Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2247
Peer-review started: April 10, 2019
First decision: May 9, 2019
Revised: June 13, 2019
Accepted: July 3, 2019
Article in press: July 3, 2019
Published online: August 26, 2019
Processing time: 138 Days and 12.7 Hours
Childhood cancer survivors are potentially at a higher risk of infection with hepatitis C virus (HCV). The effects of all-oral direct-acting antiviral therapy (DAA) on both the HCV infection as well as the state of cancer remission have not been well investigated in this population.
To test the effects of dual sofosbuvir/daclatasvir (SOF/DCV) therapy in the treatment of chronic HCV in survivors of hematologic malignancy in pediatric age group.
We conducted a prospective, uncontrolled, open-label multicenter study. A total of 20 eligible, chronic HCV, genotype-4, infected children who had been in continuous complete remission from hematologic cancer (leukemia/lymphoma) for at least one year were included in the study. All patients were treated with combined SOF/DCV for 12 wk. Patients were monitored throughout the study till 12 wk after end of treatment for safety and efficacy outcomes including the sustained virologic response 12 (SVR12) rate, hematological indices, liver and kidney functions.
The intent-to-treat SVR12 rate was 20 of 20 (100%; 95%CI: 84%-100%). All patients showed normalized liver enzymes from week-4. All hematological indices, liver and kidney functions were kept normal throughout the study. No fatalities or treatment-emergent serious or severe adverse events were reported throughout the study.
SOF/DCV combined therapy could be used safely and effectively in the treatment of chronic HCV genotype-4 infection in leukemia/lymphoma treated children. No relapses were detected during treatment and throughout the follow up period for either the original malignant disease or the HCV infection.
Core tip: Dual Sofosbuvir/Daclatasvir (SOF/DCV) treatment was found to be as effective in the treatment of chronic hepatitis C virus (HCV) in survivors of childhood hematologic malignancy as in others with no history of malignancy. No relapses detected during study for the original malignant disease or the HCV infection. The results of this study could be assuring that the treatment of chronic HCV infection with direct-acting antivirals (DAAs) might not adversely affect the state of remission of survivors of hematologic malignancy in pediatric age groups; though larger studies with different DAAs and longer follow up are needed to confirm.
- Citation: El-Shabrawi MH, Sherief LM, Yakoot M, Kamal NM, Almalky MA, AbdElgawad MM, Mahfouz AA, Helmy S, Kamal EM, Attia D, El-Khayat HR. Effects of dual sofosbuvir/daclatasvir therapy on, chronic hepatitis C infected, survivors of childhood malignancy. World J Clin Cases 2019; 7(16): 2247-2255
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2247.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2247
Although the risk of transfusion-related-infection with hepatitis C virus (HCV) has become markedly low after the implementation of routine screening of the blood supply by the year 1992[1]; risk factors for HCV acquisition reported by many studies in middle and low income countries still include among others exposures to healthcare procedures, parenteral administration of drugs, chemotherapy and blood products transfusions[2,3].
Childhood cancer survivors are potentially at a higher risk of infection with HCV as they are more exposed to receive multiple transfusions of blood products and/or intravenous drug therapy[2,3].
HCV infection was shown to be associated with significantly increased morbidity and mortality from several cancers[3-5]. while, immunosuppression from cancer and/or the cancer-related treatment could increase the risk of chronicity, activation and progression to cirrhosis in patients infected with HCV[3,6].
Apart from hepatocellular carcinoma (HCC), the prevalence of HCV antibodies was reported to be much higher in patients harboring malignant diseases than others; in particular hematological malignancies which in some reports were associated with HCV antibodies in as high as 30% of cases[7,8]. This has led The European Conference on Infection in Leukemia (ECIL-5) group of experts to recommend screening for all patients with hematological malignancies for hepatotropic viruses before hematological treatment and that patients with markers of past or current viral hepatitis should be assessed by an expert[9].
Nowadays, the direct-acting antivirals (DAAs) have changed the treatment paradigm for chronic HCV infection and improved virologic outcomes in all studied populations including HCV-infected adult patients with cancer[10]. As a result of the great advances in our understanding of cancer biology and the better survival of cancer patients with modern therapy; the populations of survivors of childhood cancer are becoming now of increased prevalence and importance worldwide. We have previously screened a group of 100 consecutive patients attending the follow up outpatient pediatric-hematology-oncology clinics in a university hospital in Egypt after achieving cure from hematologic cancers and we found that the prevalence of HCV infection is very high approaching 50% of the screened cases (unpublished data). The effects of DAAs treatment on both the HCV infection as well as the state of cancer remission have not been well studied in survivors of cancer in pediatric age groups.
There have been conflicting reports that survivors of hepatocellular carcinoma may be at increased risk of early recurrence with more aggressive course of malignancy after treatment with DAAs[11,12]. Although many other recent reports demonstrated that DAAs are not causative for de-novo cancer[13]; many hepatologists are still afraid to expose survivors of cancer (of any type) to be treated with DAAs.
We aimed to study the efficacy, safety and tolerability of dual sofosbuvir/da-clatasvir (SOF/DCV) therapy in survivors of childhood hematologic malignancy infected with chronic HCV.
This study is a part of our clinical evaluation program conducted by our group of investigators to test safety and efficacy of the new DAA therapy on different pediatric populations.
The study had been designed as a prospective, single cohort, open-label, multi-center study and conducted in an outpatient setting.
Consecutive male or female patients, from eight to seventeen years of age, who had been detected from our HCV screening program for survivors of childhood hematologic malignancies to be infected with chronic HCV (by a positive HCV antibody test and/or positive serum HCV RNA), were subjected to screening for inclusion in the study according to our common protocol eligibility criteria described in our previous study[14]. Key exclusion criteria were: Pregnancy or lactation; concurrent other causes of hepatitis or HIV virus infection; active schistosomiasis; Child-Pugh score > six; alanine or aspartate aminotransferase (AST) > seven times the upper limit of normal, albumin < 2.8 g/dL; international normalized ratio > 2.3; transient elastography (by FibroScan®) result of more than 12.5 kPa at screening and/or an AST to platelet ratio index (APRI) of more than two; platelet count less than 50 × 109/L; severe anemia [hemoglobin grade-3 or higher (< 8 g/dL)]; any malignancy; Alfa-fetoprotein (AFP) level above 200 ng/mL; critically ill or more than slight limitation of activity; unwilling to participate or to sign the informed consent.
The study common protocol was reviewed and approved by the Research Ethics Committee of Faculty of Medicine, Alexandria University (IRB00007555) according to the Declaration of Helsinki. All subjects and their parents/guardians signed the informed consents before the start of the study interventions.
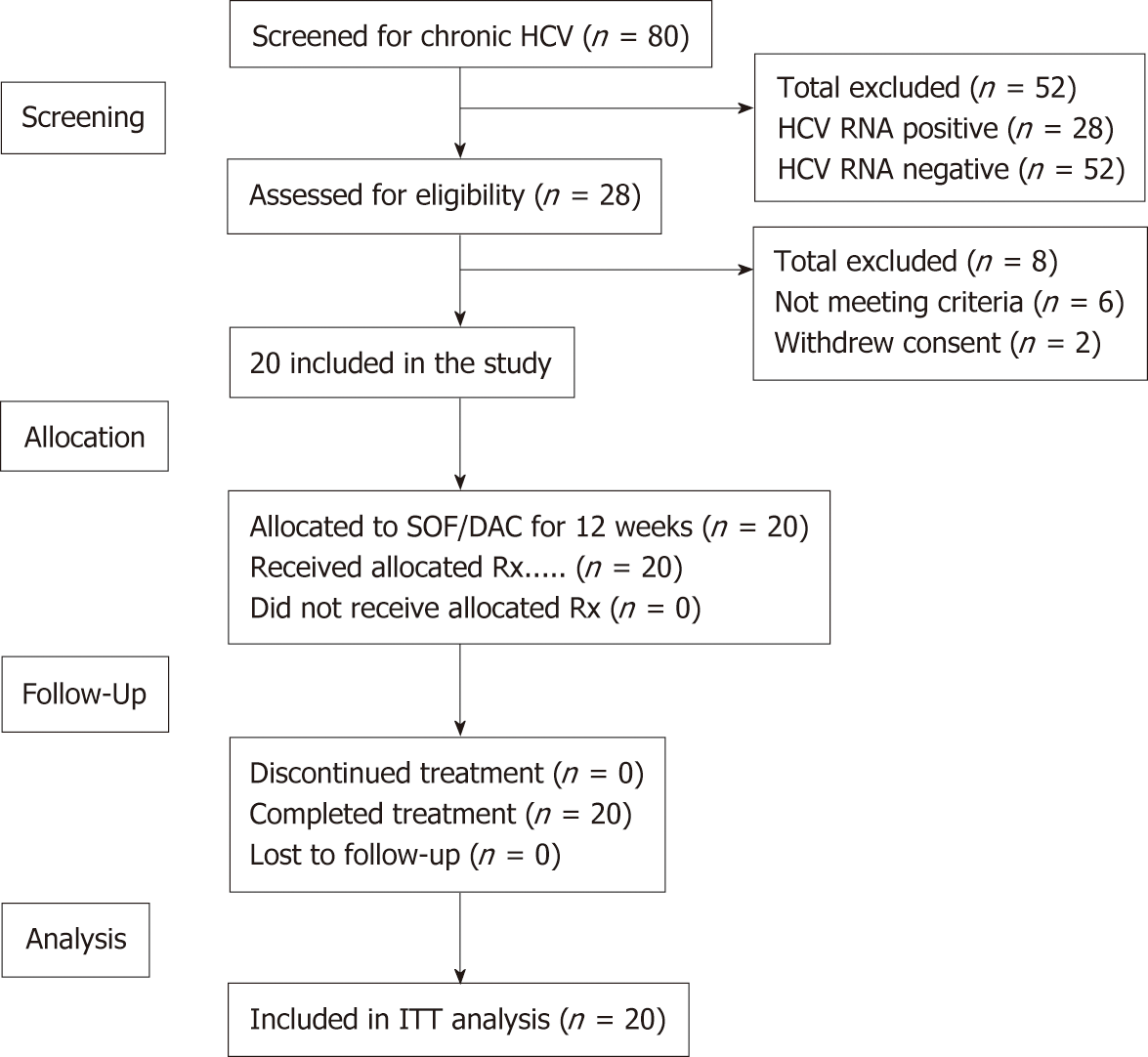
Starting from September 2017, we contacted a convenient sample of 80 survivors of pediatric hematologic cancer (who had been in continuous complete remission for at least one year after completion of their treatment protocol for their malignant disease) from 4 pediatric centers in Egypt. All were subjected to blood analysis for HCV antibodies by the third generation enzyme-linked immunosorbent assay (ELISA-III) test and those found to be HCV antibody positive were further tested for serum HCV-RNA positivity and virus load using the polymerase chain reaction quantitative measurements by COBAS Amplicor 2.0, Roche Molecular Diagnostics, Pleasanton, CA, United States (lower limit of detection of 10 IU/mL). Those with a virus load 10000 IU/mL or above were subjected to full screening for eligibility criteria. The first 20 consecutive patients who fulfilled all eligibility criteria were included in this study as a single treatment group and received the study medications and regular assessments at study visits.
Study medications: All patients were subjected to a dual therapy in the form of a weight based daily doses of Gratisovir (Sofosbuvir) plus Daclatasvir (generic products produced by Pharco Pharmaceuticals, Alexandria, Egypt) for 12 wk duration. The daily dose of Sofosbuvir tablets was based on the body weight: One 400 mg tablet once daily for body weight 35 kg or above; or a dose of 200 mg once daily for those who weighed less than 35 kg. Daclatasvir was given in a daily dose: 60 mg once daily for body weight 35 kg or above and 30 mg once daily for those who weighed less than 35 kg.
Study visits: All other procedures done during all the study visits were the same as described in our previously published report[11]. All patients were subjected to full physical examination and investigations including the complete blood count, serum bilirubin, serum albumin, alanine aminotransferase (ALT), AST, prothrombin time, serum creatinine, serum HCV-RNA level (virus load) and ultrasonographic abdominal scan during the baseline visit (week 0) and all other study visits [week 2, 4, 12 (EOT)] and 24 (12 wk after EOT). Patients were then followed up for further 12 wk (till week 36) for detection of any relapse for either the HCV infection or the original malignant disease. Tests to exclude ineligible cases, tests for virus genotyping [using GEN-C 2.0 Reverse Hybridization Strip Assay (Nuclear Laser Medicine, Settala, Italy)] as well as liver biopsy /or transient elastography by FibroScan® (Echosens, Paris, France) were performed only at the screening visit (week 0). All patients were interrogated throughout the study for any adverse events and were requested to call by phone or visit at any unscheduled time for reporting any adverse event or query.
Efficacy outcome measures: The primary efficacy outcome of this study was the proportion of patients achieving sustained virologic response 12 (SVR12) defined as serum HCV RNA below lowest level of quantification (LLOQ) at the end of 12 wk after end of treatment on intent-to-treat basis. Failure to sustain a virus load less than LLOQ either by the end of treatment or by the end of 12 wk after end of treatment is considered a virologic failure [on-treatment or post-treatment (relapse) failure] respectively.
Safety outcome measures: The primary safety outcome of this study was the incidence of any relapse or reactivation of the original treated malignant disease or any treatment-emergent adverse event (TEAE) as defined in previous study report[14]. All TEAEs were reported using the Medical Dictionary for Regulatory Activities version 20 (MedDRA v.20) lowest level and preferred terms. TEAEs deemed to have certain, probable or possible causality category according to Uppsala Monitoring Center causality categorization were considered in analysis for incidence rate and graded according to seriousness (serious/non-serious) and severity using the Common Terminology Criteria for Adverse Events v3.0.
We presented our qualitative data as counts, proportions or percentage with the confidence interval using Wilson Score Interval Method. For quantitative data, descriptive statistics are the arithmetic mean, the standard deviation, the median and the 95% confidence interval whenever found appropriate. A repeated measure ANOVA design with one “within-factor” (liver enzymes) and one group with a total of 20 subjects; each subject is measured 3 times. This design achieves 81% power to test the “within-factor” if a Geisser-Greenhouse Corrected F Test is used with a 5% significance level and the actual effect standard deviation is 0.3.
Out of the 80 cancer survivors that were screened for anti-HCV antibodies, 34 (42.5%) were found positive. The confirmed positive patients for HCV RNA were 28 (35%), all were genotype-4. Twenty patients who fulfilled all the eligibility criteria and signed the informed consent were included in this study. All received treatment with dual SOF/DAC therapy and were compliant to the protocol throughout all the study visits (Figure 1).
The baseline demographics and clinical characteristics are presented in Table 1.
| Characteristic | Patients total (n = 20) |
| Age (y): Median (range) | 11.5 (8-16) |
| Sex, male: | 10 (50) |
| Duration (yr) after end of cancer treatment | 4 (1- 9) |
| Type of cancer history | |
| ALL | 17 (85) |
| Hodgkin Lymphoma | 2 (10) |
| Non-Hodgkin Lymphoma | 1 (5) |
| Weight (kg): Median (range) | 37 (24-64.5) |
| Height (cm): Median (range) | 137.5 (114-77) |
| BMI (kg/m2): Median (range) | 17.7 (14.56-24.89) |
| HCV RNA (log10 IU/mL): Mean (SD) | 6.09 (0.72) |
| HCV RNA ≥ 800000 IU/mL | 10 (50) |
| Interferon or ribavirin treatment experience | |
| Naïve: | 19 (95) |
| Non-respondent to IFN Rx | 1 (5) |
| METAVIR Score by Fibroscan | |
| F1 | 17 (85) |
| F2 | 3 (15) |
| WBCs (× 10⁹/L): Median (range) | 7.5 (4.1-13.8) |
| Hemoglobin (g/dL): Median (range) | 12.05 (10-13.8) |
| Platelet Count (× 10⁹/L): Median (range) | 236.5 (66-444) |
| AST (IU/L): Mean (SD) | 50.7 (29.4) |
| ALT (IU/L): Mean (SD) | 54.3 (40.2) |
| S. Total Bilirubin (mg/dL): Median (range) | 0.7 (0.2-1.7) |
| S. Creatinine (mg/dL): Median (range) | 0.65 (0.2-0.8) |
The age ranged from eight to sixteen years with a mean (SD) of 11.6 (2.48) and 50% were males.
Seventeen were survivors of acute lymphoblastic leukemia (ALL); two were survivors of Hodgkin lymphoma and one of non-Hodgkin lymphoma. All had been in continuous complete remission for at least one year after the completion of their chemotherapy protocol.
All the 20 patients (100%) achieved viral negativity (serum HCV RNA below level of quantification) by the end of week 4 and remained so at the end of week 12 (end of treatment (EOT), week 24 (12 wk after EOT) and week 36 (24 wk after EOT). The intent-to-treat (ITT) sustained virologic response at week 12 after end of treatment (SVR12) rate was 20/20 (100%; 95%CI: 84%-100%).
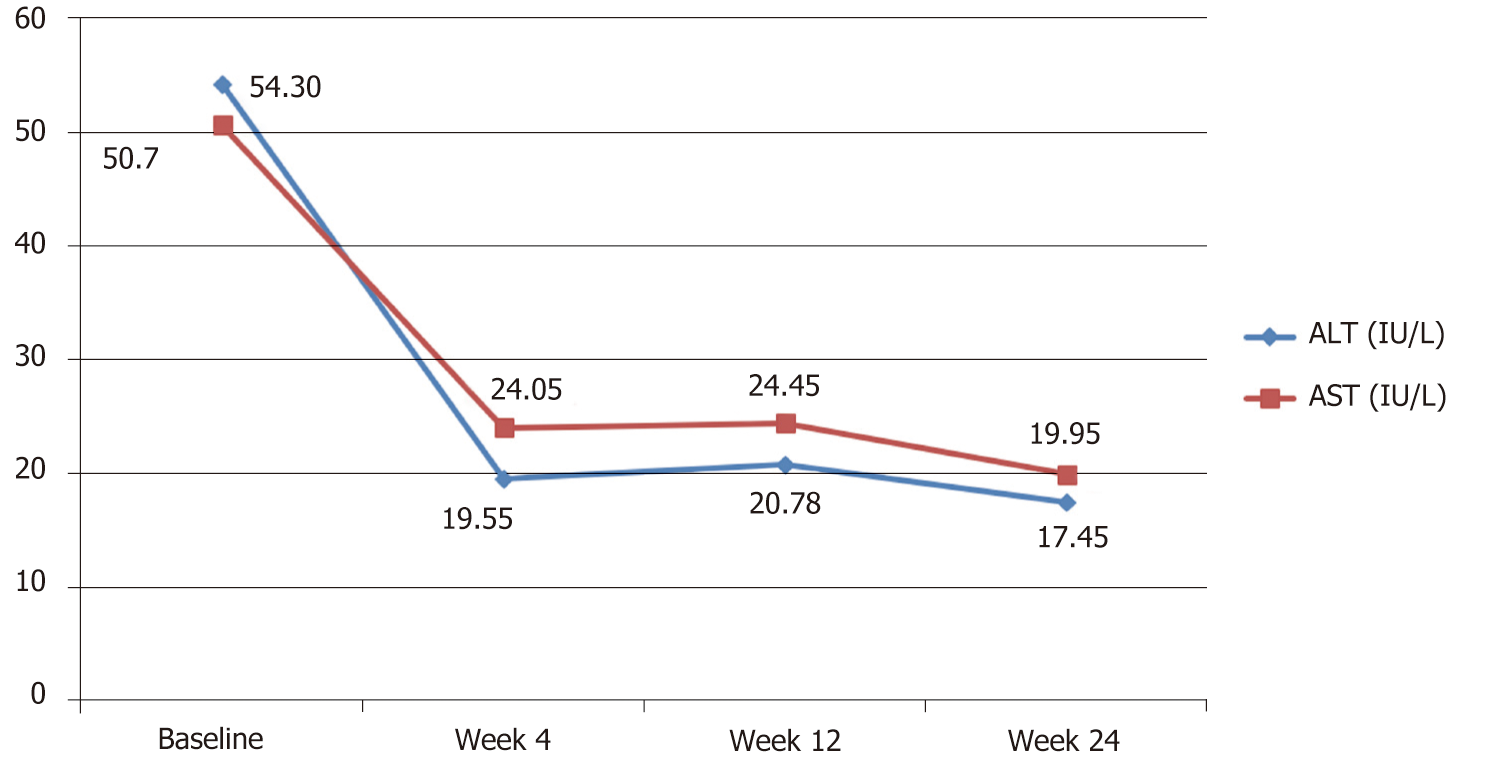
Both mean serum ALT and AST enzymes were above normal levels at baseline and showed significant reduction towards full normalization by week 4 through the end of the study (Table 2 and Figure 2).
| Variable [Mean (95%CI)] | At baseline | At wk 4 | At wk 12 | Sig. |
| Serum. ALT (IU/L) | 54.3 (35.49– 73.11) | 19.55 (11.02-28.08) | 17.45 (15.11-19.80) | < 0.05 |
| Serum AST (IU/L) | 50.7 (36.95-64.45) | 24.05 (16.40-31.70) | 19.95 (17.37-22.53) | < 0.05 |
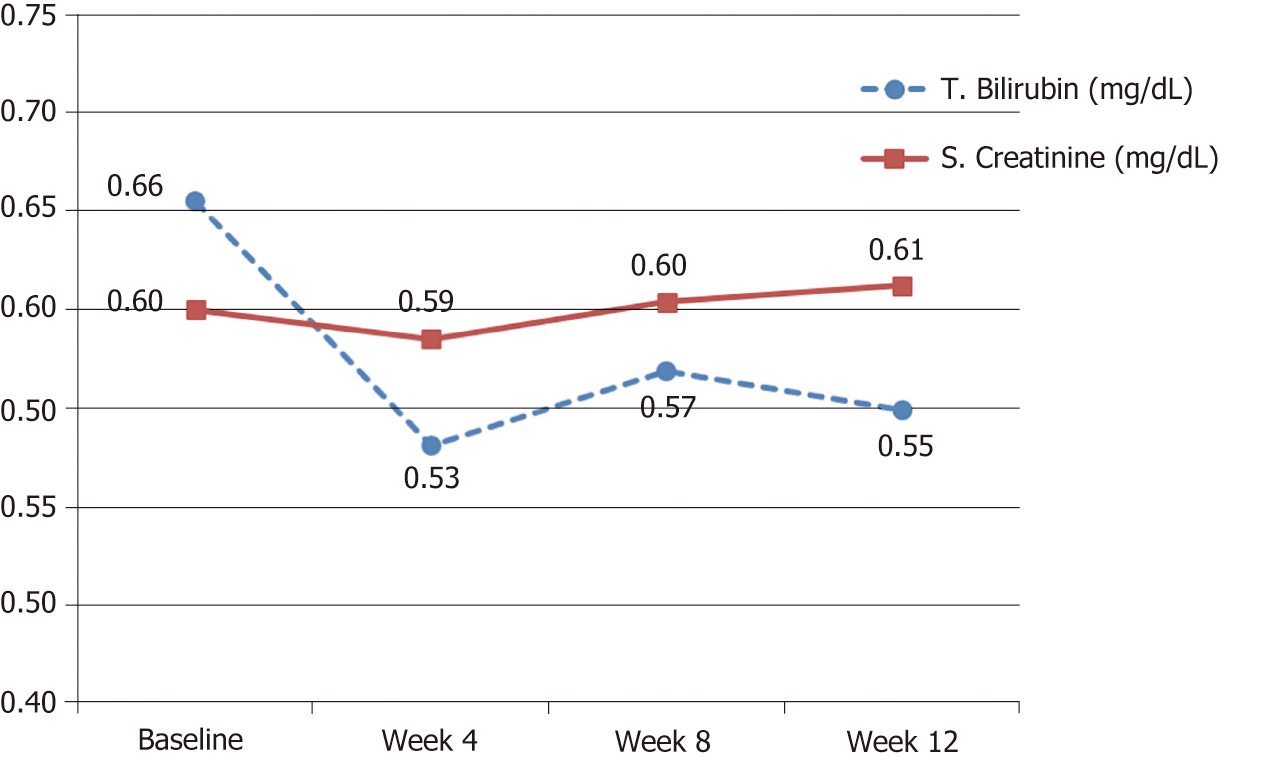
| T. Bilirubin (mg/dl) | 0.66 (0.49-0.82) | 0.53 (0.42-0.64) | 0.55 (0.45-0.65) | No |
| S. Creatinine (mg/dl) | 0.6 (0.52-0.68) | 0.59 (0.51-0.66) | 0.61 (0.54-0.68) | No |
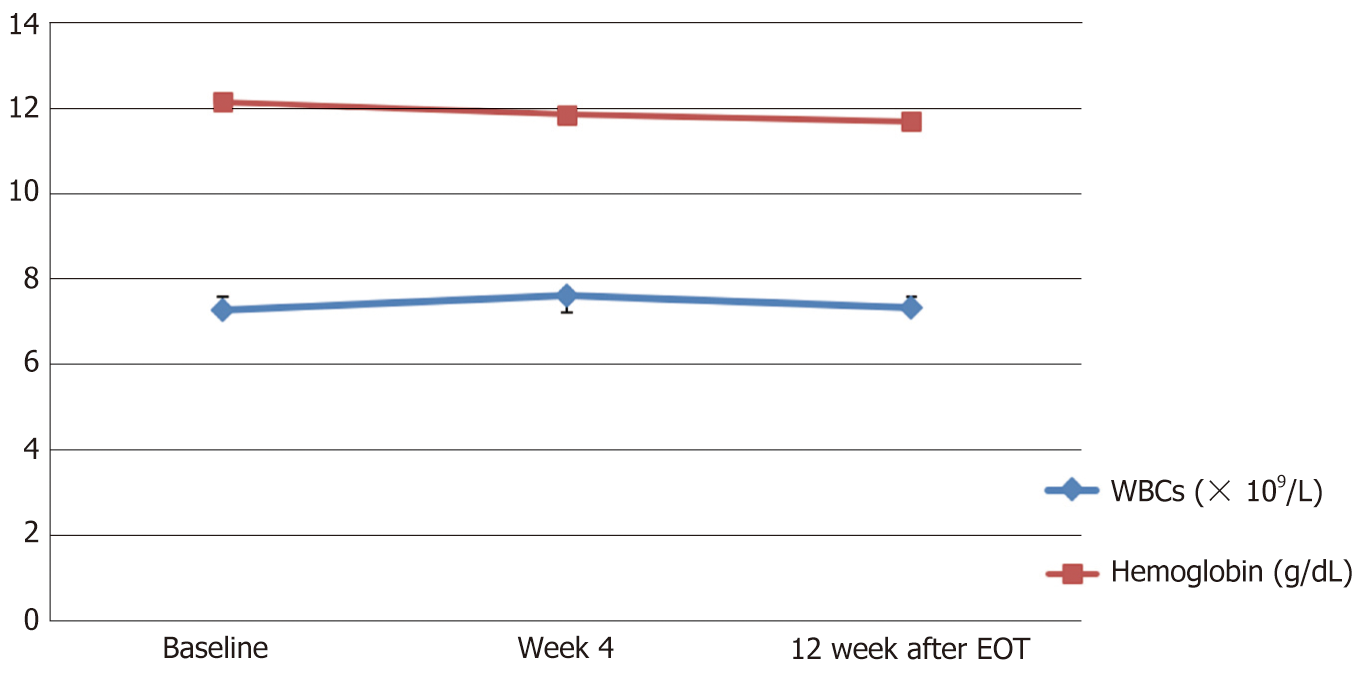
| WBCs (× 10⁹/L) | 7.27 (6.3-8.2) | 7.62 (6.8-8.5) | 7.3 (6.5-8.1) | No |
| Hemoglobin (g/dL) | 12.16 (11.7-12.6) | 11.9 (11.38-12.35) | 11.70 (11.13-12.27) | No |
| Platelets (× 10⁹/L) | 243 (203-283) | 235.5 (200-271) | 247.3 (213-281) | No |
While all other tested biochemical and hematological parameters were within the normal ranges at baseline and remained so with no significant changes throughout the study visits (Table 2 and Figures 3, 4).
No fatalities or serious adverse events were reported during the period of the study. Only seven (35%) patients reported 14 non-serious TEAEs throughout the study with causality assessment reports as possible or above. Nausea was reported by four (20%) patients, abdominal pain reported by three (15%) patients, fatigue reported by three (15%) patients, headache by 2 (10%) patients and pruritus or skin rash by 2 (10%) patients. All were mild to moderate (≤ grade-2) in severity.
No relapses were detected during treatment and throughout the follow up period for either the original malignant disease or the HCV.
To our knowledge this is the first study conducted to test the effects of dual SOF/DCV therapy on survivors of childhood malignancy, infected with chronic HCV. The SVR12 rate was 100% (95%CI: 84%-100%) which is concordant with the results of other published studies that tested efficacy of DAA agents on chronic HCV in other populations in pediatric age groups with no history of malignancy[14-17]. Also, the effects of treatment on other biochemical and hematological tests as well as other safety data matched the results of other studies conducted on other patients without history of malignancy.
Of particular importance in our findings that, there were no relapses detected for either the original malignant disease or the HCV during the study and all throughout the follow up period.
This finding is rather important and assuring to pediatricians treating survivors or patients with history of hematologic malignancy that this new class of DAA drugs (or at least SOF/DCV) might not adversely affect the state of remission of the original malignant disease, though further larger studies with longer follow up are needed.
This study also highlights the importance of screening for both survivors and patients with hematologic malignancy and goes well with the recommendations of ECIL-5[9]. Particularly that the rate of HCV infection in our screened “cancer survivors” sample (though small and non-random) was found unacceptably high 35%.
We acknowledge the small sample size and the short follow up duration of this pilot study which addresses a critical research question in a critical study population. But we hope that the results of this study might give other investigators and clinicians more confidence to include such population in their studies and real practice.
We hope that this study will prompt many other larger and longer studies to address the problem of HCV infection in both patients and survivors of cancer.
In conclusion, SOF/DCV combined therapy could be used safely and effectively in the treatment of chronic HCV genotype-4 infection in leukemia/lymphoma treated children. We detected no relapses/recurrence during treatment and throughout the follow up period for either the original malignant disease or the HCV infection.
Childhood cancer survivors are at a higher risk for infection with chronic hepatitis C (HCV). Sofosbuvir/Daclatasvir (SOF/DCV) dual therapy is currently a successful pan-genotypic option recommended by the world health organization for the treatment of adults infected with chronic HCV.
The effect of directly acting antiviral drugs (DAAs) on childhood cancer survivors infected with chronic hepatitis C (HCV) has not been well investigated. There is still hesitancy among pediatricians to start treatment with DAAs in this population for fear of negative impact on the state of complete remission.
To test the effects of SOF/DCV on both the HCV infection and the state of cancer remission in survivors of childhood hematologic malignancy.
We conducted a prospective, uncontrolled multicenter study. A total of 20 eligible, chronic HCV, genotype-4, infected children who had been in continuous complete remission from hematologic cancer (leukemia/lymphoma) for at least one year were included in the study. All patients were treated with combined SOF/DCV for 12 wk. Patients were monitored throughout the study till 12 weeks after end of treatment for safety and efficacy outcomes including the sustained virologic response 12 (SVR12) rate, hematological indices, liver and kidney functions.
The sustained virologic response rate at 12 weeks after end of treatment (SVR12) was 20 of 20 (100%; 95%CI: 84%-100%). All patients showed normalized liver enzymes from week-4. All hematological indices, liver and kidney functions were kept normal throughout the study. No fatalities or treatment-emergent serious or severe adverse events were reported throughout the study.
SOF/DCV combined therapy could be used safely and effectively in the treatment of chronic HCV genotype-4 infection in leukemia/lymphoma treated children. No relapses were detected during treatment and throughout the follow up period for either the original malignant disease or the HCV infection.
The results of this study could indicate that dual SOF/DCV therapy is effective and safe in the treatment of chronic HCV in survivors of childhood hematologic malignancy. These results could also be assuring to pediatricians that the treatment of chronic HCV infection with DAAs might not adversely affect the state of complete remission of hematologic malignancy in pediatric age groups. This is hoped to encourage treatment and more studies on this population.
Egyptian Cure Bank Charity supported the study medications
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Snyder N, Chen Z, Ahmed M, Dumitrascu DL S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Donahue JG, Muñoz A, Ness PM, Brown DE, Yawn DH, McAllister HA, Reitz BA, Nelson KE. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 434] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | El-Raziky MS, Halawa EF, Draz IH, Ali MS. Natural history and response to treatment of HCV infection among Egyptian survivors of childhood malignancy. Pediatr Hematol Oncol. 2015;32:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Torres HA, Shigle TL, Hammoudi N, Link JT, Samaniego F, Kaseb A, Mallet V. The oncologic burden of hepatitis C virus infection: A clinical perspective. CA Cancer J Clin. 2017;67:411-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Mahale P, Torres HA, Kramer JR, Hwang LY, Li R, Brown EL, Engels EA. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer. 2017;123:1202-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Allison RD, Tong X, Moorman AC, Ly KN, Rupp L, Xu F, Gordon SC, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol. 2015;63:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, Russo D, Falasca E, Botta GA, Baccarani M. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296-4301. [PubMed] |
| 8. | Torres HA, Chong PP, De Lima M, Friedman MS, Giralt S, Hammond SP, Kiel PJ, Masur H, McDonald GB, Wingard JR, Gambarin-Gelwan M. Hepatitis C Virus Infection among Hematopoietic Cell Transplant Donors and Recipients: American Society for Blood and Marrow Transplantation Task Force Recommendations. Biol Blood Marrow Transplant. 2015;21:1870-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Mallet V, van Bömmel F, Doerig C, Pischke S, Hermine O, Locasciulli A, Cordonnier C, Berg T, Moradpour D, Wedemeyer H, Ljungman P; ECIL-5. Management of viral hepatitis in patients with haematological malignancy and in patients undergoing haemopoietic stem cell transplantation: recommendations of the 5th European Conference on Infections in Leukaemia (ECIL-5). Lancet Infect Dis. 2016;16:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Torres HA, Economides MP, Kyvernitakis A, Mahale P, Hosry J, Naing A, Turturro F, Kaseb AO, Raad II, Granwehr BP. Sofosbuvir-based therapy in patients with chronic hepatitis C virus infection and malignancies: A prospective observational study of 143 patients. J Clin Oncol. 2017;35:e18152. |
| 11. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 12. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 13. | Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, Volk M, Odewole M, Scaglione S, Guy J, Said A, Feld JJ, John BV, Frenette C, Mantry P, Rangnekar AS, Oloruntoba O, Leise M, Jou JH, Bhamidimarri KR, Kulik L, Tran T, Samant H, Dhanasekaran R, Duarte-Rojo A, Salgia R, Eswaran S, Jalal P, Flores A, Satapathy SK, Wong R, Huang A, Misra S, Schwartz M, Mitrani R, Nakka S, Noureddine W, Ho C, Konjeti VR, Dao A, Nelson K, Delarosa K, Rahim U, Mavuram M, Xie JJ, Murphy CC, Parikh ND. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156:1683-1692.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 14. | Yakoot M, El-Shabrawi MH, AbdElgawad MM, Mahfouz AA, Helmy S, Abdo AM, El-Khayat HR. Dual Sofosbuvir/Daclatasvir Therapy in Adolescent Patients With Chronic Hepatitis C Infection. J Pediatr Gastroenterol Nutr. 2018;67:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K, Massetto B, Zhu Y, Kanwar B, German P, Svarovskaia E, Brainard DM, Wen J, Gonzalez-Peralta RP, Jonas MM, Schwarz K. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12-17 years old with hepatitis C virus genotype 1 infection. Hepatology. 2017;66:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Wirth S, Rosenthal P, Gonzalez-Peralta RP, Jonas MM, Balistreri WF, Lin CH, Hardikar W, Kersey K, Massetto B, Kanwar B, Brainard DM, Shao J, Svarovskaia E, Kirby B, Arnon R, Murray KF, Schwarz KB. Sofosbuvir and ribavirin in adolescents 12-17 years old with hepatitis C virus genotype 2 or 3 infection. Hepatology. 2017;66:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Quintero J, Juampérez J, Julio E, Cabello V, Mercadal-Hally M, Soler-Palacín P, Segarra Ó, Rodrigo C. [Ledipasvir/sofosbuvir combination for chronic hepatitis C infection in children and adolescents]. An Pediatr (Barc). 2019;90:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |