Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2384
Peer-review started: December 3, 2018
First decision: January 30, 2019
Revised: March 12, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: August 26, 2019
Processing time: 265 Days and 20.9 Hours
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has becoming ever more recognized in the treatment of hepatocellular carcinoma (HCC). Nevertheless, long-term survival rate and postoperative complications are far from ideal, mainly since the majority of patients treated with ALPPS surgery have large or multiple lesions and microvascular tumor thrombus.
We present the case of a 47-year-old male patient with a huge right hepatic mass and an estimated insufficient residual liver, who was successfully treated with ALPPS surgery and apatinib. Postoperative pathology revealed HCC with several significant microvascular embolisms. Twenty months after operation, no tumor reoccurrence was observed.
Our case indicated that combined targeted drug therapy with ALPPS can lead to long-term survival for patients with large HCC.
Core tip: Our aim was to explore the feasibility of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) combined with targeted therapy in the treatment of advanced hepatocellular carcinoma (HCC). Herein, we present the case of a 47-year-old male patient with a huge right hepatic mass and an estimated insufficient residual liver, who was successfully treated with ALPPS surgery and apatinib. Postoperative pathology revealed HCC with several significant microvascular embolisms. Twenty months later, no tumor reoccurrence was observed, indicating that combined targeted drug therapy with ALPPS can lead to long-term survival for patients with large HCC.
- Citation: Liu L, Li NF, Zhang Q, Lin L. Hepatocellular carcinoma successfully treated with ALPPS and apatinib: A case report. World J Clin Cases 2019; 7(16): 2384-2392
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2384.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2384
Hepatocellular carcinoma (HCC) is among the most common causes of cancer-related deaths worldwide. Currently, HCC is the second most common malignancy in cities and the first most common malignancy in the countryside of China; it is the second cancer-related mortality in males and third in females, seriously affecting the health of Chinese people[1]. Currently, the treatments for HCC have greatly progressed due to the development of technology. In addition, a great variety of treatments have been applied so far, such as targeted drug therapy, microwave and radiofrequency ablation therapy, interventional radiotherapy and so on. Nevertheless, surgical resection remains the most effective treatment, preferred by patients with HCC, among whom only 20%-30% are eligible for surgical resection[2,3]. It has also been reported that the 5-year survival rate of HCC is 40%, while the rate of resectable early HCC can reach 60%-70%[4], which means that most patients cannot undergo radical resection but can only benefit from remedial treatments such as drugs and radiation interventions, which eventually leads to the poor prognosis of HCC treatment.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a new surgical procedure that has emerged over the recent years. It was first applied to liver metastasis of colon cancer with good results[5]. ALPPS is directed at patients with large or multiple right liver tumors that cannot be resected at one time due to insufficient residual liver volume. The first step is the ligation of the affected side of the portal vein, which allows the blood supply of the portal vein to nourish the normal liver. In addition, the hepatic artery and vein of the affected side are retained to preserve parts of the function of the affected liver; the left and right livers are separated at the same time to prevent tumor invasion of the normal liver lobe. When the normal hepatic lobe volume increases to an ideal range, which usually takes 2-3 wk, the affected liver is resected to avoid the occurrence of postoperative liver failure. It has been reported that 55% of the patients with HCC in the world are Chinese[1]. Many large liver centers in China have applied ALPPS for the treatment of liver cancer over the recent years, achieving good results. The team of academician Fan Jia, Zhongshan Hospital of Shanghai Fudan University, has performed a retrospective analysis of 45 cases of hepatitis B-related HCC after ALPPS surgery from April 2013 to September 2017, showing that the long-term survival rate after ALPPS was better than that after transcatheter arterial chemoembolization (TACE), and that there was no significant difference between ALPPS and primary hepatectomy[6]. Yet, many scholars have questioned the high complication and mortality rates of ALPPS. Most of the patients who underwent ALPPS were advanced HCC. How to carry out postoperative comprehensive treatment to improve the survival rate of HCC patients is also a problem worthy of discussion. Herein, we present a case of giant HCC with cirrhosis of the right liver. Preoperative assessment of residual liver insufficiency made it difficult to perform one-off resection. Postoperative pathological examination revealed formation of multiple microvascular tumor thrombi. We performed ALPPS surgery which was subsequently combined with a targeted new drug (apatinib), eventually achieving good results. The main purpose of this study was to investigate the reasonable procedure of ALPPS in order to reduce postoperative complications and mortality, and to further improve the survival rate of patients undergoing ALPPS.
This study was approved by the ethic committee of Xiangya Hospital, Central South University and the patient has consented to the submission of the case report.
A 47-year-old male patient was admitted to the Department of Hepatobiliary and Pancreatic Surgery of Xiangya Hospital of Central South University in November 2016 due to right upper abdominal pain that lasted for one week.
He felt paroxysmal dull pain in his right upper abdomen for one week without fever, jaundice, or other symptoms.
He had a 10-year long history of hepatitis B that was not treated systematically, and a 10-year long history of gout.
His brother had a history of hepatitis B, but his parents’ histories of hepatitis B were not clear.
Physical examination revealed clear mind, moderate nutrition, and no yellow stains in the skin and sclera; no swollen lymph nodes; the abdomen was soft, and the liver and spleen were not palpable under the ribs with tapping pain in the liver area; there was no edema in lower limbs.
Laboratory tests indicated the following: AFP 25.82 ng/mL; TBIL 19.2 μmol/L, DBIL 8.5 μmol/L, ALB 42.1 g/L, ALT 158.6 U/L, AST 231.6 U/L; HBsAg (+), HBcAb(+), HBV DNA 7.89E+04. Routine blood tests, routine urine tests, routine fecal tests, the renal and coagulation functions were all normal. Preoperative evaluation was performed, which revealed an indocyanine green (ICG) retention rate at 15 min (ICG15) of 6.9% and Child-Pugh grade A liver function.
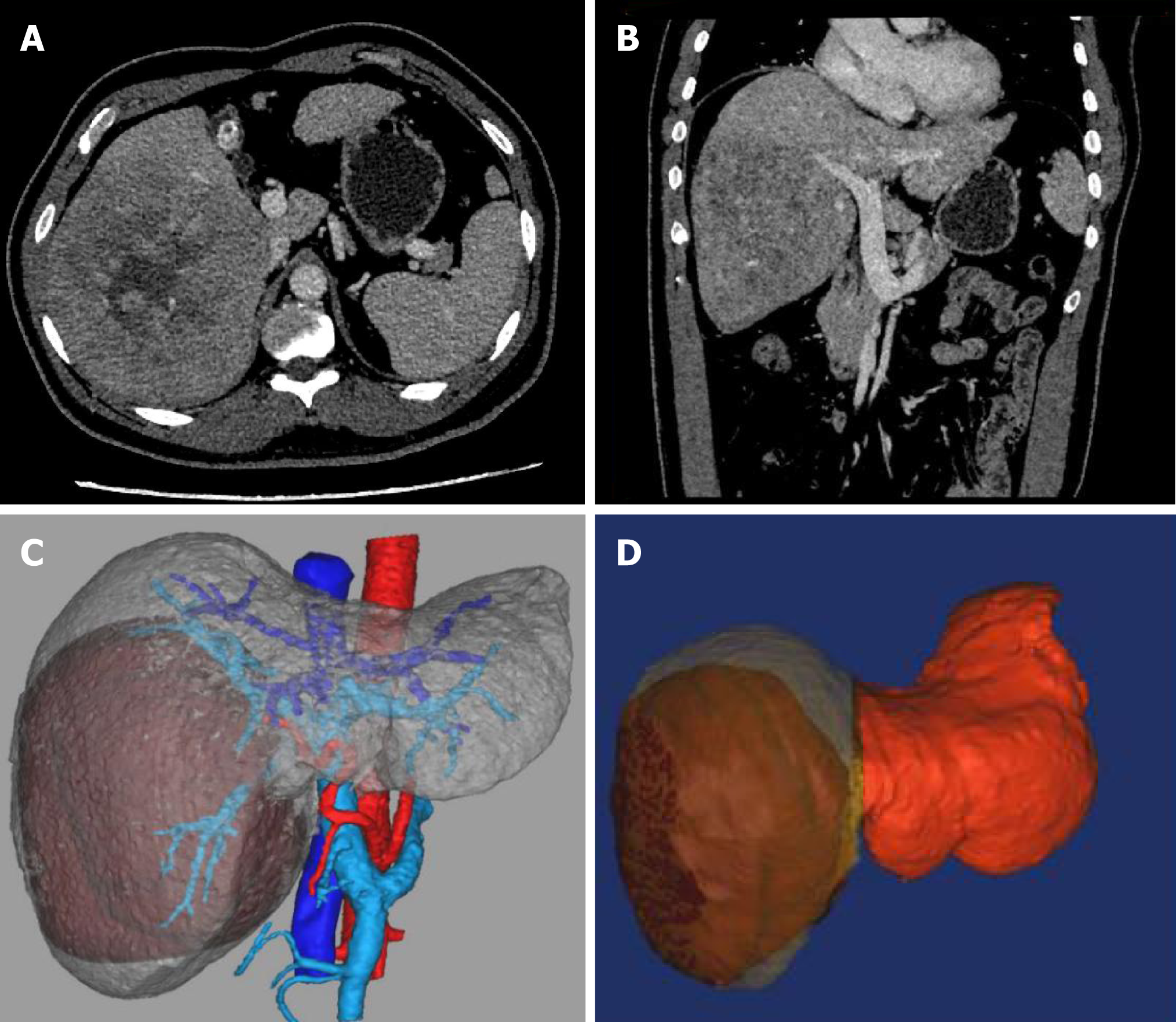
Ultrasonography and hepatic contrast-enhanced computed tomography (CT) showed cirrhosis, splenomegaly, portal hypertension, and a solid mass about 135 mm × 124 mm close to the first and second hepatic hilum, commonly observed in massive HCC (Figure 1).
The final diagnosis of the presented case was primary HCC due to hepatitis B.
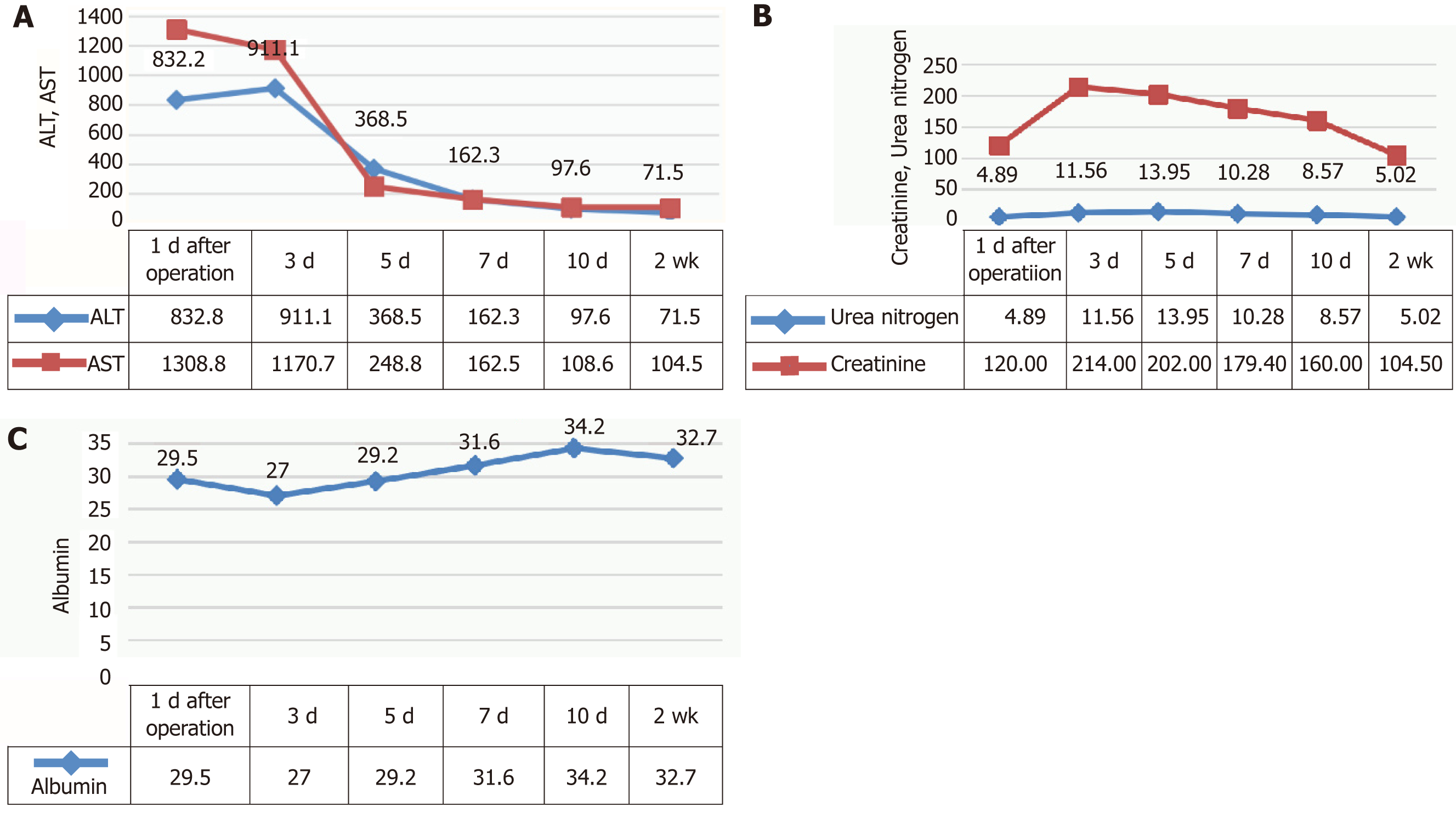
According to CT imaging, 3D modeling revealed that residual liver volume/effective liver volume was 37.6%. Considering that the patient had large HCC that required right hemi-hepatectomy, and that hepatitis virus replication was in active phase and the patient had liver cirrhosis and mild damage of liver function, we performed ALPPS. The first step of ALPPS, i.e., laparoscopic ligation of the right branch of portal vein and separation of the left and right liver, was performed after one week of treatment with magnesium isoglycyrrhizinate and entecavir. Briefly, a significant transaminase increase and albumin decrease were observed after the operation (Figure 2A). At the same time, the patient developed hepatorenal syndrome caused by oliguria, ascites, and renal dysfunction (Figure 2B). The validity of the ALPPS procedure was further confirmed by postoperative deterioration of liver function. The symptoms of ascites and oliguria were obviously improved after liver protection, diuresis, and albumin supplement, and the liver and kidney functions were close to normal. The indicators of liver and kidney function after operation are shown in Figure 2.
The liver volume increased rapidly after the first step of ALPPS. The residual liver volume/effective liver volume was 48.2% on the 9th day and 58.7% on the 16th day (Table 1 and Figure 3).
| Time | Estimated residual liver volume |
| Pre-operation | 37.6% |
| 9 d after operation | 48.2% |
| 16 d after operation | 58.7% |
The second step of ALPPS, i.e., right hepatectomy, was performed under general anesthesia on the 16th day after the first step of ALPPS. The patient was discharged on the 10th day after the operation.
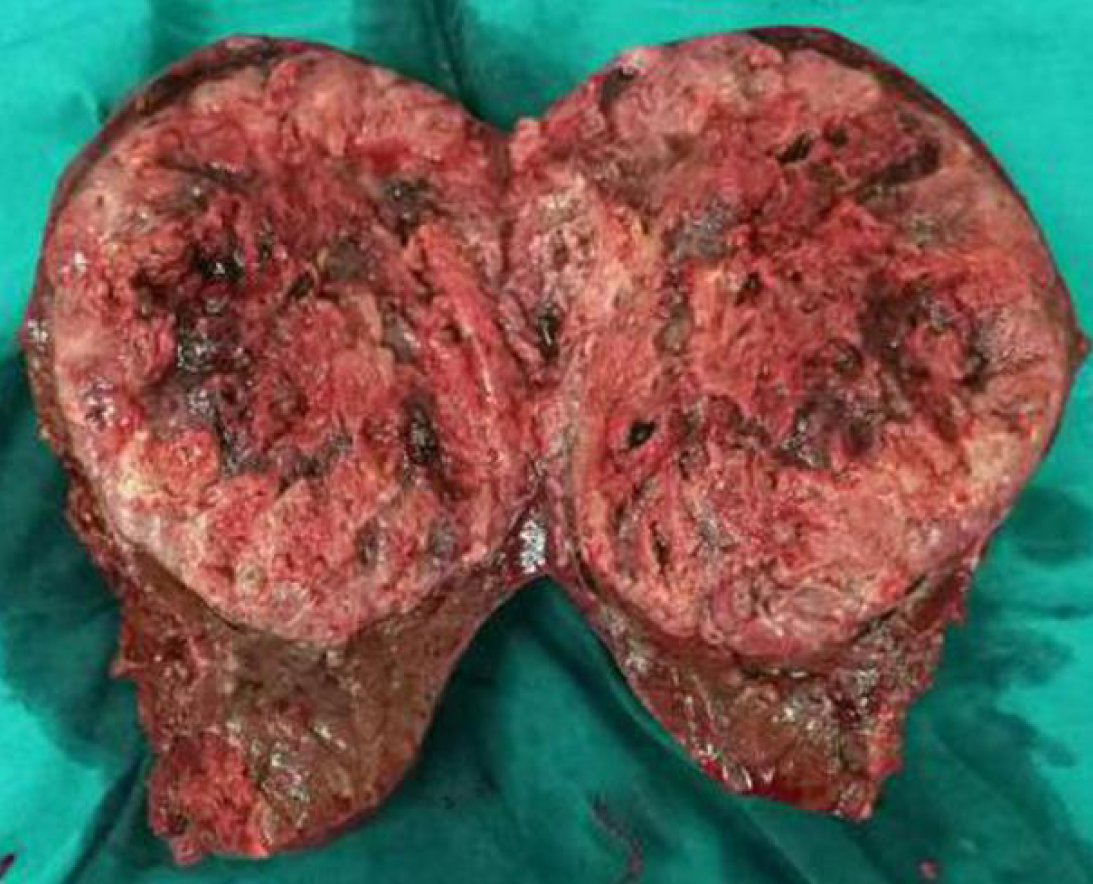
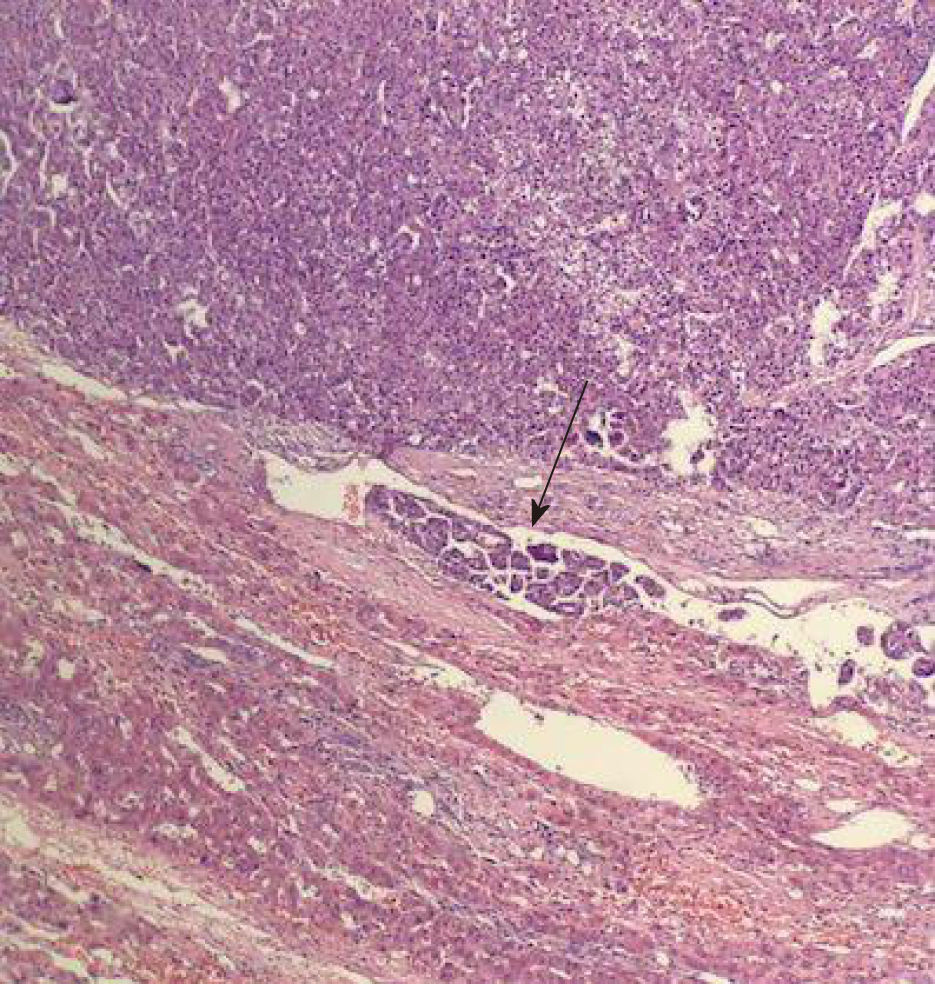
Postoperative pathological examination showed that high-grade differentiated HCC was found with more than five clear microvascular tumor thrombi adjacent to the tumor, and no tumor cells were found at the incisal margin (Figures 4 and 5).
After operation, the patient was treated with apatinib (500 mg daily) and oral administration of entecavir was continued. Mild hand-foot skin reaction and hypertension occurred during oral administration. The symptoms were improved after symptomatic treatment.
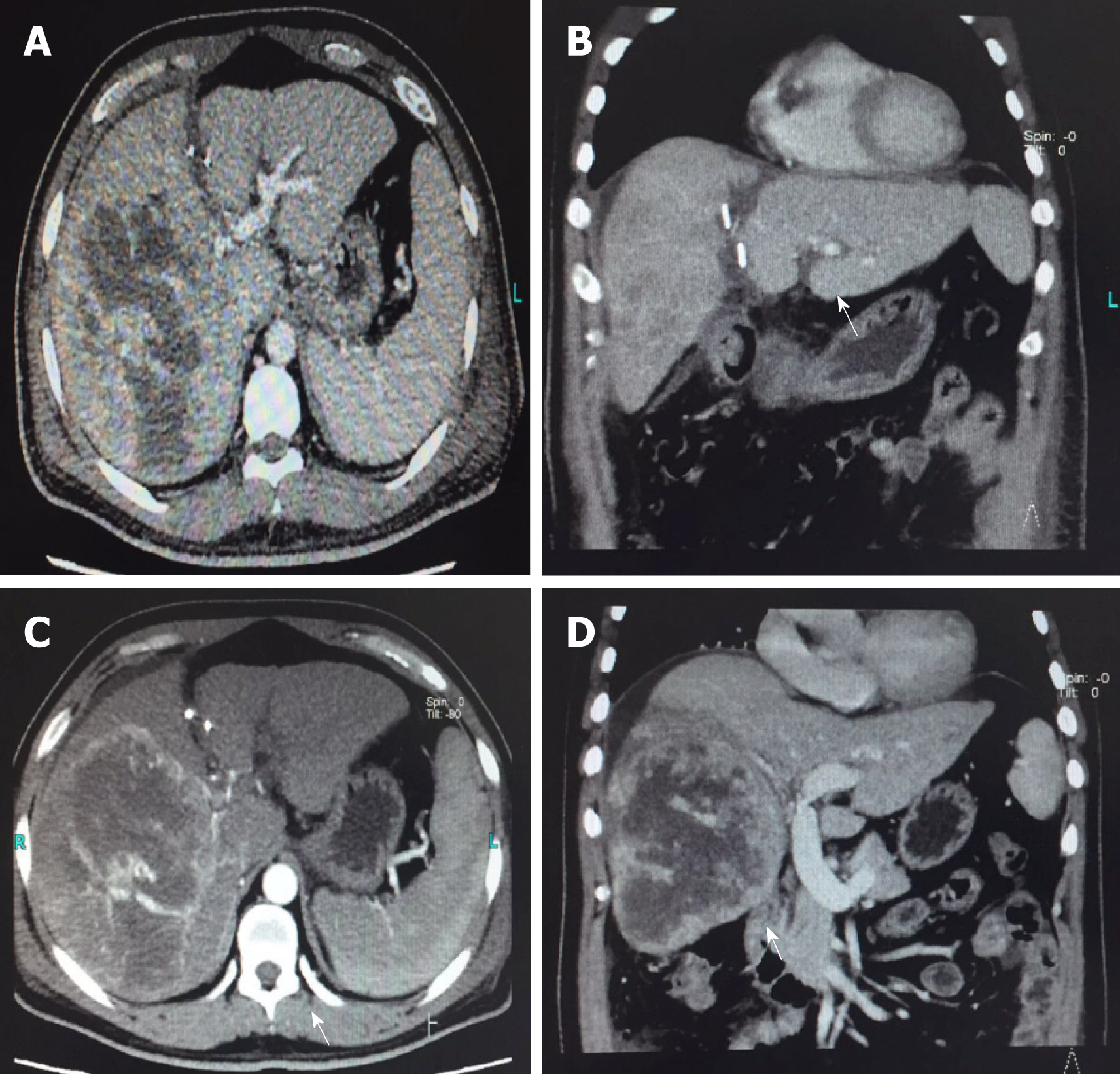
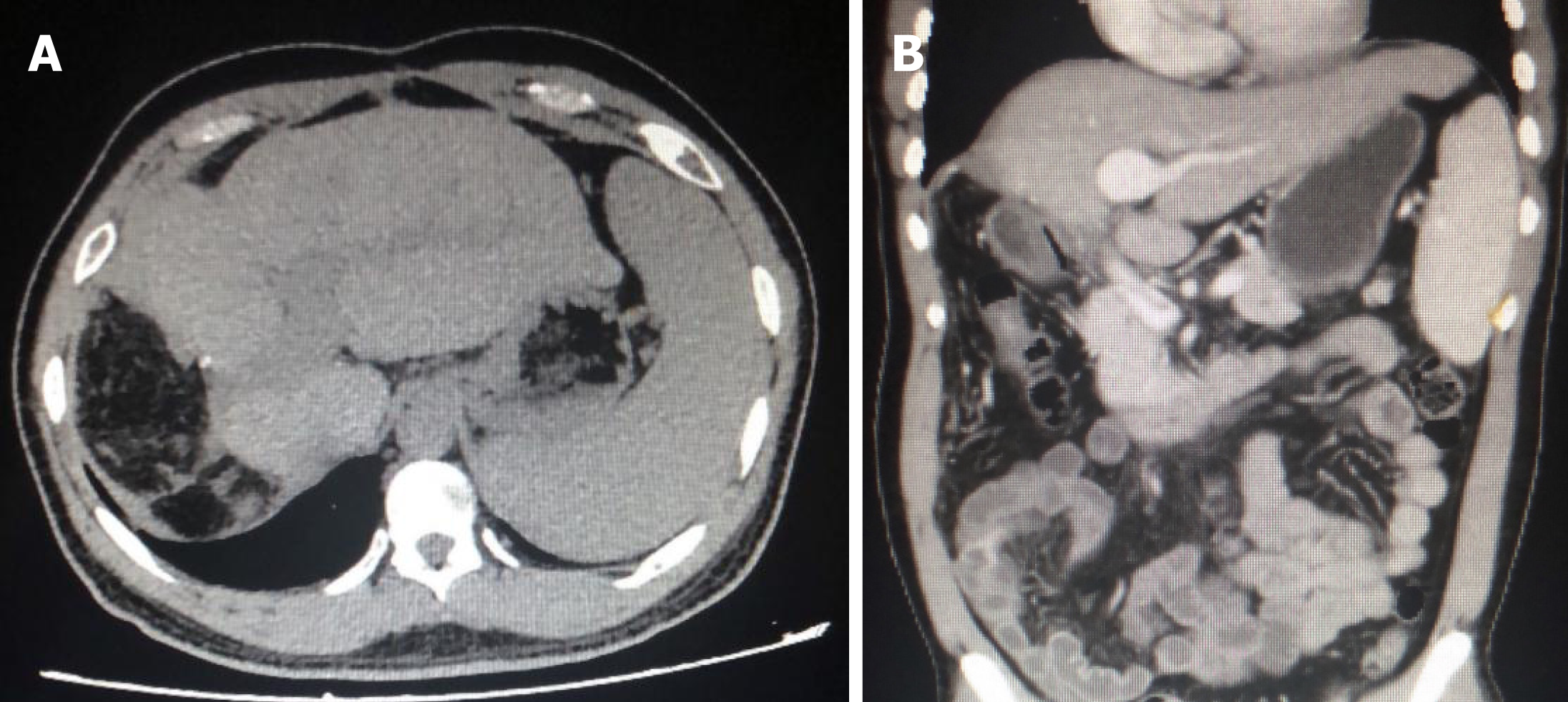
After discharge, the patient was regularly reexamined and returned to our hospital every 3 mo for systemic CT, AFP, second liver two half-and-half test, HBV DNA, liver and kidney function, blood routine test, and other related examinations. The latest follow-up time was August 2018. CT examination at the latest follow-up showed no recurrence or metastasis at 20 mo (Figure 6). The level of AFP was 5.48 ng/mL, which was significantly lower compared to 25.82 ng/mL, preoperatively.
There are three main reasons why HCC cannot be treated by resection: (1) patient’s general condition is such that he/she cannot tolerate surgery, especially due to liver condition; (2) presence of extrahepatic metastasis; and (3) the volume of residual liver is insufficient. Japanese scholars advocate the use of portal vein embolization (PVE) to solve this problem, but residual liver volume growth is slower after PVE than ALPPS. The average interval between two resections is 28 d, and 20%-50% of patients cannot reach the standard of total resection[7-9]. From January 2015 to December 2017, 20 patients underwent ALPPS at our center. The average interval between the two steps was 14 d, and only two patients did not reach the ideal volume without total radical surgery. Currently, the feasibility of ALPPS for giant HCC remains controversial. The main focus is on postoperative liver failure, complications, and mortality, whose incidence rates are significantly higher than those of TACE + PVE. A multicenter retrospective study has found that the postoperative mortality rates of ALPPS and PVE are 15% and 6%, respectively. The mortality rates in the ALPPS group are significantly higher than those in PVE group[10]. However, 20 cases of ALPPS were performed at our center with no mortalities at 90 d after operation. Preoperative estimations of residual liver volume, ICG retention test, and Child-Pugh grading combined with 3D modeling provided accurate assessment for HCC resection, minimized the risk of operation, improved the resection rate, and significantly reduced the occurrence of postoperative complications. Thus, ALPPS is a safe treatment for patients with HCC who cannot be resected in one operation. From our experience, the best candidates were patients with Child-Pugh grade A, ICG15 < 10%, residual liver volume/effective liver volume > 40% with mild liver damage or liver cirrhosis, or residual liver volume/effective liver volume > 30% with normal liver for surgery. Although there is a lack of multicenter randomized trial data on ALPPS, current studies have successfully demonstrated the feasibility of ALPPS in the treatment of HCC.
As we all know, patients undergoing ALPPS are mostly large HCC or multiple HCC patients. Pawlik's study has shown that the occurrence of microvascular invasion (MVI) is positively correlated with tumor size. MVI rates were 25%, 40%, 55%, and 63% in patients with tumor diameter < 3 cm, 3.1-5 cm, 5.1-6.5 cm, and > 6.5 cm, respectively (P < 0.005). The size and quantity of HCC were important predictors of MVI[11]. Meanwhile, MVI was also closely related to poor prognosis (including high recurrence rate and low long-term survival rate) in HCC patients. Sumie et al[12] have classified the patients into MVI-free group, mild MVI group (1-5), and severe MVI group (> 5) according to the number of MVI. The results showed that the higher the classification, the shorter the disease-specific relapse-free survival. Patients with HCC undergoing ALPPS are at high risk for MVI, so it is necessary to treat them with systemic therapy. In the present study, pathological examination of the patient showed that there were more than five distinct microvascular invasions around the tumor. For the high-risk recurrence factors, we used targeted therapy after operation. The drug of our choice was apatinib (500 mg/d). Apatinib is a direct multi-target RTK blocker for HCC, which effectively inhibits the activity, proliferation, and metastasis of tumor cells and promotes the apoptosis of cells[13]. Its price is relatively cheap, and a number of clinical studies have confirmed its safety and efficacy in the treatment of HCC[14-17]. Mild hand-foot skin reaction and hypertension occurred in the course of oral administration, which were improved after symptomatic treatment. The patient’s 20-mo tumor-free survival confirmed that apatinib associated with ALPPS resection greatly prolonged the survival of the HCC patient who could not have the tumor resected at one time and who had a vascular tumor thrombus. The patient had greatly benefited from this treatment, compared with patients with advanced HCC whose survival period was less than 10 mo.
Based on this case and from our experience with ALPPS, ALPPS is an effective treatment for patients with HCC. Preoperative evaluation was based on the patient's general condition, Child classification of liver function, calculation of residual volume/effective volume of liver, and ICG retention test. The mortality rate was significantly reduced, and combination with systemic therapy, such as targeted therapy, is expected to prolong the survival of patients. Apatinib has shown good results in the treatment of HCC. In view of the huge number of patients with advanced HCC in China, future studies should further expand the sample size to demonstrate its efficacy that would benefit more patients.
We wish to thank our patient for agreeing to share his case.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goral V, Vij M S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Yao L, Tang W. Screening for and surveillance of high-risk patients with HBV-related chronic liver disease: promoting the early detection of hepatocellular carcinoma in China. Biosci Trends. 2013;7:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 851] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 4. | Song P, Feng X, Zhang K, Song T, Ma K, Kokudo N, Dong J, Tang W. Perspectives on using des-γ-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr. 2013;2:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 5. | Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development. Hepatobiliary Pancreat Dis Int. 2017;16:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, Shi Y, Xiao Y, Ding Z, Yang X, Tang M, Tang Z, Wang J, Lau WY, Fan J, Zhou J. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Unresectable Hepatitis B Virus-related Hepatocellular Carcinoma: A Single Center Study of 45 Patients. Ann Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Aoki T, Kubota K. Preoperative portal vein embolization for hepatocellular carcinoma: Consensus and controversy. World J Hepatol. 2016;8:439-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Piron L, Deshayes E, Escal L, Souche R, Herrero A, Pierredon-Foulongne MA, Assenat E, le Lam N, Quenet F, Guiu B. [Portal vein embolization: Present and future]. Bull Cancer. 2017;104:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Glantzounis GK, Tokidis E, Basourakos SP, Ntzani EE, Lianos GD, Pentheroudakis G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur J Surg Oncol. 2017;43:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H, de Santibaňes E, Clavien PA. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 11. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, Abdalla EK. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, Kakuma T, Torimura T, Sata M. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Li X, Xu A, Li H, Zhang B, Cao B, Huang J. Novel role of apatinib as a multi-target RTK inhibitor in the direct suppression of hepatocellular carcinoma cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Zhu H, Ma X, Zhao Y, Duo J. The excellent antitumor effect of apatinib alone as second-line therapy in a patient with sorafenib-refractory hepatocellular carcinoma: A case report. Medicine (Baltimore). 2018;97:e11214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Yu WC, Zhang KZ, Chen SG, Liu WF. Efficacy and Safety of apatinib in patients with intermediate/advanced hepatocellular carcinoma: A prospective observation study. Medicine (Baltimore). 2018;97:e9704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Lu W, Jin XL, Yang C, Du P, Jiang FQ, Ma JP, Yang J, Xie P, Zhang Z. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol Ther. 2017;18:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Gou Q, Xu R, Chen X, Zhou Z. Efficacy and safety of sorafenib versus apatinib in the treatment of intermediate and advanced hepatocellular carcinoma: a comparative retrospective study. Onco Targets Ther. 2018;11:3407-3413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |