Published online Sep 26, 2019. doi: 10.12998/wjcc.v7.i18.2712
Peer-review started: June 18, 2019
First decision: July 30, 2019
Revised: August 6, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 26, 2019
Processing time: 104 Days and 4.3 Hours
Radical gastrectomy with D2 lymph node (LN) dissection is the standard surgical procedure for patients with resectable gastric cancer (GC). In the fifteenth edition of the Japanese Classification of Gastric Carcinoma, the 14v LN (LNs along the root of the superior mesenteric vein) was defined as the regional gastric LN. The efficacy of 14v LN dissection during radical distal gastrectomy for lower-third GC remains controversial.
To analyze whether the addition of 14v LN dissection improved the survival of patients with lower-third GC.
The data from 65 patients who underwent 14v LN dissection and 65 patients treated without 14v LN dissection were selected using the propensity score-matched method from our institute database constructed between 2000 and 2012. Overall survival was compared between the groups.
Overall survival was similar between patients with 14v LN metastasis and those with distant metastasis (P = 0.521). Among patients with pathological stage IIIA disease, those who were treated with 14v LN dissection had a significantly higher overall survival than those treated without it (P = 0.020). Multivariate analysis showed that age < 65 years and pT2-3 stage were independent favorable prognostic factors for prolonged overall survival in patients with pathological stage IIIA disease. Patients with No. 1, No. 6, No. 8a, or No. 11p LN metastasis were at higher risk of having 14v LN metastasis.
Adding 14v LN dissection to D2 dissection during radical distal gastrectomy may improve the overall survival of patients with pathological stage IIIA lower-third GC.
Core Tip: The efficacy of 14v lymph node (LN) dissection during radical distal gastrectomy for lower-third gastric cancer (GC) remains controversial. The present propensity score-matched study indicated that among pathological stage lower-third GC IIIA patients, 14v LN dissection resulted in longer survival compared to treatment without it. The overall survival of patients with 14v LN metastasis was similar with that of patients with distant metastasis.
- Citation: Zheng C, Gao ZM, Sun AQ, Huang HB, Wang ZN, Li K, Gao S. Prognostic significance of 14v-lymph node dissection to D2 dissection for lower-third gastric cancer. World J Clin Cases 2019; 7(18): 2712-2721
- URL: https://www.wjgnet.com/2307-8960/full/v7/i18/2712.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i18.2712
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer death worldwide[1]. Radical gastrectomy with D2 lymph node (LN) dissection is the standard surgical procedure for patients with resectable GC[2-8]. LNs along the root of the superior mesenteric vein are defined as the 14v LNs. In the fifteenth edition of the Japanese Classification of Gastric Carcinoma, the 14v LN was defined as the regional gastric LN[9]. The fifth edition of the Japanese Gastric Cancer Treatment Guidelines states that D2 gastrectomy does not include dissection of the 14v LNs, but D2 (+14v LN) dissection may be beneficial for tumors with apparent metastasis to the No. 6 LN[10,11].
The prognosis of patients with 14v LN metastasis is poor[12-14]. Whether metastasis to the 14v LNs are classified as regional gastric LN metastasis or distant metastasis (M1) remains controversial. An et al[12] found that the 14v LN should be excluded from regional gastric LNs, as the survival of patients with 14v LN metastasis was similar with that of patients with M1 stage disease. The efficacy of prophylactic 14v LN dissection during radical distal gastrectomy for lower-third gastric cancer (LTGC) remains unclear[15,16].
Therefore, the aims of the present study were to (A) compare the prognosis of patients with 14v LN metastasis and those with M1 stage disease; (B) evaluate the prognostic significance of adding 14v LN dissection to D2 dissection during radical distal gastrectomy for patients with LTGC; and (C) aid in patient selection for 14v LN dissection.
Between January 2000 and December 2012, data from 1510 patients with GC who underwent distal gastrectomy at the Department of Surgical Oncology, First Affiliated Hospital of China Medical University were collected retrospectively. The eligibility criteria were as follows: (A) Diagnosis of gastric adenocarcinoma; (B) Presence of primary tumors in the lower third of the stomach; (C) Undergoing distal gastrectomy; (D) Receiving at least D2 LN dissection; (E) Absence of microscopic residual tumor; (F) No history of gastrectomy or other malignancy; (G) No history of preoperative chemotherapy or radiotherapy; and (H) Absence of distant metastasis.
A total of 96 patients with M1 stage disease satisfied the inclusion criteria but were only included for comparing the prognosis of M1 stage patients with 14v LN metastasis. Ultimately, 1004 patients were included in this study. Of these patients, 65 underwent 14v LN dissection [the 14vD (+) group], and the remaining 939 patients did not undergo 14v LN dissection [the 14vD (-) group]. The 14vD (+) group included patients with 14v LN metastasis and those without 14v LN metastasis. After propensity score matching, we included 65 patients in the 14vD (+) group and 65 patients in the 14vD (-) group.
There were no predefined indications for adding 14v LN dissection to lymphadenectomy. The decision to perform 14v LN dissection was made at the surgeon’s discretion[12]. The TNM stage was defined according to the AJCC guidelines, eighth edition[17]. The extent of lymphadenectomy and LN stations were defined according to the fifteenth edition of the Japanese Classification of Gastric Carcinoma[9]. Eligible patients underwent postoperative chemotherapy with 5-fluorouracil or platinum-based regimens.
The entire study population was followed up via phone and/or outpatient clinic consultation until death or the last follow-up date (December 31, 2017). The Institutional Ethics Committee of China Medical University approved this study. As this was a retrospective study, formal patient consent was not required.
All statistical analyses were performed with the Statistical Package for the Social Sciences version 24.0 for Windows (SPSS Inc., Chicago, IL, United States). The chi-squared test was used for categorical variables. Overall survival (OS) was analyzed using Kaplan–Meier analysis and compared using the log-rank test. Univariate analysis was performed using the log-rank test. Multivariate analysis for prognostic factors was conducted using the Cox proportional hazard model. The hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox proportional hazard model. A two-tailed P-value < 0.05 was considered statistically significant.
Propensity score matching was used to reduce the effects of selection bias and potential confounding factors. Propensity scores were calculated using a logistic regression model for the following covariates: Age, gender, pT stage, and pN stage. Patients in the 14vD (+) group were matched in a 1:1 ratio with those in the 14vD (-) group using imposed propensity scores with a 0.02 caliper width. We performed propensity score matching using SPSS 24.0 (SPSS Inc., Chicago, IL, United States).
Table 1 shows the comparison of the clinicopathological characteristics of the 14vD (+) and the 14vD (-) groups (n = 65 each). Of the 65 patients in the 14vD (+) group, 8 (12.31%) had 14v LN metastasis. There were no significant differences in age, gender, tumor size, histologic grade, pT stage, pN stage, pTNM stage, and postoperative chemotherapy between the 14vD (+) and 14vD (-) groups (all P > 0.05).
| Characteristics | Propensity score matched patients (n = 130) | P-value | ||
| 14vD (-) (n = 65) | 14vD (+) (n = 65) | |||
| LNM (-) (n = 57) | LNM (+) (n = 8) | |||
| Age group | 0.323 | |||
| < 65 yr | 45 (69.2) | 43 (66.2) | 7 (10.8) | |
| ≥ 65 yr | 20 (30.8) | 14 (21.5) | 1 (1.5) | |
| Gender | 0.149 | |||
| Male | 36 (55.4) | 37 (56.9) | 7 (10.8) | |
| Female | 29 (44.6) | 20 (30.8) | 1 (1.5) | |
| Tumor size | ||||
| < 4 cm | 33 (50.8) | 27 (41.5) | 3 (4.6) | 0.559 |
| ≥ 4 cm | 32 (49.2) | 30 (46.2) | 5 (7.7) | |
| Histologic grade | 0.537 | |||
| Differentiated | 17 (26.2) | 11 (16.9) | 3 (4.6) | |
| Undifferentiated | 48 (73.8) | 46 (70.8) | 5 (7.7) | |
| pT stage | 0.488 | |||
| T1 | 9 (13.9) | 6 (9.2) | 0 | |
| T2 | 16 (24.6) | 17 (26.2) | 2 (3.1) | |
| T3 | 13 (20.0) | 19 (29.3) | 1 (1.5) | |
| T4a | 26 (40.0) | 14 (21.5) | 5 (7.7) | |
| T4b | 1 (1.5) | 1 (1.5) | 0 | |
| pN stage | 0.788 | |||
| N0 | 27 (41.6) | 22 (33.8) | 0 | |
| N1 | 8 (12.3) | 6 (9.2) | 1 (1.5) | |
| N2 | 14 (21.5) | 15 (23.2) | 1 (1.5) | |
| N3a | 13 (20.0) | 14 (21.5) | 4 (6.2) | |
| N3b | 3 (4.6) | 0 | 2 (3.1) | |
| pTNM stage | 0.288 | |||
| IA | 8 (12.3) | 3 (4.6) | 0 | |
| IB | 11 (16.9) | 7 (10.8) | 0 | |
| IIA | 3 (4.6) | 10 (15.4) | 0 | |
| IIB | 12 (18.5) | 13 (20.0) | 0 | |
| IIIA | 16 (24.6) | 14 (21.5) | 3 (4.6) | |
| IIIB | 13 (20.0) | 10 (15.4) | 4 (6.2) | |
| IIIC | 2 (3.1) | 0 | 1 (1.5) | |
| postoperative chemotherapy | 0.856 | |||
| Yes | 24 (36.9) | 22 (33.8) | 3 (4.6) | |
| No | 41 (63.1) | 35 (53.9) | 5 (7.7) | |
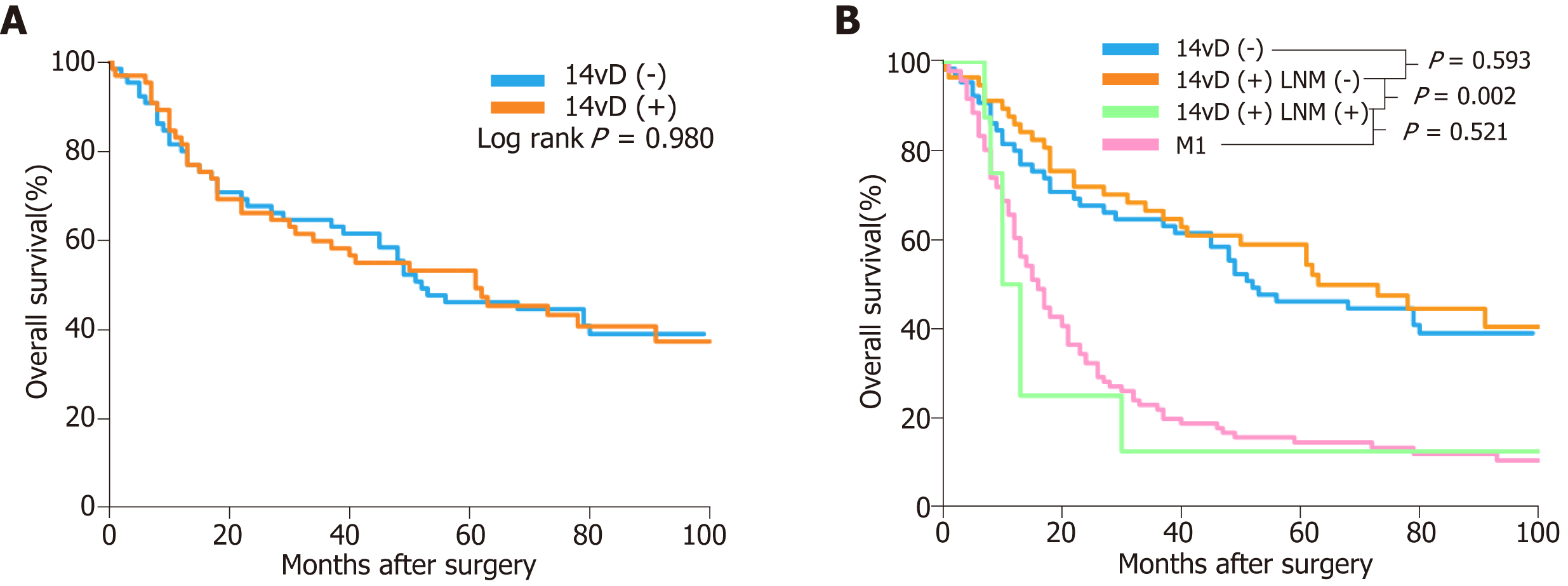
OS was similar between the 14vD (+) and 14vD (-) groups (HR: 1.01, 95%CI: 0.64–1.58, P = 0.980; Figure 1A). After stratified analysis, patients with 14v LN metastasis had a significantly shorter OS than the 14vD (+) and 14vD (-) groups (HR: 3.35, 95%CI: 1.51–7.45, P = 0.002; Figure 1B). The OS of patients with 14v LN metastasis in the 14vD (+) group was similar to that of patients with M1 stage disease (HR: 0.79; 95%CI 0.38–1.65; P = 0.521; Figure 1B).
Univariate and multivariate survival analyses for the entire population Univariate analysis indicated that pT stage and pN stage were prognostic factors for OS. Multivariate analysis showed that pT stage and pN stage were independent prognostic factors, while the status of 14v dissection was not a prognostic factor (Table 2).
| Variable | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P-value | Hazard ratio (95%CI) | P-value | |
| Status of 14v dissection | 0.980 | 0.900 | ||
| 14vD (-) | 1 (ref) | 1 (ref) | ||
| 14vD (+) | 1.01 (0.64-1.58) | 0.97 (0.59-1.60) | ||
| Age group | 0.569 | 0.234 | ||
| < 65 yr | 1 (ref) | 1 (ref) | ||
| ≥ 65 yr | 1.15 (0.71-1.86) | 1.40 (0.80-2.45) | ||
| Gender | 0.781 | |||
| Male | 1 (ref) | 1 (ref) | 0.721 | |
| Female | 0.94 (0.60-1.46) | 0.91 (0.56-1.50) | ||
| Tumor size | 0.455 | 0.448 | ||
| < 4 cm | 1 (ref) | 1 (ref) | ||
| ≥ 4 cm | 1.19 (0.76-1.86) | 0.82 (0.50-1.56) | ||
| Histologic grade | 0.275 | 0.162 | ||
| Differentiated | 1 (ref) | 1 (ref) | ||
| Undifferentiated | 1.34 (0.80-2.24) | 1.51 (0.85-2.71) | ||
| pT stage | 0.004 | 0.033 | ||
| T1 | 1 (ref) | 1 (ref) | ||
| T2 | 4.88 (1.13-20.99) | 4.97 (1.11-22.28) | ||
| T3 | 8.32 (1.96-35.29) | 6.58 (1.45-29.92) | ||
| T4 | 9.17 (2.2-38.21) | 7.87 (1.84-33.74) | ||
| pN stage | 0.002 | 0.018 | ||
| N0 | 1 (ref) | 1 (ref) | ||
| N1 | 2.29 (1.07-4.90) | 2.08 (0.92-4.67) | ||
| N2 | 2.18 (1.20-3.97) | 2.01 (1.06-3.81) | ||
| N3a | 3.11 (1.71-5.63) | 2.82 (1.49-5.36) | ||
| N3b | 4.30 (1.46-12.65) | 3.90 (1.22-12.45) | ||
| Postoperative chemotherapy | 0.799 | 0.368 | ||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 1.06 (0.67-1.70) | 0.78 (0.46-1.33) | ||
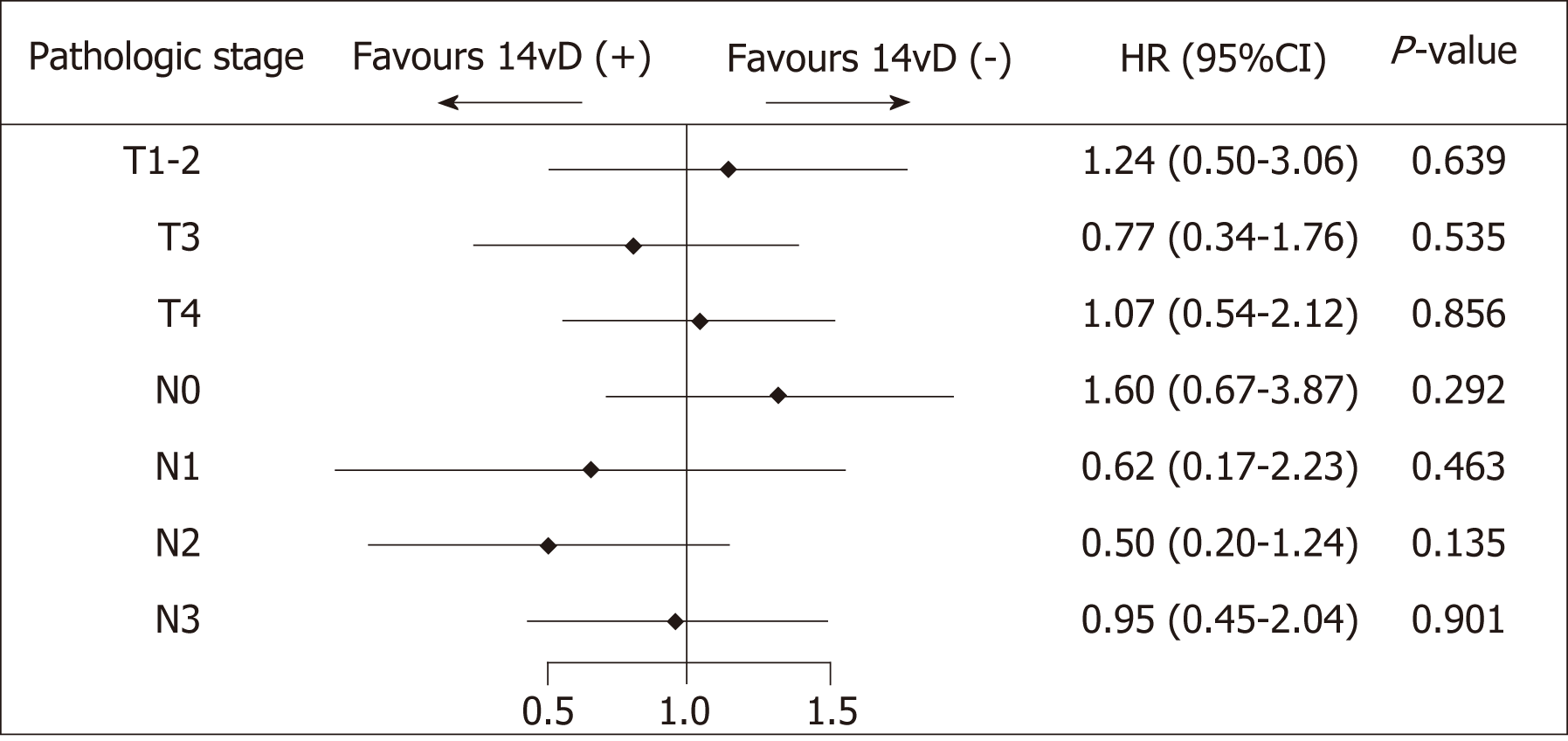
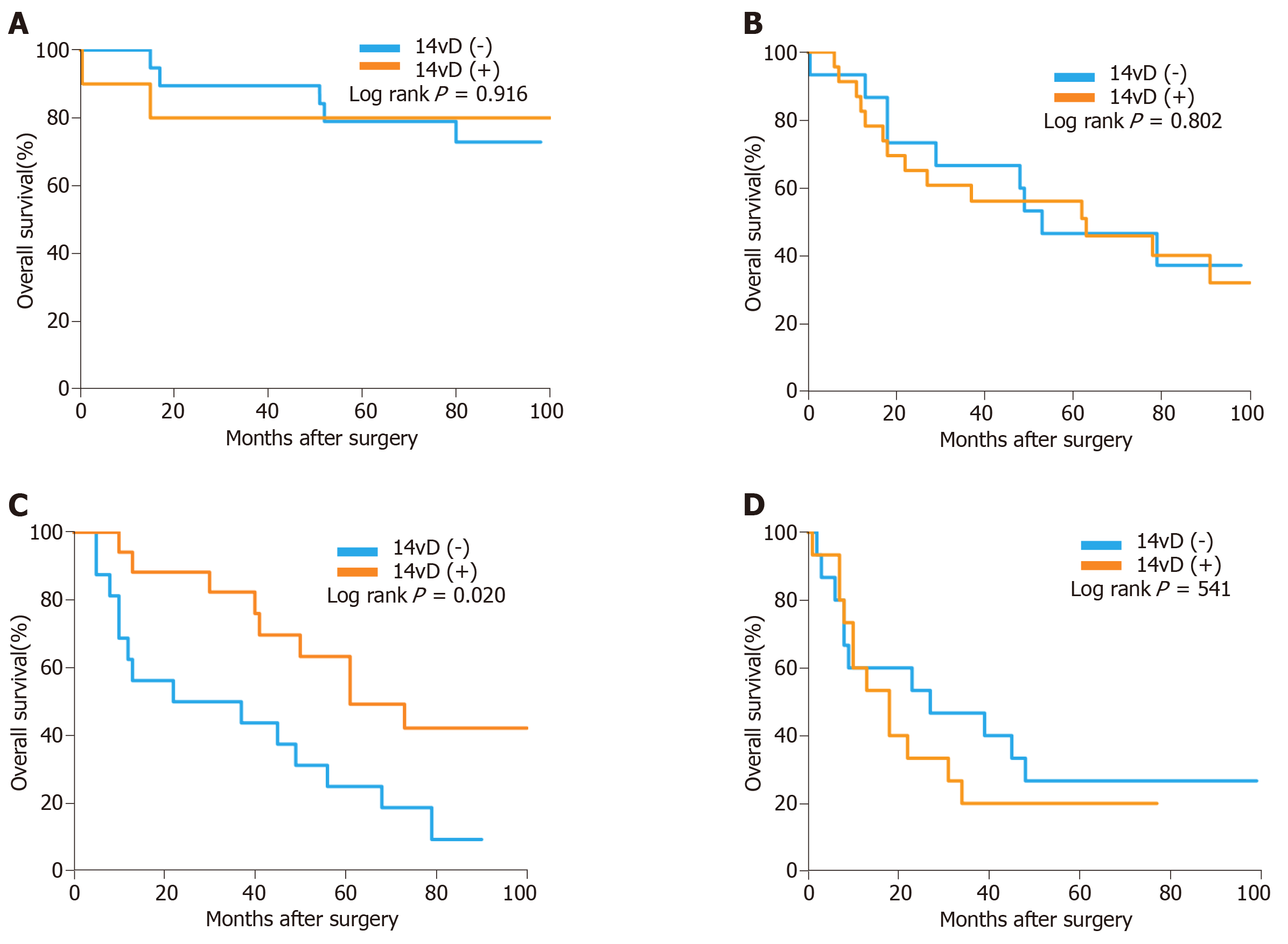
The forest plot showed that the OS of the 14vD (+) group was similar to that of the 14vD (-) group considering the pathological tumor stage and LN stage (Figure 2). Figure 3 shows the OS according to the status of 14v dissection for each pathological stage. Among patients with pathological stages I, II, and IIIB/IIIC GC, OS was not significantly different between the 14vD (+) and 14vD (-) groups (P = 0.916, P = 0.802, and P = 0.541, respectively); however, the 14vD (+) group had better OS compared with the 14vD (-) group for pathological stage IIIA GC (P = 0.020).
On univa5riate analysis, the status of 14v dissection significantly affected the prognosis. Multivariate analysis indicated that the independent prognostic factors for prolonged OS were age < 65 years (P = 0.018) and pT2-3 stage (P = 0.006; Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P-value | Hazard ratio (95%CI) | P-value | |
| Status of 14v dissection | 0.027 | 0.342 | ||
| 14vD (-) | 1 (ref) | 1 (ref) | ||
| 14vD (+) | 0.39 (0.17-0.90) | 0.61 (0.22-1.70) | ||
| Age group | 0.331 | 0.018 | ||
| < 65 yr | 1 (ref) | 1 (ref) | ||
| ≥ 65 yr | 1.53 (0.65-3.64) | 7.23 (1.41-37.06) | ||
| Gender | 0.986 | 0.930 | ||
| Male | 1 (ref) | 1 (ref) | ||
| Female | 0.99 (0.44-2.24) | 1.05 (0.36-3.09) | ||
| Tumor size | 0.091 | 0.091 | ||
| < 4 cm | 1 (ref) | 1 (ref) | ||
| ≥ 4 cm | 0.46 (0.19-1.13) | 0.37 (0.12-1.17) | ||
| Histologic grade | 0.220 | 0.091 | ||
| Differentiated | 1 (ref) | 1 (ref) | ||
| Undifferentiated | 1.71 (0.73-4.02) | 2.71 (0.85-8.60) | ||
| pT stage | 0.154 | 0.006 | ||
| T2-3 | 1 (ref) | 1 (ref) | ||
| T4 | 1.35 (0.89-2.04) | 14.15 (2.11-95.06) | ||
| pN stage | 0.886 | 0.335 | ||
| N0-1 | 1 (ref) | 1 (ref) | ||
| N2 | 0.86 (0.33-2.25) | 1.77 (0.55-5.72) | ||
| N3a | 0.74 (0.22-2.49) | 3.69 (0.64-21.37) | ||
| Postoperative chemotherapy | 0.936 | 0.586 | ||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 0.96 (0.39-2.37) | 0.75 (0.27-2.11) | ||
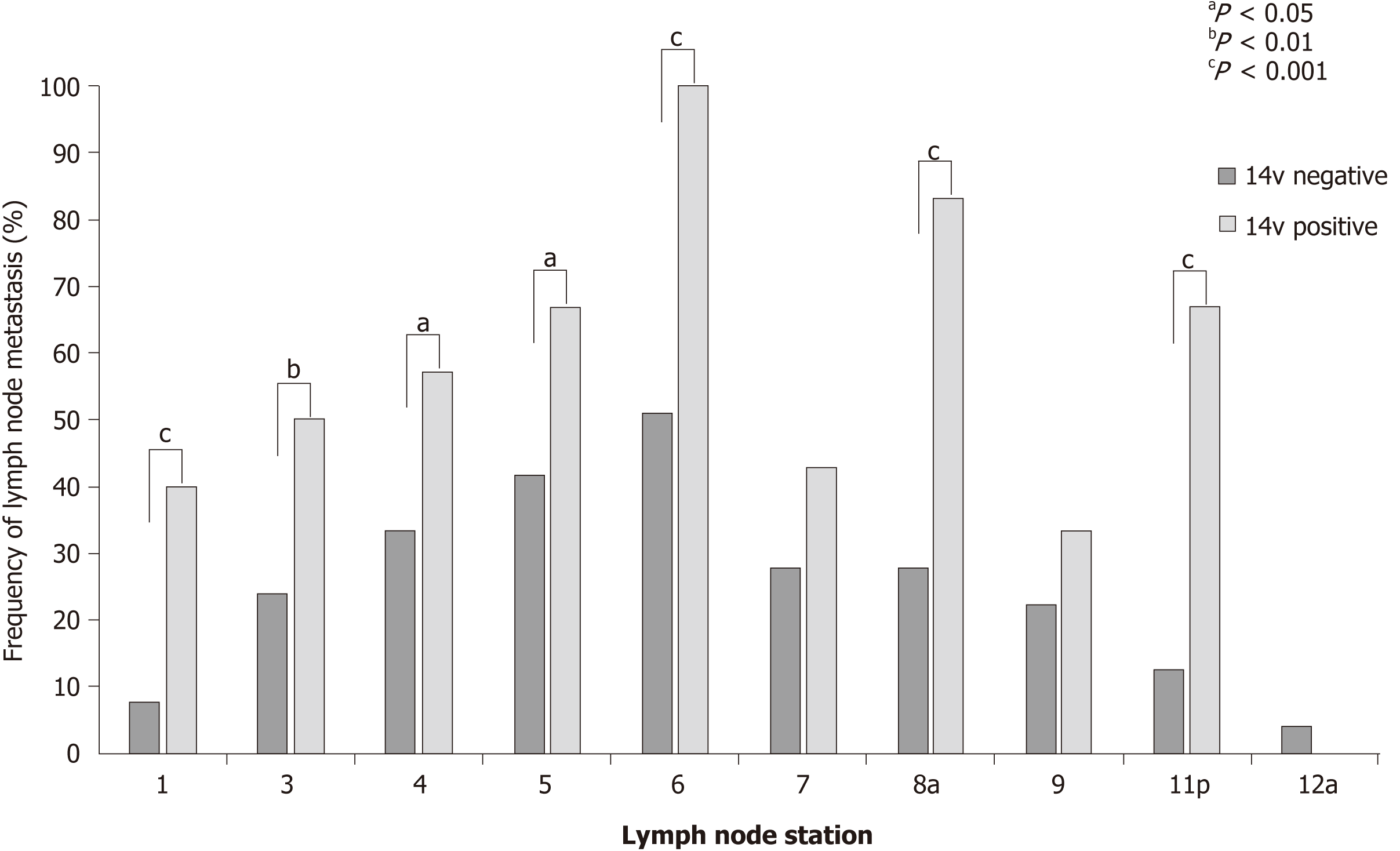
Tumors with 14v LN metastasis metastasized more often to LN stations 1, 3, 4, 5, 6, 8a, and 11p. This difference was significant for LN stations 1, 6, 8a, and 11p (P < 0.001). These results may indicate that the presence of 14v LN metastasis can be predicted based on the presence of metastasis to LN stations 1, 6, 8a, and 11p (Figure 4).
In the present study, we found that the OS of patients with 14v LN metastasis was comparable to that of patients with M1 stage tumors, similar to previous findings[12]. However, previous studies have also shown that GC patients with 14v LN metastasis without other distant metastasis had a significantly better OS compared to patients with M1 stage GC (P < 0.001)[18,19]. Given the differences in the results, we cannot directly classify patients with 14v metastasis as having M1 stage GC, and we cannot ignore the potential survival benefits of 14v LN dissection[20,21]. Therefore, it is important to select appropriate candidates who will benefit from the addition of 14v LN dissection. Some studies supported the addition of 14v LN dissection to D2 gastrectomy for patients with LTGC[19,20,22-31]. Eom et al[24] showed that 14v LN dissection was an independent prognostic factor for patients with clinical stage III/IV GC in the middle or lower third of the stomach. Liang et al[25] argued that 14v LN dissection might improve the 3-year OS for distal pathological stage IIIB/IIIC GC. Additionally, Chen et al[19] found that adding laparoscopic 14v dissection to laparoscopic-assisted radical distal gastrectomy might improve the OS of cT2-3 patients.
In the present study, we found that, among patients with pathological stage IIIA GC, the 14vD (+) group had better OS compared with the 14vD (-) group (P = 0.020). The TNM stage used in our study was defined according to the AJCC eighth edition, while the sixth edition was used in the study by Eom et al[24] and the seventh edition in the studies by Liang et al[27] and Edge et al[32,33]. Moreover, our study demonstrated that adding 14v LN dissection had survival benefits for stage IIIA patients, and these results are similar to those obtained by Eom et al[24] and Liang et al[27].The latter studies evaluated patients with advanced-stage tumors, while the patients in our study had early stage GC. To the best of our knowledge, the present study is the first to evaluate the role of adding 14v LN dissection for patients with different pathological stage GC according to the AJCC eighth edition. We found no effect of adding 14v LN dissection on the OS of patients with pathological stage I and II LTGC. This result could probably be attributed to the rarity of 14v LN metastasis in these diseases. In the studies by An et al[12] and Kong et al[34-36], the incidence of 14v LN metastasis was 0% and 2%-3% in stages I and II GC, respectively. Considering the low incidence of 14v LN metastasis in pathological stage I/II LTGC, 14v LN dissection was not recommended for these patients. Moreover, in the current study, 14v LN dissection did not result in better OS of patients with pathological stage IIIB and IIIC LTGC probably because patients with stage IIIB and IIIC GC have more extensive tumor invasion and tend to develop systemic disease.
The 14v LNs are anatomically downstream of the No. 6 LN considering the lymphatic flow for patients with LTGC. In theory, once the No. 6 LN is invaded, there is a high risk of metastasis to the 14v LNs. An et al[12]. reported that metastasis to the No. 6 LN was a useful predictive factor for 14v LN metastasis, with an accuracy rate of 99.0% and false-negative rate of 1.9%[10]. In the present study, all patients with 14v LN metastasis had No. 6 LN metastases. Thus, our study results may indicate that the presence of 14v LN metastasis could be predicted based on the presence of metastasis to LN stations 1, 6, 8a, and 11p.
The present study has some limitations. First, this was a retrospective cohort study, and the clinicopathological features were different between the two groups. Therefore, we performed propensity score matching analysis to minimize these differences caused by nonrandom assignments. Second, the number of patients was small, especially for subgroup analysis, thereby possibly influencing the results. Third, we could not obtain information about surgical-related safety assessment, postoperative complications, and postoperative mortality; therefore, it was impossible to compare whether the risk of 14v LN dissection increased. Accordingly, in the future, high-quality multicenter clinical randomized controlled studies are needed to evaluate the effect of 14v LN dissection on OS.
In conclusion, the present study demonstrated that OS was similar between patients with 14v LN metastasis and those with M1 stage disease. Patients with No. 1, No. 6, No. 8a, or No. 11p LN metastasis were at a higher risk of 14v LN metastasis. The addition of 14v LN dissection to D2 dissection during radical distal gastrectomy may improve the OS of patients with pathological stage IIIA LTGC.
In the fifteenth edition of the Japanese Classification of Gastric Carcinoma, the 14v lymph node (LN) (LNs along the root of the superior mesenteric vein) was defined as the regional gastric LN.
The efficacy of 14v LN dissection during radical distal gastrectomy for lower-third gastric cancer (GC) remains controversial.
To analyze whether the addition of 14v LN dissection improved the survival of patients with lower-third GC.
Using the propensity score-matched method from our institute database constructed between 2000 and 2012, overall survival (OS) was compared between the patients with and without 14v LN dissection.
OS was similar between patients with 14v LN metastasis and those with distant metastasis. Among patients with pathological stage IIIA disease, those who were treated with 14v LN dissection had a significantly higher OS than those treated without it.
Adding 14v LN dissection to D2 dissection during radical distal gastrectomy may improve the OS of patients with pathological stage IIIA lower-third GC.
In the future, high-quality multicenter clinical randomized controlled studies are needed to evaluate the effect of 14v LN dissection on OS.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kositamongkol P, Higgins PD S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55817] [Article Influence: 7973.9] [Reference Citation Analysis (132)] |
| 2. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1308] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 4. | Mohri J, Katada C, Ueda M, Sugawara M, Yamashita K, Moriya H, Komori S, Hayakawa K, Koizumi W, Atsuda K. Predisposing Factors for Chemotherapy-induced Nephrotoxicity in Patients with Advanced Esophageal Cancer Who Received Combination Chemotherapy with Docetaxel, Cisplatin, and 5-fluorouracil. J Transl Int Med. 2018;6:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Schizas D, Kapsampelis P, Mylonas KS MD. Adenosquamous Carcinoma of the Esophagus: A Literature Review. J Transl Int Med. 2018;6:70-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G, Morino M; Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 7. | Douridas GN, Pierrakakis SK. Is There Any Role for D3 Lymphadenectomy in Gastric Cancer? Front Surg. 2018;5:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Yarema R, de Manzoni G, Fetsych T, Ohorchak M, Pliatsko M, Bencivenga M. On the road to standardization of D2 lymph node dissection in a European population of patients with gastric cancer. World J Gastrointest Oncol. 2016;8:489-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Association JGC. Japanese classification of gastric carcinoma. 15 version ed. Tokyo: Kinbara Publishing Co 2017; . |
| 10. | Association JGC. Japanese gastric cancer treatment guidelines. 5 version ed. Tokyo: Kinbara Publishing Co 2018; . |
| 11. | Gibiino G, Larghi A. EUS-guided fine-needle biopsy for histological examination: Is it time to change our sampling technique? Endosc Ultrasound. 2018;7:71-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | An JY, Pak KH, Inaba K, Cheong JH, Hyung WJ, Noh SH. Relevance of lymph node metastasis along the superior mesenteric vein in gastric cancer. Br J Surg. 2011;98:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Masuda TA, Sakaguchi Y, Toh Y, Aoki Y, Harimoto N, Taomoto J, Ikeda O, Ohga T, Adachi E, Okamura T. Clinical characteristics of gastric cancer with metastasis to the lymph node along the superior mesenteric vein (14v). Dig Surg. 2008;25:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Zhang J, Zou S, Luo R, Zhu Z, Wang Z, Xu H, Huang B. Is it worthy of adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for distal gastric cancers with No. 6 lymph node metastasis? Clin Transl Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Morita S, Fukagawa T, Fujiwara H, Katai H. Questionnaire survey regarding the current status of super-extended lymph node dissection in Japan. World J Gastrointest Oncol. 2016;8:707-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kumagai K, Sano T, Hiki N, Nunobe S, Tsujiura M, Ida S, Ohashi M, Yamaguchi T. Survival benefit of "D2-plus" gastrectomy in gastric cancer patients with duodenal invasion. Gastric Cancer. 2018;21:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P. Gastric Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. |
| 18. | Wu L, Zhang C, Liang Y, Wang X, Ding X, Liang H. Risk factors for metastasis to No.14v lymph node and prognostic value of 14v status for gastric cancer patients after surgery. Jpn J Clin Oncol. 2018;48:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Chen QY, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Huang CM. Safety and prognostic impact of prophylactic laparoscopic superior mesenteric vein (No. 14v) lymph node dissection for lower-third gastric cancer: a propensity score-matched case-control study. Surg Endosc. 2018;32:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Blouhos K, Boulas KA, Tsalis K, Hatzigeorgiadis A. Right-sided bursectomy as an access plane for aesthetic resection of the posterior leaf of the lesser sac from the head of the pancreas en block with the No. 6 and 14v lymph nodes in advanced lower third gastric cancer. Surgery. 2015;158:1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dietrich CF, Bibby E, Jenssen C, Saftoiu A, Iglesias-Garcia J, Havre RF. EUS elastography: How to do it? Endosc Ultrasound. 2018;7:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Tokunaga M, Ohyama S, Hiki N, Fukunaga T, Inoue H, Yamada K, Sano T, Yamaguchi T, Nakajima T. Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion: is the posterior pancreatic head lymph node dissection beneficial? Ann Surg Oncol. 2009;16:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 335] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Eom BW, Joo J, Kim YW, Reim D, Park JY, Yoon HM, Ryu KW, Lee JY, Kook MC. Improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer. Surgery. 2014;155:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Liang Y, Wu L, Wang X, Ding X, Liu H, Li B, Wang B, Pan Y, Zhang R, Liu N, Liang H. Positive impact of adding No.14v lymph node to D2 dissection on survival for distal gastric cancer patients after surgery with curative intent. Chin J Cancer Res. 2015;27:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Therapeutic Value of Lymph Node Dissection Along the Superior Mesenteric Vein and the Posterior Surface of the Pancreatic Head in Gastric Cancer Located in the Lower Third of the Stomach. Yonago Acta Med. 2018;61:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Liang H, Deng J. Evaluation of rational extent lymphadenectomy for local advanced gastric cancer. Chin J Cancer Res. 2016;28:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Yu P, Du Y, Xu Z, Huang L, Cheng X. Comparison of D2 and D2 plus radical surgery for advanced distal gastric cancer: a randomized controlled study. World J Surg Oncol. 2019;17:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Choudhary NS, Bodh V, Kumar N, Puri R, Sarin H, Guleria M, Piplani T, Krishan S, Rai R, Sud R. Yield of endoscopic ultrasound-guided fine needle aspiration for subcentimetric lymph nodes: A comparison to larger nodes. Endosc Ultrasound. 2017;6:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Cooray M, Nistor I, Pham J, Bair D, Arya N. Accuracy of endoscopic ultrasound-fine needle aspiration of solid lesions over time: Experience from a new endoscopic ultrasound program at a Canadian community hospital. Endosc Ultrasound. 2017;6:187-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Dietrich CF. The resectable pancreatic ductal adenocarcinoma: To FNA or not to FNA? A diagnostic dilemma, introduction. Endosc Ultrasound. 2017;6:S69-S70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Lh S. TNM classification of malignant tumors. New York: John Wiley Sons 2002; . |
| 33. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6460] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 34. | Kong SH, Yoo MW, Kim JW, Lee HJ, Kim WH, Lee KU, Yang HK. Validation of limited lymphadenectomy for lower-third gastric cancer based on depth of tumour invasion. Br J Surg. 2011;98:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Yamane S, Katada C, Tanabe S, Azuma M, Ishido K, Yano T, Wada T, Watanabe A, Kawanishi N, Furue Y, Kondo Y, Komori S, Ishiyama H, Hayakawa K, Koizumi W. Clinical Outcomes in Patients with Cancer of Unknown Primary Site Treated by Gastrointestinal Oncologists. J Transl Int Med. 2017;5:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Yasumoto M, Okabe Y, Ishikawa H, Kisaki J, Akiba J, Naito Y, Ishida Y, Ushijima T, Tsuruta O, Torimura T. A case of gastric wall implantation caused by EUS-FNA 22 months after pancreatic cancer resection. Endosc Ultrasound. 2018;7:64-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |