Published online Aug 6, 2019. doi: 10.12998/wjcc.v7.i15.2049
Peer-review started: February 20, 2019
First decision: May 31, 2019
Revised: June 11, 2019
Accepted: June 20, 2019
Article in press: June 21, 2019
Published online: August 6, 2019
Processing time: 169 Days and 3.7 Hours
Anaplastic large cell lymphoma (ALCL) is a type of T-cell lymphoma that can be divided into two categories: anaplastic lymphoma kinase-positive (ALK+) and ALK-negative. Gastrointestinal ALK+ ALCL is rare. Multiple lymphomatous polyposis (MLP) is thought to be a representative form of gastrointestinal lesion in mantle cell lymphoma, and T-cell lymphomas seldom show this feature. Here, we report the first known case of ALK+ ALCL with gastroduodenal involvement to present with MLP.
The patient was a 43-year-old man who was complained of a mass in the left inguinal area and was performed open biopsy. ALK+ ALCL was diagnosed pathologically. Computed tomography scan demonstrated multiple lymph node lesions in the abdomen - pelvis/inguinal region, and scattered nodular lesions in both lung fields. He did not complain of gastrointestinal symptoms. While, esophagogastroduodenoscopy identified MLP lesions from the antrum of the stomach to the descending portion of the duodenum and mild thickened folds on the corpus of the stomach, and biopsy showed invasion of ALK+ ALCL. We treated this patient with six cycles of CHOEP (Cyclophosphamide, Doxorubicin, Vincristine, Etoposide, and Prednisone) chemotherapy. At the conclusion of treatment, there was complete remission. Numerous white scars were found on the stomach, endoscopically consistent with a remission image of lymphoma. The endoscopic features of this case were thought to be similar to those of MCL.
The macroscopic/endoscopic features of gastrointestinal ALK+ ALCL may be more similar to those of B-cell lymphomas rather than T-cell lymphomas.
Core tip: Anaplastic large cell lymphoma (ALCL) encompasses two distinct categories: anaplastic lymphoma kinase-positive (ALK+) and ALK-negative. ALK+ ALCL cases rarely involve the gut. However, in a very small number of case reports, gastrointestinal ALK+ ALCL exhibits a fungating growth pattern, similar to that of B-cell lymphomas rather than T-cell lymphomas. Multiple lymphomatous polyposis (MLP) is thought to be a typical form of gastrointestinal lesion in mantle cell lymphoma, but it develops in other B-cell lymphomas. T-cell lymphomas seldom present with MLP. Here, we report the first known case in the world of ALK+ ALCL with gastroduodenal involvement presenting with MLP.
- Citation: Saito M, Izumiyama K, Ogasawara R, Mori A, Kondo T, Tanaka M, Morioka M, Miyashita K, Tanino M. ALK-positive anaplastic large cell lymphoma presenting multiple lymphomatous polyposis: A case report and literature review. World J Clin Cases 2019; 7(15): 2049-2057
- URL: https://www.wjgnet.com/2307-8960/full/v7/i15/2049.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i15.2049
Anaplastic large cell lymphoma (ALCL) was first described in 1985 by Stein et al[1]. According to the 4th edition (2008) of the WHO classification[2], primary systemic ALCL can be divided into two distinct categories: anaplastic lymphoma kinase-positive (ALK+) and ALK-negative. Majority of ALK+ ALCL contain a t(2;5) chromosomal translocation. The t(2;5) translocation fuses a distal part of the ALK gene on chromosome 2p23 with the promoter and a proximal domain of the nucleophosmin (NPM1) gene on chromosome 5q35[3]. The most common extranodal sites of ALK+ ALCL are the skin, bone and soft tissue. ALK+ ALCL cases involving the gut are rare[2].
Multiple lymphomatous polyposis (MLP) is thought to be a representative form of gastrointestinal lesion in mantle cell lymphoma (MCL)[2,4,5]. Here, we report an extremely rare case of ALK+ ALCL with gastroduodenal involvement presenting as MLP.
A 43-year-old Japanese man suffering from fever, night sweats, general fatigue and weight loss complained of a mass in the left inguinal area. The patient did not complain of gastrointestinal symptoms.
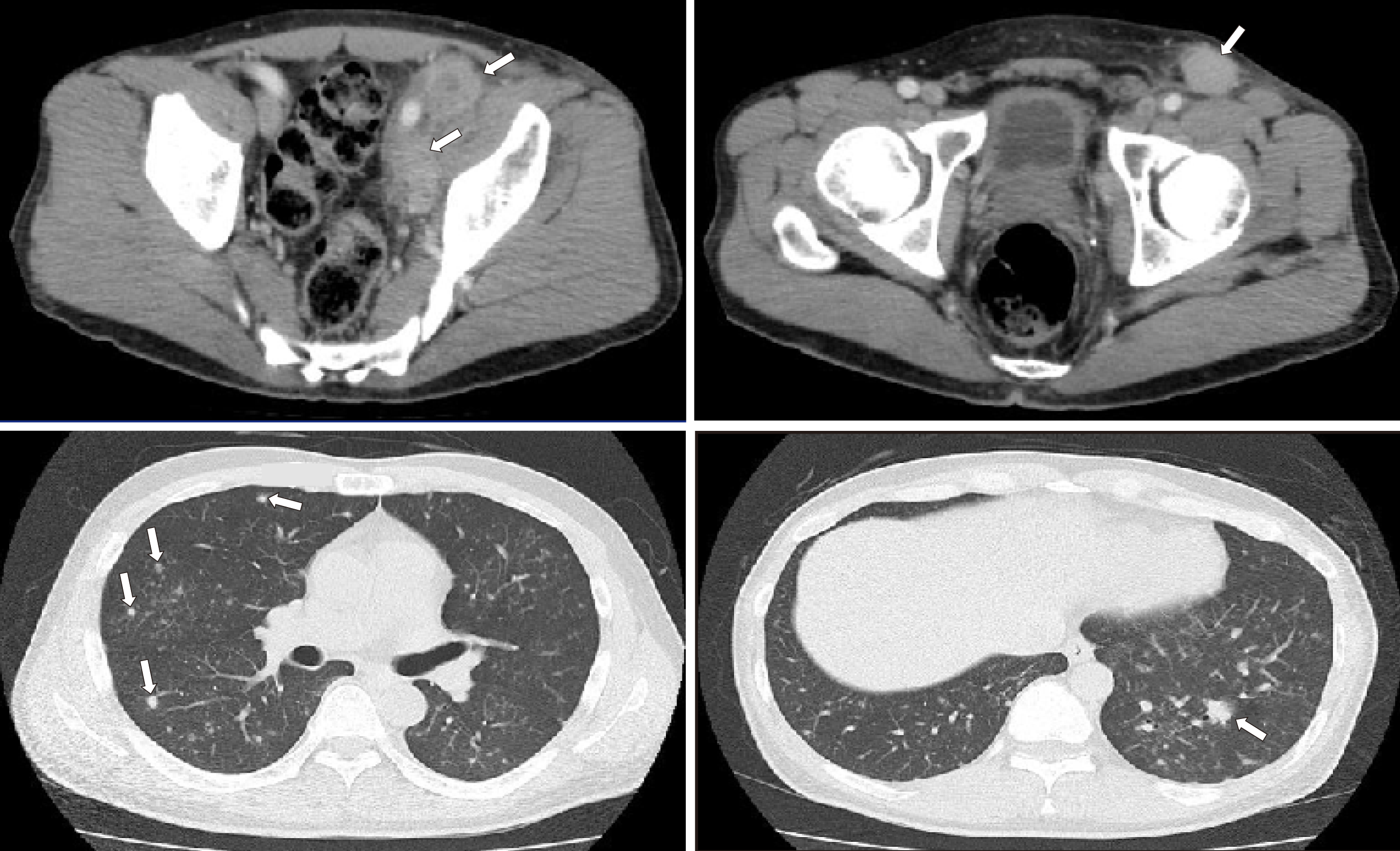
The patient was admitted to a local hospital. Computed tomography (CT) scan demonstrated multiple lymph node lesions continuing to the mesentery and para-aortic/left common iliac artery/left inguinal region (Figure 1). Scattered nodular lesions were found in both lung fields (Figure 1). Following needle biopsy of the left inguinal lesion, malignant lymphoma was suspected, and the patient was transferred to our department.
He had no chronic illness.
Left inguinal lymph nodes were significantly swollen.
WBC and CRP showed markedly high values, and elevated platelet count, liver dysfunction, and hypoalbuminemia were also observed. In addition, the level of soluble interleukin-2 receptor (normal range: 121-613 U/mL), which was severely elevated according to the measurement of the previous hospital, had further increased over approximately one week (7120 → 12500 U/mL) (Table 1).
| WBC | 21.5 × 109/L | TP | 5.8 g/dL |
| St | 3% | Alb | 2.3 g/dL |
| Seg | 73% | GOT | 117 IU/L |
| Lym | 16% | GPT | 252 IU/L |
| Mon | 4% | LDH | 275 IU/L |
| Eos | 4% | ALP | 753 IU/L |
| RBC | 3.99 × 1012/L | γ-GTP | 170 IU/L |
| Hb | 12.1 g/dL | T-Bil | 0.2 mg/dL |
| Hct | 36.3% | CRP | 13.00 mg/dL |
| Plt | 695 × 109/L | s-IL-2R | 12500 U/mL |
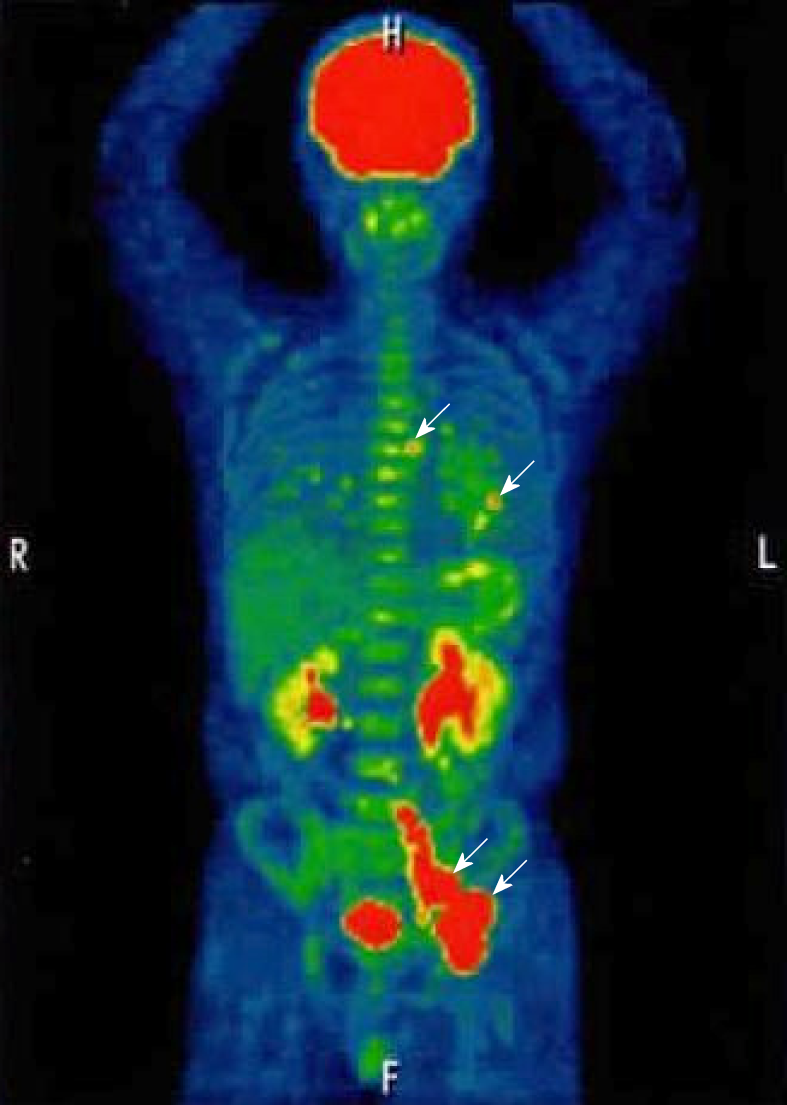
As measured by positron emission tomography (PET)/CT scan, the mean standardized uptake value was 18.1 in the abdomen – pelvis/inguinal nodes and 3.5 in the lung fields and the mediastinal nodes (Figure 2).
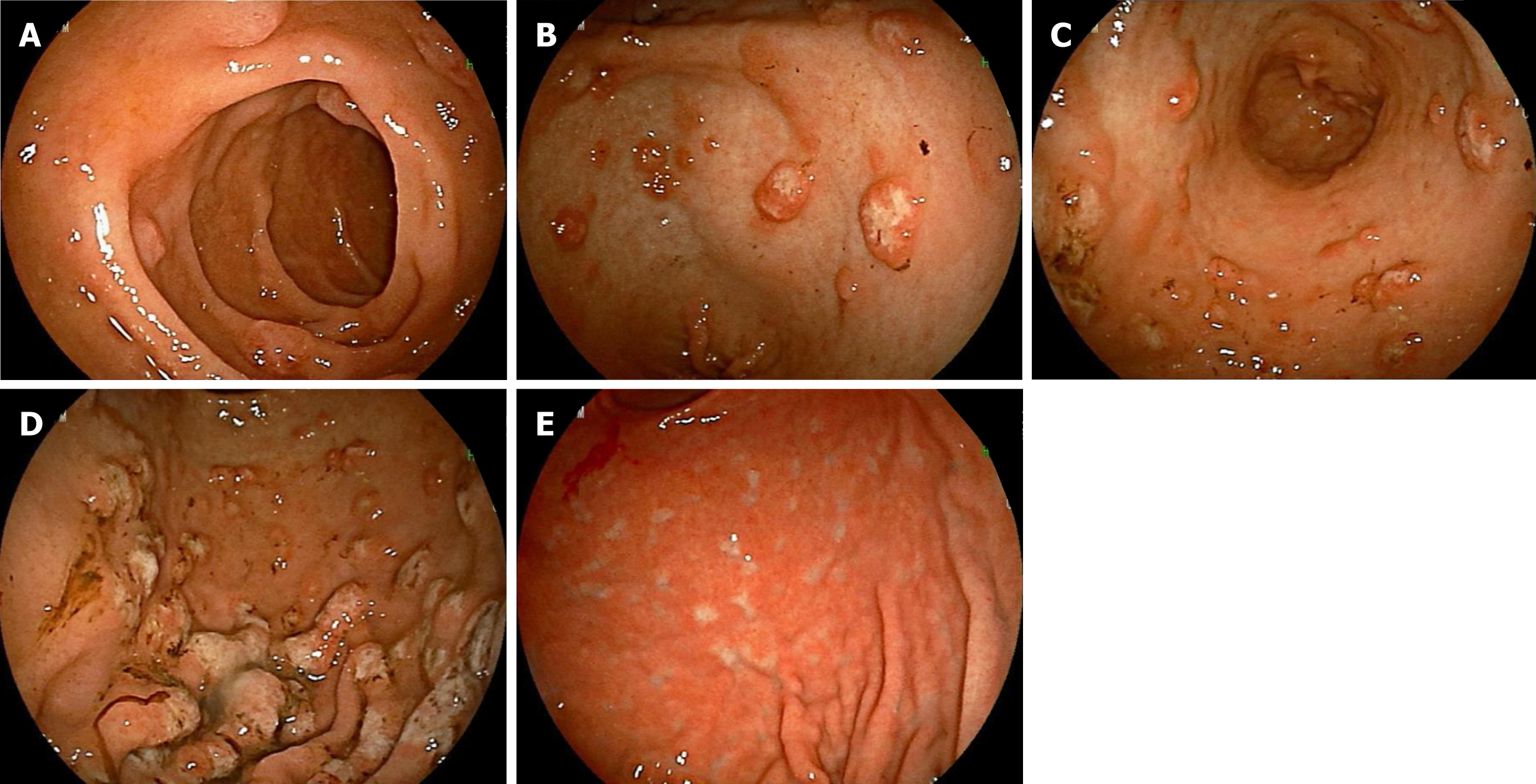
Esophagogastroduodenoscopy findings: In the descending portion of the duodenum, multiple polypoid lesions of 2-3 mm in diameter were observed (Figure 3A). Various large and small polypoid lesions were observed in the antrum of the stomach (Figure 3B and Figure 3C). These lesions are consistent with MLP continuously extending from the antrum of the stomach to the descending portion of the duodenum. Mucosal folds in the corpus of the stomach were slightly thickened, and its surface had changed to a white tone (Figure 3D).
The biopsies of both gastric and duodenal lesions proved the invasion of lymphoma cells, as below mentioned. These lesions could not be detected by PET/CT scan. Colonoscopy and bone marrow aspiration showed no involvement of lymphoma lesions.
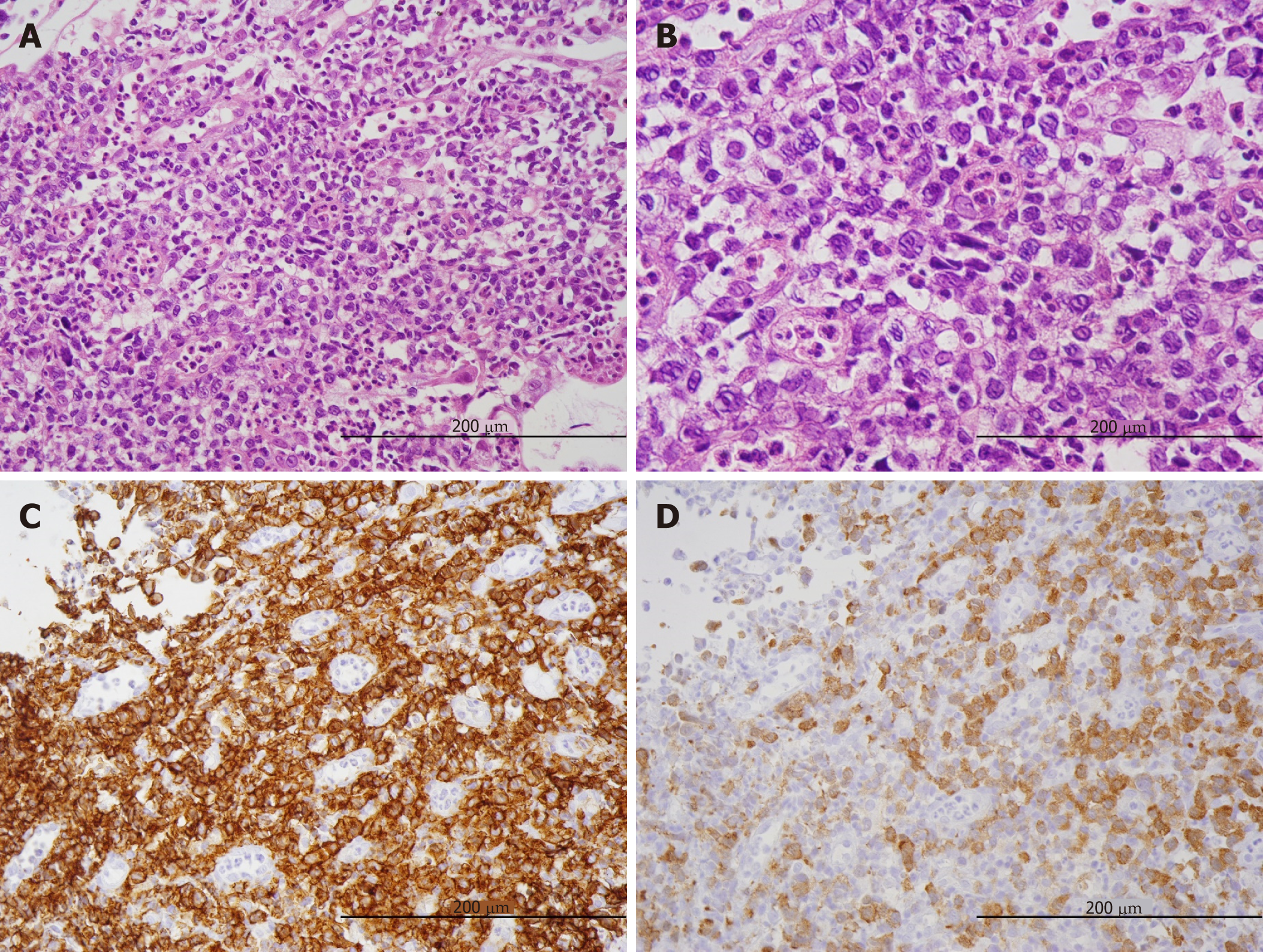
Left inguinal lymph nodes were examined by open biopsy. Medium to large-sized abnormal lymphoid cells were observed to be invasively proliferating. CD25, CD30, T-cell intracellular antigen-1, and ALK tested positive by immunostaining. Meanwhile, CD3, CD20 and the Epstein-Barr encoding region in situ hybridization tested negative. Based on the histopathology, ALK+ ALCL was diagnosed. In the gastric and duodenal biopsy samples, abnormal lymphoid cells with irregular nuclei grew diffusely (Figure 4A), and mitotic figures were observed in high numbers (Figure 4B). Immunohistochemically, CD30 tested strongly positive (Figure 4C), ALK (monoclonal antibody, ALK1) tested positive in nuclear and cytoplasmic pattern (Figure 4D), and the Ki-67 proliferative (MIB1) index was > 80% (not shown).
ALK+ ALCL that involved multiple lymph node lesions in the abdomen - pelvis/inguinal region and mediastinum, also both lung fields and the gastroduodenal lesions (Stage IV).
We treated this patient with six cycles of CHOEP chemotherapy (Cyclophosphamide 750 mg/m2, Doxorubicin 50 mg/m2, Vincristine 1.4 mg/m2, Etoposide 100 mg/m2 x 3 d, and Prednisone 50 mg/m2 x 5 d) every three weeks. After administration of two cycles, the lymphoma lesions including lung field nodules shrank remarkably and disappeared at CT examination.
At the conclusion of treatment, there was complete remission. Numerous white scars were found in the stomach, endoscopically consistent with a remission image of lymphoma (Figure 3E). He is followed-up as outpatient with no treatment. There was no evidence of recurrence for more than 1 year and 10 mo since the end of the final treatment.
ALCL is a clinically, morphologically, and immunophenotypically heterogeneous T-cell lymphoma. Depending on the presence or absence of ALK expression, ALK+ ALCL was classified as a biologically homogeneous disease unit independent of ALK-negative ALCL in the 4th edition (2008) of the WHO classification[2]. ALK+ ALCL is more prevalent in children and young adults, and the ALK fusion protein is associated with a good prognosis[6]. In contrast, ALK-negative ALCL has a poor prognosis similar to peripheral T-cell lymphoma, not otherwise specified, and angioimmunoblastic T-cell lymphoma[7]. ALK staining pattern is nuclear and cytoplasmic in cases of t(2;5)/NPM-ALK translocation, and membranous or diffuse/granular cytoplasmic in the remaining cases with a variant translocation involving at 2p23[2,8]. ALK staining in our patient showed a nuclear and cytoplasmic pattern. G-banded karyotype of the lymph node lesions showed complex chromosomal abnormalities with +2, der(2;21)(q10;q10),+7, however, translocation at 2p23 could not be confirmed. ALK+ ALCL frequently involves both lymph nodes and extranodal sites. The most commonly involved extranodal sites include the skin, bone, soft tissue, liver and lungs as we reported here[2].
Because gastrointestinal T-cell lymphomas are rare, little is known about the clinicopathological characteristics of primary gastrointestinal T-cell lymphomas. Kim et al[9] investigated the endoscopic differences between B- and T-cell lymphomas and observed that B-cell lymphomas presented more often as fungating (54% of cases), while T-cell lymphomas were frequently ulcerative (47%), and only 13% showing a fungating pattern. Regardless of ALK expression, primary gastrointestinal ALCL is rarer[10]. According to a recent review on gastrointestinal ALCL, the rate of ALK expression among gastrointestinal ALCL was low at 24%[11]. As far as we can determine, ALK+ ALCL of the gut has been reported in only eleven cases[11-20]. The clinical features are summarized in Table 2 for a total of 12 gastrointestinal ALK+ ALCL cases, including our patient. Of these, four cases were reported to involve the esophagus[12-15], all showing fungating tumors. Of the remaining cases, two involved the stomach[11,16], one involved the duodenum[17], one involved both the stomach and duodenum[18], and three involved the small intestine (jejunum/ileum)[11,19,20]. Only three of these last seven cases showed macroscopic findings by resection or endoscopy; the morphology showed a mass formation pattern, such as a submucosal or bulky tumor. Although the number of cases is very small, gastrointestinal ALK+ ALCL often seems to involve the esophagus, in which lymphomas rarely develop, and frequently exhibits a fungating growth pattern similar to that of B-cell lymphomas.
| Case | Age / sex | Ref. | Occurrence site | Gross/ Endoscopic findings | ALK staining pattern | 2p23(FISH /RT-PCR) | Treatment | Outcome |
| 1 | 56/M | [12] | Esophagus | Large mass | Cytoplasmic | chemo. + auto-SCT | Alive (3 mo) | |
| 2 | 34/F | [13] | Esophagus | Fungating tumor | Nuclear and cytoplasmic | chemo. + auto-SCT | Alive (24 mo) | |
| 3 | 37/M | [14] | Esophagus | Submucosal mass | Nuclear and cytoplasmic | resection + chemo. | Alive (14 mo) | |
| 4 | 3/M | [15] | Esophagus | Wall thickening | Nuclear and cytoplasmic | chemo. | (undescribed) | |
| 5 | 53/M | [11] | Stomach | (Undescribed) | Cytoplasmic | resection + chemo. | Alive (84 mo) | |
| 6 | 72/F | [16] | Stomach | (Undescribed) | (Undescribed) | + (FISH) | resection | Alive (84 mo) |
| 7 | 36/M | [17] | Duodenum | (Undescribed) | Nuclear and cytoplasmic | + (RT-PCR) | resection + chemo. | Alive (24 mo) |
| 8 | 21/M | [18] | Stomach and Duodenum | Submucosal tumor | Nuclear and cytoplasmic | chemo. | (undescribed) | |
| 9 | 10/M | [11] | Small intestine | (Undescribed) | Nuclear and cytoplasmic | resection + chemo. | Alive (75 mo) | |
| 10 | 17/M | [19] | Jejunum | Polypoidal mass | Cytoplasmic | resection + chemo. | Alive (18 mo) | |
| 11 | 32/M | [20] | Jejunum/Ileum (junction) | Massive tumor | Nuclear and cytoplasmic | resection + chemo. | Alive (5 mo) | |
| 12 | 43/M | Our case | Stomach and Duodenum | MLP and gastric mocosal thickening | Nuclear and cytoplasmic | chemo. | Alive (22 mo) |
The term ‘‘MLP’’ was introduced in 1961 by Cornes to describe malignant lymphoma that presented as multiple polypoid tumors, from 2 mm to several centimeters, affecting long segments of the gastrointestinal tract[21]. Histopathologically, these polyps originate from the mantle zone of the lymphoid follicle of the mucosa-associated lymphoid tissue (MALT)[22]. Therefore, the most frequent lymphoma presenting with MLP is MCL[4,5], and it also develops in other B-cell lymphomas, such as MALT lymphoma and follicular lymphoma[23]. Furthermore, T-cell lymphomas seldom show this feature (11%)[24]. Several cases of adult T-cell leukemia/lymphoma presenting with MLP have been reported in Japan, which is an endemic area of human T-cell lymphotropic virus type 1 infection[25,26]. To the best of our knowledge, including ALK-negative, our case is the first observation of ALCL presenting with MLP. However, in contrast to what has been seen in MCL, the MLP presented in this case with, irregular sized-polyps arranged irregularly. In addition, no MLP lesion was found in the large intestine, which is frequently involved in MCL[27]. In our case, MLP lesions could not be detected with PET/CT scans. As we previously reported in a case of MCL[28], it appears that MLP lesions do not infiltrate the deep layer and instead maintain involvement with the surface layer of the gastrointestinal mucosa, even in the case of ALCL. The characteristic endoscopic findings of MCL are not only MLP, but also thickening of gastric mucosal folds has been reported[5]. In our case, thickened folds of the stomach were also seen, and the endoscopic features were thought to be similar to those of MCL.
We reported a case of gastroduodenal ALK+ ALCL presenting with MLP. Although there is a limit in considering the macroscopic/endoscopic features of gastrointestinal ALK+ ALCL patients through this very rare case, the morphological features of gastrointestinal ALK+ ALCL may be similar to those of B-cell lymphomas rather than T-cell lymphomas. It is necessary to further accumulate and study the gastrointestinal ALK+ ALCL patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghoch ME, Gurzu S, Paydas S, Sergi C, Yu S S-Editor: Ma YJ L-Editor: A E-Editor: Xing YX
| 1. | Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858. [PubMed] [Cited in This Article: ] |
| 2. | Delsol G, Jaffe ES, Falini B, Gascoyne RD, Müller-Hermelink HK, Stein H, Campo E, Kinney MC, Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA. Anaplastic large cell lymphoma (ALCL), ALK-positive. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA. WHO Classification of Tumours of Haematopietic and Lymphoid Tissues. Lyon, France: IARC press; 2008; 312-316. [Cited in This Article: ] |
| 3. | Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281-1284. [PubMed] [Cited in This Article: ] |
| 4. | Ruskoné-Fourmestraux A, Audouin J. Primary gastrointestinal tract mantle cell lymphoma as multiple lymphomatous polyposis. Best Pract Res Clin Gastroenterol. 2010;24:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Saito M, Mori A, Irie T, Tanaka M, Morioka M, Ozasa M, Kobayashi T, Saga A, Miwa K, Tanaka S. Endoscopic follow-up of 3 cases with gastrointestinal tract involvement of mantle cell lymphoma. Intern Med. 2010;49:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, Pileri S, Falini B. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681-3695. [PubMed] [Cited in This Article: ] |
| 7. | ten Berge RL, de Bruin PC, Oudejans JJ, Ossenkoppele GJ, van der Valk P, Meijer CJ. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology. 2003;43:462-469. [PubMed] [Cited in This Article: ] |
| 8. | Falini B, Pulford K, Pucciarini A, Carbone A, De Wolf-Peeters C, Cordell J, Fizzotti M, Santucci A, Pelicci PG, Pileri S, Campo E, Ott G, Delsol G, Mason DY. Lymphomas expressing ALK fusion protein(s) other than NPM-ALK. Blood. 1999;94:3509-3515. [PubMed] [Cited in This Article: ] |
| 9. | Kim YH, Lee JH, Yang SK, Kim TI, Kim JS, Kim HJ, Kim JI, Kim SW, Kim JO, Jung IK, Jung SA, Jung MK, Kim HS, Myung SJ, Kim WH, Rhee JC, Choi KY, Song IS, Hyun JH, Min YI. Primary colon lymphoma in Korea: a KASID (Korean Association for the Study of Intestinal Diseases) Study. Dig Dis Sci. 2005;50:2243-2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Kim DH, Lee D, Kim JW, Huh J, Park SH, Ha HK, Suh C, Yoon SM, Kim KJ, Choi KD, Ye BD, Byeon JS, Song HJ, Jung HY, Yang SK, Kim JH, Myung SJ. Endoscopic and clinical analysis of primary T-cell lymphoma of the gastrointestinal tract according to pathological subtype. J Gastroenterol Hepatol. 2014;29:934-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lee YY, Takata K, Wang RC, Yang SF, Chuang SS. Primary gastrointestinal anaplastic large cell lymphoma. Pathology. 2017;49:479-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Joshi A, Fields P, Simo R. Anaplastic lymphoma of the cervical esophagus presenting as a tracheoesophageal fistula. Head Neck. 2008;30:1264-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Yaakup H, Sagap I, Fadilah SA. Primary oesophageal Ki (CD30)-positive ALK+ anaplastic large cell lymphoma of T-cell phenotype. Singapore Med J. 2008;49:e289-e292. [PubMed] [Cited in This Article: ] |
| 14. | Wu N, Pang L, Chen Z, Wang Y, Ma Q, Chen G, Chen J, Huang J. Primary esophageal CD30-positive ALK-positive anaplastic large cell lymphoma: a case report and literature review. J Gastrointest Cancer. 2011;42:57-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hryhorczuk AL, Harris MH, Vargas SO, Lee EY. Anaplastic large cell lymphoma of the esophagus in a pediatric patient. Pediatr Radiol. 2012;42:627-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Iwamizu-Watanabe S, Yamashita Y, Yatabe Y, Nakamura S, Mori N. Frequent expression of CD30 antigen in the primary gastric non-B, non-Hodgkin lymphomas. Pathol Int. 2004;54:503-509. [PubMed] [Cited in This Article: ] |
| 17. | Carey MJ, Medeiros LJ, Roepke JE, Kjeldsberg CR, Elenitoba-Johnson KS. Primary anaplastic large cell lymphoma of the small intestine. Am J Clin Pathol. 1999;112:696-701. [PubMed] [Cited in This Article: ] |
| 18. | Ishii H, Isomoto H, Taniguchi H, Kinoshita N, Matsushima K, Taguchi J, Miyazaki Y, Nakao K. Education and Imaging: Gastrointestinal: gastroduodenal involvement of ALK-positive anaplastic large cell lymphoma. J Gastroenterol Hepatol. 2011;26:933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Sadiya N, Ghosh M. Primary ALK positive anaplastic large cell lymphoma of T-cell type of jejunum: Report of a rare extranodal entity with review of literature. Arch Int Surg. 2014;4:50-53. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Cao Q, Liu F, Li S, Liu N, Li L, Li C, Peng T. Primary rare anaplastic large cell lymphoma, ALK positive in small intestine: case report and review of the literature. Diagn Pathol. 2016;11:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Cornes JS. Multiple lymphomatous polyposis of the gastrointestinal tract. Cancer. 1961;14:249-257. [PubMed] [Cited in This Article: ] |
| 22. | Isaacson PG, MacLennan KA, Subbuswamy SG. Multiple lymphomatous polyposis of the gastrointestinal tract. Histopathology. 1984;8:641-656. [PubMed] [Cited in This Article: ] |
| 23. | Kodama T, Ohshima K, Nomura K, Taniwaki M, Nakamura N, Nakamura S, Kohno S, Yamamoto J, Karube K, Yamasita Y, Shirakusa T, Kikuchi M. Lymphomatous polyposis of the gastrointestinal tract, including mantle cell lymphoma, follicular lymphoma and mucosa-associated lymphoid tissue lymphoma. Histopathology. 2005;47:467-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Vetro C, Bonanno G, Giulietti G, Romano A, Conticello C, Chiarenza A, Spina P, Coppolino F, Cunsolo R, Raimondo FD. Rare gastrointestinal lymphomas: The endoscopic investigation. World J Gastrointest Endosc. 2015;7:928-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Itsuno M, Makiyama K, Muta K, Furukawa K, Hara K, Tabata S, Soda H, Ikeda S, Takashima H, Fukuda Y. Adult T-cell leukemia with multiple lymphomatous polyposis of the gastrointestinal tract. Endoscopy. 1995;27:700-703. [PubMed] [Cited in This Article: ] |
| 26. | Hokama A, Tomoyose T, Yamamoto Y, Watanabe T, Hirata T, Kinjo F, Kato S, Ohshima K, Uezato H, Takasu N, Fujita J. Adult T-cell leukemia/lymphoma presenting multiple lymphomatous polyposis. World J Gastroenterol. 2008;14:6584-6588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Romaguera JE, Medeiros LJ, Hagemeister FB, Fayad LE, Rodriguez MA, Pro B, Younes A, McLaughlin P, Goy A, Sarris AH, Dang NH, Samaniego F, Brown HM, Gagneja HK, Cabanillas F. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003;97:586-591. [PubMed] [Cited in This Article: ] |
| 28. | Saito M, Miyazaki M, Tanino M, Tanaka S, Miyashita K, Izumiyama K, Mori A, Irie T, Tanaka M, Morioka M, Tsukamoto E. ¹⁸F-FDG PET/CT imaging for a gastrointestinal mantle cell lymphoma with multiple lymphomatous polyposis. World J Gastroenterol. 2014;20:5141-5146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |