Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2611
Peer-review started: March 15, 2019
First decision: July 30, 2019
Revised: August 9, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 6, 2019
Processing time: 177 Days and 22.5 Hours
Aplasia cutis congenita (ACC) in newborns is a condition in which congenital defects or hypoplasia is present in part of the epidermis, dermis and even subcutaneous tissue (including muscle and bones). First reported by Cordon in 1767, ACC is a rare disease with a low incidence of 1/100000 to 3/10000. Currently, there are 500 cases reported worldwide. ACC can be accompanied by other malformations. The onset mechanism of the disease remains unknown but is thought to be correlated to factors such as genetics, narrow uterus, foetal skin and amniotic membrane adhesion, use of teratogenic drugs in early pregnancy and viral infection.
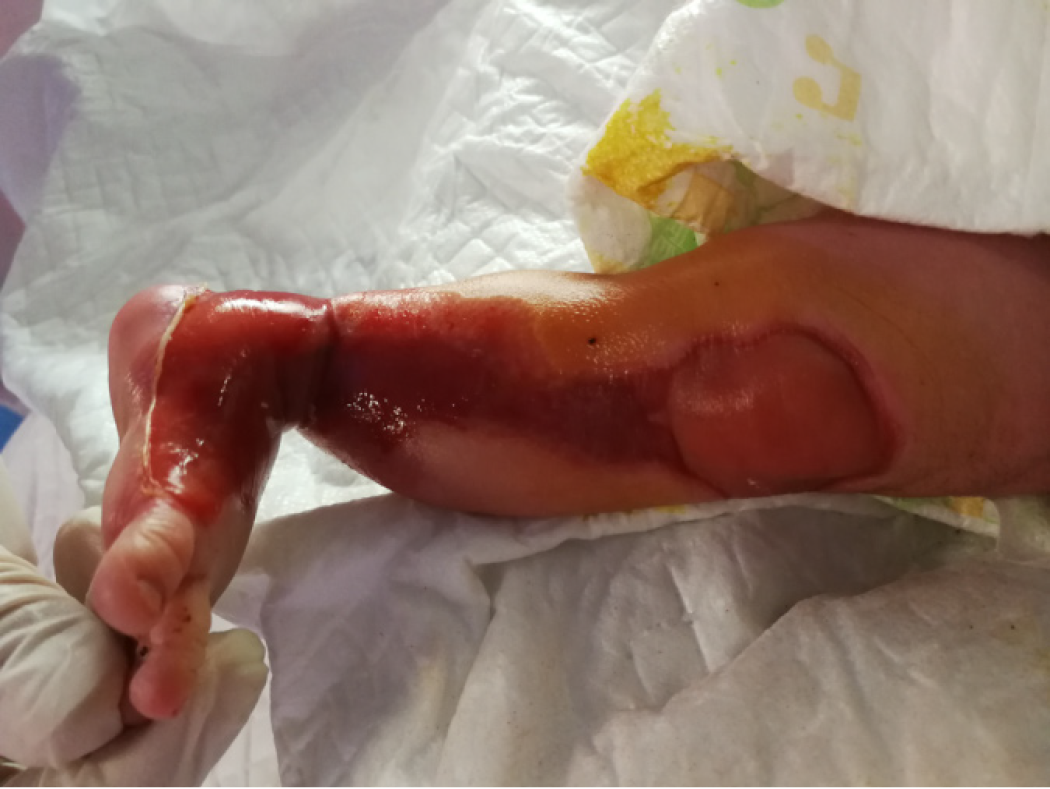
In August 2018, we treated a newborn with ACC on the left lower limbs using a combination of ionic silver dressing and moist exposed burn ointment (MEBO) and achieved a satisfactory treatment outcome. The skin defects were observed on the external genitals and on areas from the left foot to 3/4 of the upper left side. Subcutaneous tissue and blood vessels were observed in the regions with skin defects. The following treatments were provided. First, the wound was rinsed with 0.9% sodium chloride solution followed by disinfection with povidone-iodine twice. And then MEBO was applied to the wound at a thickness of approximately 1 mm. After applying ionic silver dressing, the wound was covered with sterile gauze. The wound dressing was replaced every 2-3 d. At the 4-mo follow-up, the treatment outcome was satisfactory. There was minimal scar tissue formation, and limb function was not impaired.
The combination of ionic silver dressing and MEBO to ACC is helpful.
Core tip: Aplasia cutis congenita (ACC) is a rare disease in China and abroad. There is not yet a well-developed treatment for this disease. Combination of ionic silver dressing and moist exposed burn ointment in treating ACC is an useful and effective method. This treatment is worthy of being promoted in clinical practice.
- Citation: Lei GF, Zhang JP, Wang XB, You XL, Gao JY, Li XM, Chen ML, Ning XQ, Sun JL. Treating aplasia cutis congenita in a newborn with the combination of ionic silver dressing and moist exposed burn ointment: A case report. World J Clin Cases 2019; 7(17): 2611-2616
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2611.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2611
Aplasia cutis congenita (ACC) is a rare disease in China and abroad. Patients with this disease often have other abnormalities or malformations. According to the literature, the incidence of ACC is approximately 1 in 100000, but the mortality rate is as high as 18%. Currently, there is not yet a well-developed treatment for this disease. A critical step in treating ACC is a prompt skin grafting to cover the wound[1]. Although skin grafting is important for patients with large areas of skin defect, this surgical process can aggravate the patient’s pain and suffering. Recently, with more in-depth studies on the treatment of ACC in newborns, methods employed in treating burn wounds have yielded beneficial therapeutic effects for treating ACC[2]. Amongst the non-surgical methods, wound protection, infection prevention and nutrient supplemen-tation are key to ensure treatment outcomes[2]. Herein, a case of ACC treated in our hospital is reported along with a retrospective analysis of the clinical data and discussion on lessons learned.
In August 2018, a female infant presented to our hospital with the skin defects on the external genitals and areas from the left foot to 3/4 of the upper left side.
Patient’s symptoms were observed after birth
The patient was a female infant born at 37+6 wk via C-section. Intrauterine distress was not observed. However, premature rupture of foetal membranes and bloody amniotic fluid were present. The baby was found with the umbilical cord around her neck. There was no abnormality in the placenta, and no asphyxia was observed. The Apgar scores were 10 at 1-10 min after birth. The birth weight was 2800 g.
The 28-year-old mother was in her second pregnancy but first birth. A miscarriage had previously occurred at age 25. The mother performed a urine pregnancy test 35 d after menstruation and received a colour ultrasound 54 d after menstruation. The results suggested intrauterine pregnancy, and the foetal size was consistent with the gestational age. Foetal movement was sensed at week 16 and remained active until delivery. The thyroid function test at week 18 displayed no abnormalities. Foetal chromosome (T21, T18, T13) screening at week 22 indicated that the baby was at low risk of aneuploidy. A four-dimensional colour ultrasound examination at week 26 found no abnormalities. After admission for delivery, no abnormalities were observed by various examinations on the mother including a routine blood test, routine urine test, coagulation test, infectious disease tests, hepatitis B, liver and kidney functions and electrocardiogram. The family did not have a history of congenital conditions.
The results of the physical examination of the infant after birth are described next. The infant appeared to be born full term with normal appearance and responded well to stimulation. Skin defects were observed on the external genitals and on areas from the left foot to 3/4 of the upper left side. Subcutaneous tissue and blood vessels were observed in the regions with skin defects (Figure 1).
Laboratory blood tests were normal.
The ultrasound, lower limbs computed tomography (CT) scan, high-resolution chest CT scan, and head magnetic resonance imaging were normal.
The baby was diagnosed with ACC and was transferred to the Division of Neonatology for further treatment.
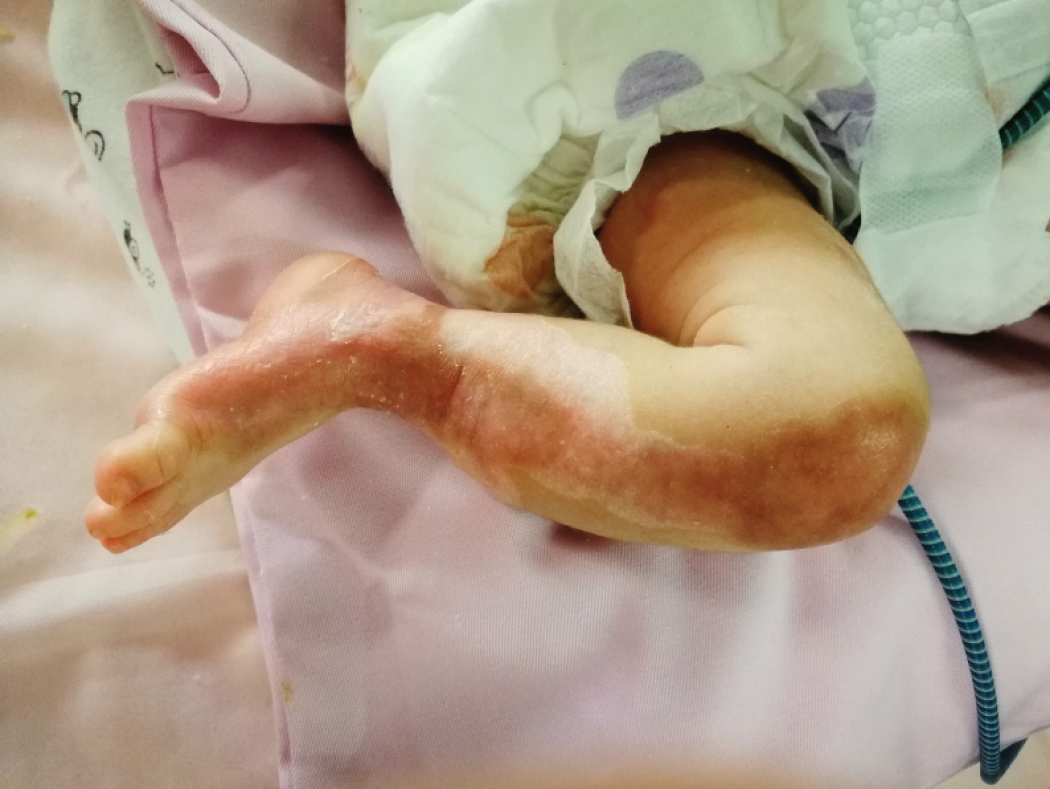
The following treatments were provided: (1) Wound treatment: The wound was rinsed with 0.9% sodium chloride solution followed by disinfection with povidone-iodine twice. Moist exposed burn ointment (MEBO) was applied to the wound at a thickness of approximately 1 mm. The surface covered by MEBO was extended beyond the edges of the wound. After applying ionic silver dressing, the wound was covered with sterile gauze. The wound dressing was replaced every 2-3 d; (2) Infection prevention: Intravenous injections of second-generation cephalosporin were provided for 7 d; and (3) Nutrition support: An intravenous infusion of 10% glucose was given along with premium powdered formulas. The condition of the infant was complicated by hypoproteinaemia, which was corrected by the infusion of albumin. With the above-described treatment, the skin on the lower limbs of the patient recovered after 26 d (Figure 2), and the patient was discharged. The patient was prescribed multi-sulfonic acid mucopolysaccharide cream to prevent scar tissue formation and soften existing scar tissue.
During treatment, the functions of the limbs were preserved; limb shortening was not observed. No blisters were observed on the skin. A histopathological examination was not performed. At the 4-mo follow-up, the treatment outcome was satisfactory. There was minimal scar tissue formation, and limb function was not impaired.
ACC is a rare neonatal disease with a low incidence rate. The incidences are typically sporadic. However, the condition may be genetic. The cause of ACC is not known. According to the literature, the most common factors of ACC include foetal chromosomal or genetic abnormalities (particularly BMS1 and UBA2 genetic abnormalities)[3], trauma[4], amniotic fluid or amniotic membrane abnormalities (e.g., foetal skin and amniotic membrane adhesion)[5], intrauterine complications (e.g., infection)[6], vascular lesions (e.g., thrombus and vascular diseases)[7] and the use of teratogenic drugs during pregnancy (e.g., cocaine, methotrexate, angiotensin inhibitor)[8]. ACC is diagnosed mainly based on clinical manifestations: Clear skin defects are observed on regions of the body at birth; the defects can be accompanied by defects or hypoplasia of subcutaneous tissues (including muscle and bones); the shape of the defected skin is irregular, which can be sheet-like, in patches, or irregular shapes; and the size and depth of the skin defects also vary. Pathophysiological examination often suggests defects in the epidermis and dermis. Total or partial defects can also be found in the lipids of the subcutaneous tissue. ACC can be accompanied by other diseases or complications such as epidermolysis bullosa (EB), cheilopalatognathus and polycystic kidney disease. In 1986, Frieden[9] classified ACC in newborns into 9 groups based on the location and features of the lesions and the accompanying conditions: Scalp ACC without multiple anomalies, scalp ACC with associated limb abnormalities, scalp ACC with associated epidermal and organoid nevi, ACC overlying embryologic malformations, ACC with associated foetus papyraceus or placental infarcts, ACC associated with EB, ACC localized to extremities without blistering, ACC caused by specific teratogens and ACC associated with malformation syndromes. Scalp lesions are the most common and comprise 70% of defects, followed by defects in limbs and the trunk. Some cases can be accompanied by defects in the oral mucosal membrane or perineal mucosal membrane and skeletal malformation in limbs[10].
Conservative and surgical methods can be employed as treatment for ACC. Conservative treatment includes the application of topical ointments such as silver sulfadiazine dressing, antibiotics, etc. Surgical treatments include split-thickness or full-thickness skin grafts, acellular dermal matrices, autologous epithelial transplantation, tissue expansion, cranioplasty, etc. The selection of the treatment method depends on the width, depth, location and involved subcutaneous tissues of the defects. Conservative treatments are recommended if the defect is only at the skin level without damage to the bones and if the area of the defect is less than 3 cm2. Conversely, skin grafts are necessary when the defected area is large and involves critical organs or tissues such as the scalp while also accompanied by the exposure of large blood vessels or sagittal sinus. The skin defects in limbs often display clearly defined boundaries and typically involve the epidermis and dermis. Approximately 85% of ACC cases are simple defects without complications. However, the indications for selecting between conservative versus surgical treatment remain controversial. Bigliardi et al[11] reported a case of ACC in the right lower limb. The defect involved the thigh, knee and lower leg to the first and second toes. Ulcer-like defects were observed, and the blood vessels were clearly visible. The wounds recovered after 3 mo of silver sulfadiazine topical cream application. In a case reported by Pająk et al[12], a skin defect in the right lower limb was 4.0 cm × 1.5 cm in size. In addition to antibiotic ointment and ionic silver dressing, suturing was performed. Although the patient recovered after one month of treatment, a linear scar formed. These case reports suggest that for ACC that does not affect bone tissues, conservative treatment might provide a better outcome. However, surgical treatment is necessary when a large area of defect is present.
The major ingredients of MEBO include sesame oil, beeswax, berberine, phellodendron and baicalin. MEBO provides a moist environment to the wound tissue. An appropriate level of humidity is beneficial for tissue recovery. Linoleic acid, multiple amino acids, vitamins and trace elements are essential for the survival of cells. These ingredients can effectively activate the transformation of residual skin tissue to stem cells and allows in situ cultivation. The newly formed skin tissues can then adhere to the adjacent hyperplasia tissues to promote natural recovery. Additionally, MEBO can relieve mild local and systemic inflammation, promote wound recovery and improve recovery quality.
Ionic silver dressing is a 3D foaming structure that allows the effective absorption of exudate and water vapor. This absorption can minimize impregnation of the wound, which then inhibits the growth of bacteria and lowers the risk of infection. Ionic silver dressing is a natural broad spectrum antimicrobicide that contains silver compound, which provides high bacterial inhibition with its slow-release abilities. In the process of treating the case reported herein, a high amount of exudate and the bleeding of the newly formed blood vessel were observed on days 4-7. The exudate and bleeding were greatly reduced with the application of ionic silver dressing.
The treatment of our case demonstrated that the combination of MEBO and ionic silver dressing can effectively promote skin growth and prevent infection. This treatment method does not involve surgical operation; thus, it reduces the pain and suffering of the patient while simultaneously reducing the financial burden. This treatment is worthy of being promoted in clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karaman A S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Alfayez Y, Alsharif S, Santli A. A Case of Aplasia Cutis Congenita Type VI: Bart Syndrome. Case Rep Dermatol. 2017;9:112-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Cherubino M, Maggiulli F, Dibartolo R, Valdatta L. Treatment of multiple wounds of aplasia cutis congenita on the lower limb: a case report. J Wound Care. 2016;25:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Marble M, Guillen Sacoto MJ, Chikarmane R, Gargiulo D, Juusola J. Missense variant in UBA2 associated with aplasia cutis congenita, duane anomaly, hip dysplasia and other anomalies: A possible new disorder involving the SUMOylation pathway. Am J Med Genet A. 2017;173:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Marcovici I. Aplasia cutis congenita presenting as vacuum-extractor-related trauma. Int J Gynaecol Obstet. 2015;129:267-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Brzezinski P, Pinteala T, Chiriac AE, Foia L, Chiriac A. Aplasia cutis congenita of the scalp--what are the steps to be followed? Case report and review of the literature. An Bras Dermatol. 2015;90:100-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Šimić D, Prohić A, Puizina Ivić N, Zeljko Penavić J, Tomić T. Aplasia Cutis Congenita in a Newborn Child Associated with Two Fetus Papyraceous. Acta Dermatovenerol Croat. 2015;23:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Choi MS, Choi JH, Ki SH, Jun YH. Aplasia Cutis Congenita Associated With Aplasia of the Superficial Temporal Artery. J Craniofac Surg. 2016;27:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Sachs C, Tebacher-Alt M, Mark M, Cribier B, Lipsker D. [Aplasia cutis congenita and antithyroid drugs during pregnancy: Case series and literature review]. Ann Dermatol Venereol. 2016;143:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Frieden IJ. Aplasia cutis congenita: a clinical review and proposal for classification. J Am Acad Dermatol. 1986;14:646-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 306] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Harvey G, Solanki NS, Anderson PJ, Carney B, Snell BJ. Management of aplasia cutis congenita of the scalp. J Craniofac Surg. 2012;23:1662-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Bigliardi PL, Braschler C, Kuhn P, Sigrist J, Buechner S, Rufli T. Unilateral aplasia cutis congenita on the leg. Pediatr Dermatol. 2004;21:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Pająk A, Szczygieł A, Paluszyńska D, Królak-Olejnik B. Congenital skin aplasia on the lower limb in a premature infant with ELBW--case report. Ital J Pediatr. 2014;40:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |