Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2556
Peer-review started: May 8, 2019
First decision: May 31, 2019
Revised: July 30, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 6, 2019
Processing time: 136 Days and 0 Hours
Acquired factor V deficiency is a rare secondary hemorrhagic disease, which can lead to a severe bleeding disorder.
We report a 47-year-old hemodialysis patient who presented with severe hemorrhagic pleural effusion and hemorrhagic pericardial effusion associated with lymphatic leakage. The laboratory examination revealed decreased factor V activity (2% of population average value). With decreased lymphatic leakage, factor V activity increased (to 46%). Lymph drainage correlated with prothrombin time and active partial thrombin time. The cause of the disease favored an acquired disease. The common causes which trigger factor V inhibitors were excluded. An inhibitor was not detected. It is possible that there was a clotting factor inhibitor leaking with the lymph in the drainage. Inhibitor production may be due to immune dysfunction caused by persistent lymphatic drainage, or that coagulation inhibitors were produced, drained with the lymph, and partly cleared by hemodialysis.
In this case, we have firstly reported factor V deficiency associated with lymphatic leakage in a hemodialysis patient.
Core tip: Acquired factor V deficiency (AFVD) is a rare secondary hemorrhagic disease, which can lead to serious bleeding disorder. We report a new AVFD case of a hemodialysis patient with severe serous cavity hemorrhagic effusion, associated with potentially secondary to lymphatic leakage, and factor V inhibitor detection is negative. This is the first report regarding the association between coagulation factor V deficiency and lymphatic drainage in hemodialysis patient of chronic kidney disease patient. Careful follow-up of blood coagulation is needed in patients under the treatment of lymphatic drainage.
- Citation: Zhao WB, Chen YR, Luo D, Lin HC, Long B, Wu ZY, Peng H. Severe serous cavity bleeding caused by acquired factor V deficiency associated with lymphatic leakage in a hemodialysis patient: A case report. World J Clin Cases 2019; 7(17): 2556-2561
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2556.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2556
Acquired factor V deficiency (AFVD) is a rare secondary hemorrhagic disease that can lead to a severe bleeding disorder. Streiff et al[1] reported AVFD for the first time in 1955. A recent literature review cited approximately 200 cases of AFVD[2]. AFVD is primarily due to the development of factor V inhibitors, i.e. antibodies against factor V. Exposure to bovine thrombin, antibiotics, surgical procedures, tumors, autoimmune diseases, infections, transplantations, and disseminated intravascular coagulation (DIC) may trigger factor V inhibitors in previously healthy patients[3].
The clinical symptoms of AFVD have been reported as variable, and range from asymptomatic to a severe bleeding disorder and thrombosis[4]. We report here a case of AVFD in a hemodialysis patient with a severe serous cavity hemorrhagic effusion secondary to lymphatic leakage, and no factor V inhibitor was detected. Even though the case occurred several years ago, we are of the opinion that the case is clinically meaningful. A large volume of lymphatic drainage can lead to a coagulation disorder, which may not arouse suspicion.
The patient was a 47-year-old man who was admitted to our hospital on 7 January 2011 with a 2-year history of recurrent edema of the eyelids and lower extremities and a 7-d history of shortness of breath and severe hypertension. He was hospitalized due to exacerbation of limb and scrotal edema, as well as decreased urine output. The patient had no previous medical history. There was no family history of hemorrhagic disease and chronic kidney disease (CKD).
Physical examination revealed mild pitting edema in both lower extremities.
Laboratory testing revealed the following: blood urea nitrogen, 60.26 mmol/L; serum creatinine, 1026 µmol/L; prothrombin time (PT), 14.3 s; active partial thrombin time (APTT), 40 s; hemoglobin, 63 g/L; white blood cell (WBC) count, 6.12 × 109/L; neutrophilic granulocyte percentage (N%), 79.2%; and platelet count, 176 × 109/L. Chest X-ray: Lung was negative. On 8 January 2011, a right femoral venous catheterization was performed to facilitate hemodialysis treatment. In the absence of ultrasonic guidance, the puncture needled the right femoral artery and resulted in a right common femoral artery pseudo aneurysm with a size of 63 mm × 37 mm. The patient received non-heparin dialysis during dialysis treatment. On 28 January 2011, after a right femoral artery pseudoaneurysm resection and rupture repair, the lump resolved. One week post-operatively, a gradually increasing 2 cm × 3 cm mass without swelling, tenderness, and a fluctuating sensation was not found in the medial aspect of the surgical incision with a light yellow clear liquid exudate on the surface. After a diagnostic puncture and catheterization, 500 mL of light red liquid was drained under negative pressure (Table 1). A lymphatic fistula was diagnosed and the lymph liquid consisted of the following: WBC count, 3.0 × 106/L; red blood cell (RBC) count, 44 × 106/L; glucose, 7.31 mmol/L; total protein, 5.3 g/L; and lactate dehydrogenase, 88 g/L.
| Lymphatic Fistula fluid | Pericardial fluid | Thoracic drainage fluid | |
| WBC (106/L) | 3 | 4960 | 3320 |
| RBC (109/L) | 44 | 4620 | 1380 |
| N (%) | 5 | 70 | 60 |
| Protein (g/L) | 5.3 | 56.7 | 45.7 |
| GLU (mmol/L) | 7.31 | 3.15 | 7.18 |
| LDH (U/L) | 88 | 539 | 261 |
| TB-Ab | Negative | Negative | Negative/Positive |
| Bacterial growth smear | Negative | Negative | Negative |
| Acid-fast bacilli smear | Negative | Negative | Negative |
Five days later, the coagulation time was significantly prolonged after lymphatic drainage on the right side; a vitamin K1 supplement proved ineffective. The hemoglobin level was significantly decreased and the patient had a small amount of nasal mucosa bleeding and large bloody pericardial and pleural effusions. Laboratory testing revealed the following: albumin, 35.7 g/L; PT, 52.8 s; APTT, 180.1 s; fibrinogen, 4.67 g/L; coagulation factor II, 72%; coagulation factor VII, 84%; and coagulation factor X, 64% (Table 2). Factor V was deficient (2% of population average) and no inhibitor was detected. A total of 1140 mL of fluid was drained by pericardial aspiration, with a red and turbid appearance. In addition, 2400 mL of fluid was drained via thoracic drainage and had a deep red appearance without clots. Fluid cultures were obtained and negative.
| Normal range | February 23, 2011 | March 3, 2011 | March 24, 2011 | April 20, 2011 | March 13, 2018 | |
| PT (s) | 11.0-15.0 | 52.8 | 29.2 | 18.9 | 16.2 | |
| PT (%) | 70-120 | 13 | 27 | 58.4 | 67 | |
| PT-INR | 6.15 | 2.82 | 1.85 | 1.29 | ||
| APTT (s) | 31.5-43.5 | 180.1 | 86.8 | 49.2 | 49 | |
| TT (s) | 14-21 | 15.6 | ||||
| Fib | 2.00-4.00 | 4.67 | ||||
| FII (%) | 70-120 | 72 | ||||
| FV (%) | 70-120 | 2 | 8 | 15 | 46 | 98.9 |
| FVII (%) | 70-120 | 84 | ||||
| FX (%) | 70-120 | 64 | ||||
| PC:AC | 70-120 | 92 |
The final diagnosis is AFVD and chronic kidney disease stage 5D.
The main treatment measures included maintenance hemodialysis, supplementing fresh frozen plasma, and reducing the lymphatic drainage.
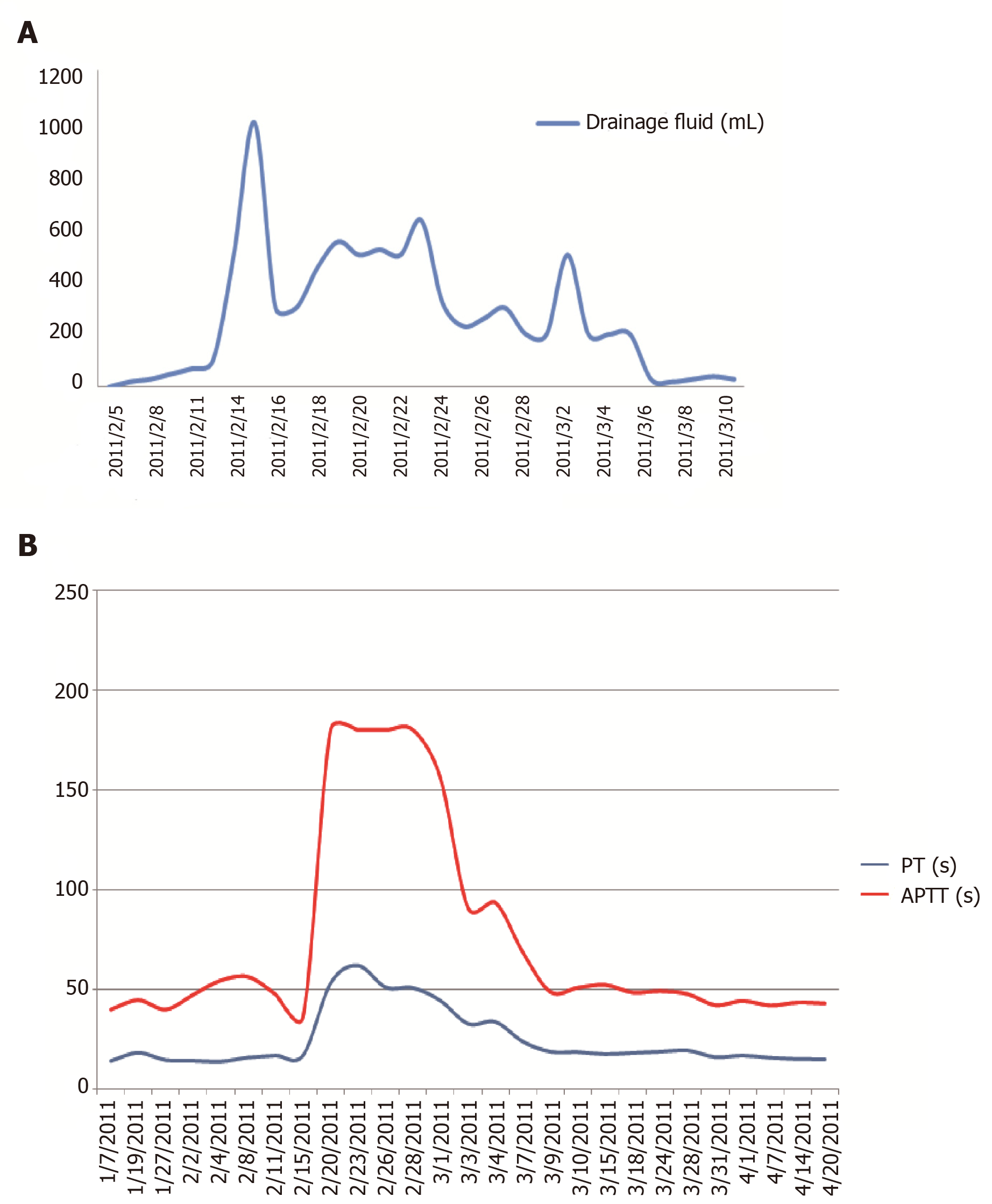
Since 5 February 2011, the patient has received treatment with a daily infusion of 200-600 mL of fresh frozen plasma. After applying a pressure dressing, the lymphatic drainage decreased, the skin bleeding resolved, the pericardial and pleural effusions decreased, coagulation function improved, and PT and APTT decreased. On 3 March 2011, the coagulation factor V level was 8% (Table 2, Figure 1). On 20 April 2011, coagulation factor V level improved (to 46%). The amount of lymph drainage was correlated with the PT and APTT.
The patient improved clinically and coagulation factor V level (to 46%) after the follow-up for three months. On 13 March 2018, coagulation factor V level was 98.9%.
We report a patient with chronic kidney disease who exhibited mucosal hemorrhage and multiple hemorrhagic effusions in the serous cavity associated with lymphatic leakage due to acquired coagulation factor V deficiency (Figure 1).
The patient had no exposure to bovine thrombin and no manifestations of DIC. There was no history of antibiotic use and no evidence of antibiotics contributing to the coagulant function abnormality. AVFD occurred two weeks after surgery, but there was no direct evidence of an association with the surgical procedure. The patient had no autoimmune diseases previously reported which could have led to AVFD.
In this case, the lymphatic vessel injury caused lymphatic leakage. Negative pressure drainage has been reported to be a method to treat lymphatic leakage[5]. After constant lymphatic drainage, the patient exhibited significant malnutrition, weight loss of approximately 10 kg, and decreased serum albumin and immunoglobulin concentrations. Biochemical testing revealed the existence of clotting factor deficiency that may have been due to immune dysfunction caused by persistent lymphatic drainage, or coagulation inhibitor production and coagulation inhibitor partly cleared by hemodialysis, resulting in no detection of inhibitors[3,6]. The lymphmay also be due to lymphatic drainage of the clotting factors rather than immune factors with producing an inhibitor, but the composition of the lymphatic drainage was not tested.
Coagulation function was improved after supplementation by fresh frozen plasma. Eight days after reduced drainage following the application of a pressure dressing, coagulation factor V concentration increased to 8%, the bleeding tendency and bloody effusions in the pericardial and pleural cavities were resolved, and after removal of the drainage tube, coagulation factor V concentration increased to 46%. It has been reported that high-dose immunoglobulin treatment rapidly improved coagulation factor V deficiency caused by immune disorder[6]. The patient in the current study was treated with human immunoglobulin (10 g/d for four consecutive days) from 25 February 2011, which was ineffective. Because no coagulation factor inhibitors were detected, hormone and immunosuppressive drugs were not used[7].The amount of lymph drainage was positively correlated with the PT and APTT values, indicating that there was a quantitative relationship between the amount of lymph drainage and coagulation function (coagulation factor V deficiency).
This is the first report regarding an association between coagulation factor V deficiency and lymphatic drainage in a chronic kidney disease patient for hemodialysis. Careful follow-up of blood coagulation is needed in patients under treatment with lymphatic drainage associated with various diseases and operation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheungpasitporn W S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Streiff MB, Ness PM. Acquired FV inhibitors: a needless iatrogenic complication of bovine thrombin exposure. Transfusion. 2002;42:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Wang X, Qin X, Yu Y, Wang R, Liu X, Ji M, Zhou M, Chen C. Acquired factor V deficiency in a patient with a urinary tract infection presenting with haematuria followed by multiple haemorrhages with an extremely low level of factor V inhibitor: a case report and review of the literature. Blood Coagul Fibrinolysis. 2017;28:334-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ang AL, Kuperan P, Ng CH, Ng HJ. Acquired factor V inhibitor. A problem-based systematic review. Thromb Haemost. 2009;101:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Kalafatis M, Simioni P, Tormene D, Beck DO, Luni S, Girolami A. Isolation and characterization of an antifactor V antibody causing activated protein C resistance from a patient with severe thrombotic manifestations. Blood. 2002;99:3985-3992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lemaire V, Brilmaker J, Kerzmann A, Jacquemin D. Treatment of a groin lymphatic fistula with negative pressure wound therapy. Eur J Vasc Endovasc Surg. 2008;36:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | de Raucourt E, Barbier C, Sinda P, Dib M, Peltier JY, Ternisien C. High-dose intravenous immunoglobulin treatment in two patients with acquired factor V inhibitors. Am J Hematol. 2003;74:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Kessler CM, Knöbl P. Acquired haemophilia: an overview for clinical practice. Eur J Haematol. 2015;95 Suppl 81:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |