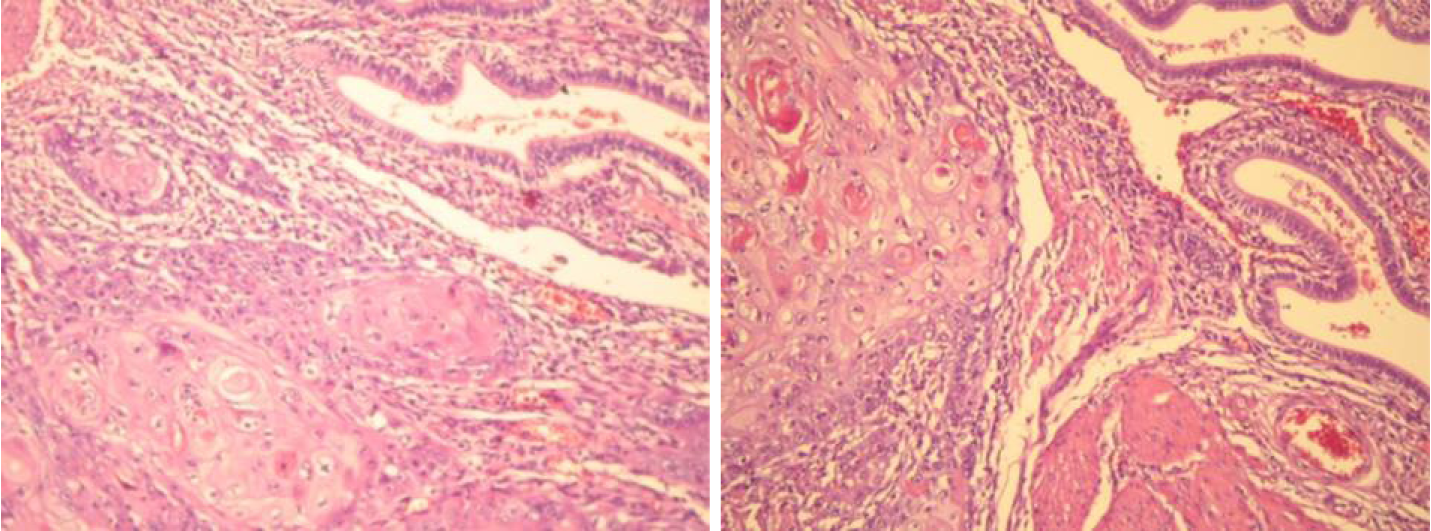
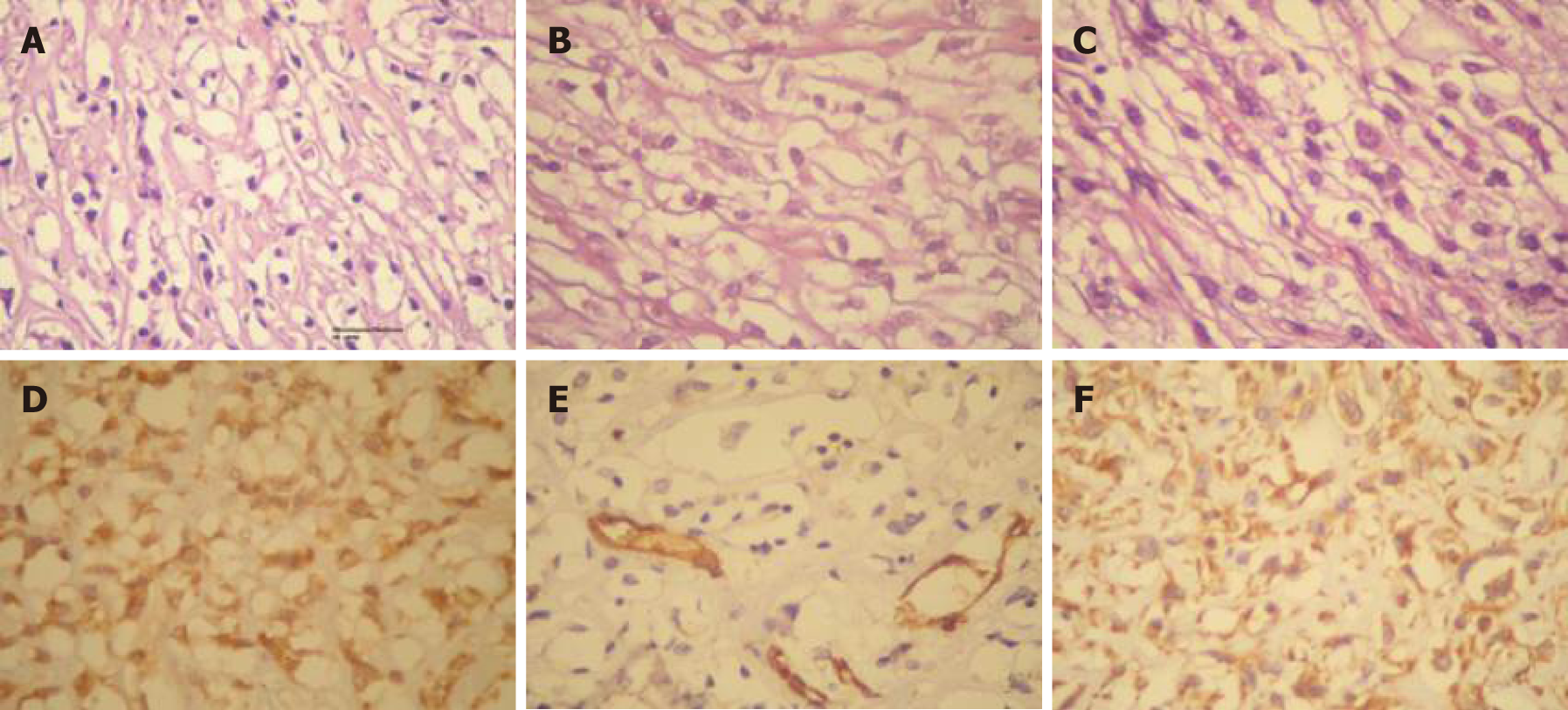
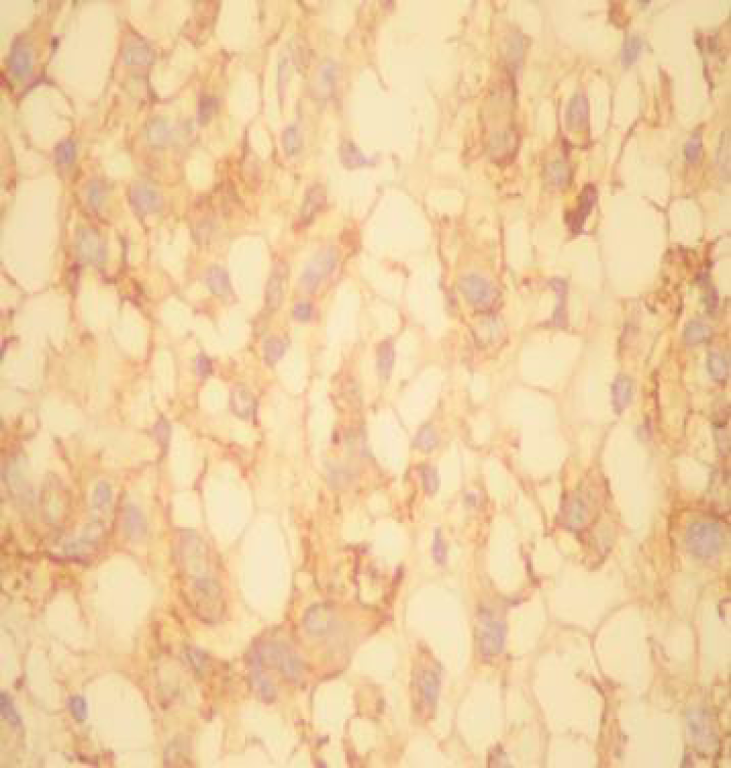
Anatomopathological: Well-differentiated SCC of the gallbladder (Figure 4). Immunohistochemistry confirmed the diagnosis (Figure 5 and Figure 6).
Patient was discharged on the sixth postoperative day and then followed up with an oncologist who indicated chemotherapy and radiotherapy. Patient died 6 mo after the procedure.
Due to the rarity of pure gallbladder SCC, its etiology is still not well understood. Muto et al[7] analyzed 1000 bile vesicles and did not find the presence of mucosal metaplasia contrary to the SCC theory if it originated from this metaplasia. A second study attempted to clarify this etiology by analyzing approximately 600 gallbladder neoplasms with 41 of them been pure SCC. The authors suggested a classification by the presence of epidermoid cells: Spinocellular differentiation, pure SCC, adeno-squamous carcinoma, and focal SCC[2].
Regardless of the etiology, the gallbladder neoplasm is very aggressive. Because the survival outcomes of gallbladder SCC are worse than that found in gallbladder adenocarcinomas, it has been reported in most cases for months and is related to tumor staging and resection performed[5].
In most of the reports the carcinomas found were anatomopathological findings, and the diagnosis of cancer was rarely suspected. When there was suspicion, there are reports of diagnostic biopsy. It is known that this procedure increases the risk of tumor implantation in the path of the needle and should be avoided. In the diagnostic suspicion after imaging, surgical removal with free margins should be indicated in order to achieve R0 resection with removal of adjacent organs if necessary. Freezing of the surgical specimen should always be performed to better program the surgical procedure with the use of more adequate resections[2,3,8,9]. In the present study, the resection performed was considered by the team as R0, but no intraoperative freezing was performed. Anatomopathological examination demonstrated free margins without compromised lymph nodes (16 lymph nodes resected).
In the Roa et al[2] study, SCC and adeno-squamous carcinomas were three times less frequent than gallbladder adenocarcinomas.
Pure gallbladder SCC is rare and should be suspected in the presence of large volume tumors in the right hypochondrium. Radical surgical treatment remains the only chance for a cure. Its survival results are usually low giving a poor prognosis for the disease.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo YM S-Editor: Wang JL L-Editor: Filipodia E-Editor: Liu JH
| 1. | Karasawa T, Itoh K, Komukai M, Ozawa U, Sakurai I, Shikata T. Squamous cell carcinoma of gallbladder--report of two cases and review of literature. Acta Pathol Jpn. 1981;31:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 2. | Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, Saka B, Losada H, Bagci P, Adsay NV. Squamous cell and adenosquamous carcinomas of the gallbladder: clinicopathological analysis of 34 cases identified in 606 carcinomas. Mod Pathol. 2011;24:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Roppongi T, Takeyoshi I, Ohwada S, Sato Y, Fujii T, Honma M, Morishita Y. Minute squamous cell carcinoma of the gallbladder: a case report. Jpn J Clin Oncol. 2000;30:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Hanada M, Shimizu H, Takami M. Squamous cell carcinoma of the gallbladder associated with squamous metaplasia and adenocarcinoma in situ of the mucosal columnar epithelium. Acta Pathol Jpn. 1986;36:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Soyama A, Tajima Y, Kuroki T, Tsuneoka N, Ohno S, Adachi T, Eguchi S, Kanematsu T. Radical surgery for advanced pure squamous cell carcinoma of the gallbladder: report of a case. Hepatogastroenterology. 2011;58:2118-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Rooholamini SA, Tehrani NS, Razavi MK, Au AH, Hansen GC, Ostrzega N, Verma RC. Imaging of gallbladder carcinoma. Radiographics. 1994;14:291-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Muto Y, Uchimara M, Waki S, Hayashi T, Samejima K, Okamoto K. Clinicopathologic study of adenosquamous carcinoma of the gall bladder and bile duct. Jpn J Cancer Clin. 1982;28:440–444. |
| 8. | Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, Coimbra FJ, Jarnagin WR. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015;17:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 9. | Chakrabarti I, Giri A, Ghosh N. Cytohistopathological correlation of a case of squamous cell carcinoma of gallbladder with lymph node metastasis. Turk Patoloji Derg. 2014;30:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |