Published online Jul 26, 2019. doi: 10.12998/wjcc.v7.i14.1844
Peer-review started: March 19, 2019
First decision: April 18, 2019
Revised: April 30, 2019
Accepted: May 11, 2019
Article in press: May 11, 2019
Published online: July 26, 2019
Processing time: 129 Days and 14.3 Hours
Metastases to adrenal glands originate principally from lung, breast, or gastrointestinal cancers, followed by malignant melanoma and thyroid neoplasms. We present an unusual case of uterine cancer metastasizing to the adrenal glands with a review of the English literature on the management of this rare disease.
A 53-year-old Caucasian woman with a history of endometrial cancer (grade 2; International Federation of Gynecology and Obstetrics III A) was hospitalized in November 2017 for a left adrenal mass found on a follow-up computed tomography scan 3 years after her gynecological surgery. Laboratory test results were normal. A laparoscopic left adrenalectomy was performed. The postoperative course was uneventful, and no chemotherapy was administered. The pathological report confirmed an adrenal endometrioid metastasis. At 36 mo of follow-up, the patient is alive and well, with no evidence of recurrent disease. A literature review identified only 11 previously-published cases of adrenal metastases from uterine cancer.
Adrenal metastasis from uterine cancer is very rare. Laparoscopic adrenalectomy may be an effective treatment in selected cases of localized adrenal metastasis.
Core tip: Isolated adrenal metastasis from endometrial cancer is uncommon but should be suspected in patients with history of gynecological cancer. We report a case of large, metachronous adrenal metastasis from endometrial cancer successfully treated with laparoscopic resection. Mini-invasive surgery is safe and feasible with good oncological results also for metastatic lesions of adrenal gland.
- Citation: Da Dalt G, Friziero A, Grego A, Serafini S, Fassina A, Blandamura S, Sperti C. Adrenal metastasis from endometrial cancer: A case report. World J Clin Cases 2019; 7(14): 1844-1849
- URL: https://www.wjgnet.com/2307-8960/full/v7/i14/1844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i14.1844
Secondary tumors are the second most common cause of adrenal cortex neoplasms, and carcinoma (especially in the advanced stage) accounts for more than 90% of secondary tumors[1]. In order of frequency, the most common primary sites of the metastatic carcinoma are: Lung cancer, breast cancer, and gastrointestinal cancers, followed by malignant melanoma and thyroid neoplasms. The metastatic involvement of adrenal glands by endometrial carcinoma is rare, and there is some controversy over the most appropriate treatment due to the lack of specific guidelines. The prognosis for patients with secondary tumors involving the adrenal glands is generally poor, but survival rates seem to improve in some cases after surgical resection. The National Comprehensive Cancer Network (NCCN) guidelines on malignancies of the adrenal glands recommend a direct open approach, while laparoscopic adrenalectomy is the gold standard in Europe, and the open approach is reserved for masses more than 6-8 cm in diameter, cases of extra-adrenal tissue invasion, or patients with contraindications to laparoscopy[2,3].
We present an unusual case of metachronous uterine cancer metastasizing to an adrenal gland that was successfully treated with laparoscopic resection. A review of cases previously reported in the English literature was undertaken to ascertain what is currently known about the treatment of this unusual condition. A comprehensive search was run in PubMed (Medline) and Scopus as at December 2018 using the keywords: “adrenal gland neoplasms”, adrenal metastasis”, “uterine cancer”, “endometrial cancer”. The “related articles” function was used to widen the search and all abstracts, studies, and citations retrieved were reviewed.
A 53-year-old Caucasian woman was admitted in November 2017 for an adrenal mass.
In 2014 she had undergone total hysterectomy, bilateral oophorectomy and pelvic lymphadenectomy for a grade 2, International Federation of Gynecology and Obstetrics (FIGO) stage III A endometrioid endometrial cancer (EEC). Postoperatively, she was treated with six cycles of carboplatin-paclitaxel chemotherapy. She had no known family history of colon cancer, and endometrial cancer related to Lynch syndrome was ruled out.
The patient had a history of laparoscopic uterine myomectomy and tonsillectomy when she was younger.
Physical examination revealed abdominal tenderness with moderate palpation of the left mid-quadrant of the abdomen.
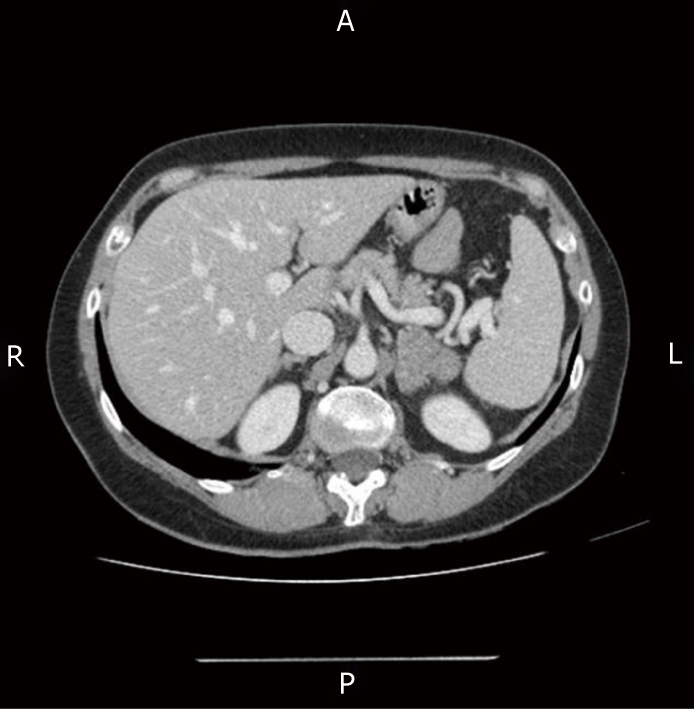
Computed tomography (CT) of the chest and abdomen revealed a solid left adrenal mass with malignant features (6 cm in diameter, with parenchymal invasion) (Figure 1).
Laboratory test findings, including hormonal assays, were unremarkable.
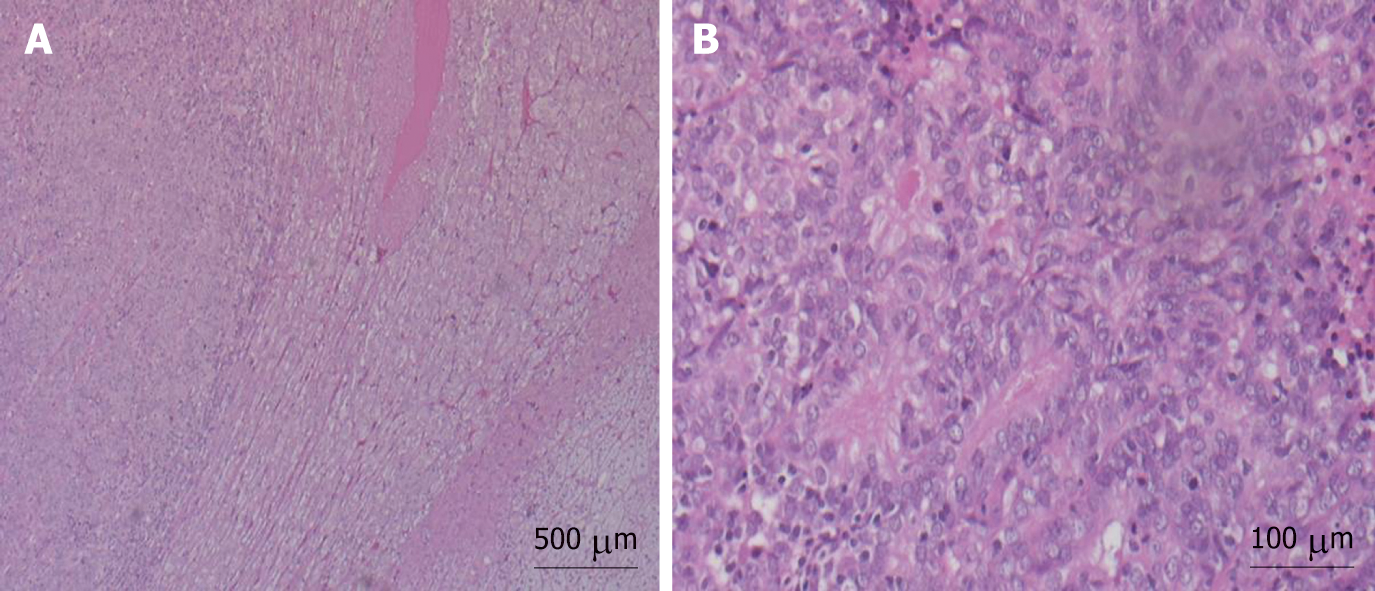
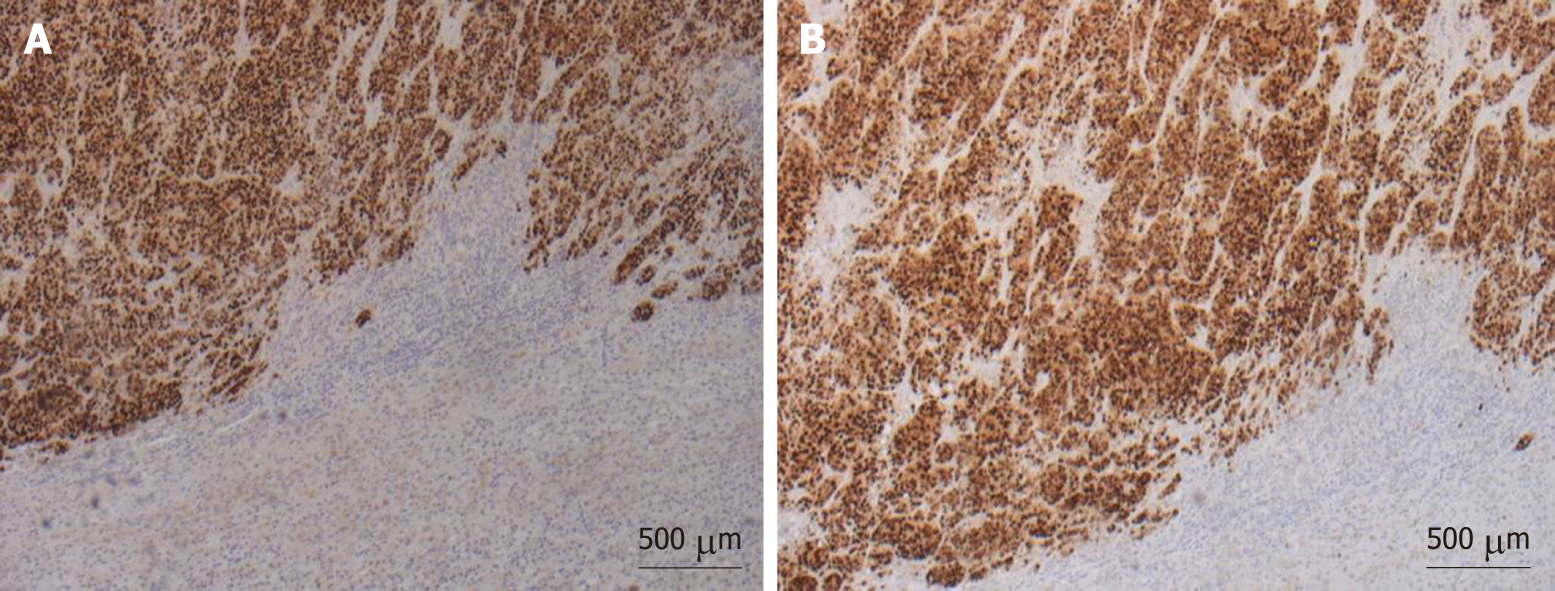
Macroscopic examination of the resected specimen showed the left adrenal gland (7 × 4 × 3 cm) almost entirely substituted by a solid, off-white neoplastic mass (6.8 cm in largest diameter). Microscopic sections showed massive adrenal and peri-adrenal endometrioid metastasis (Figure 2). Immunohistochemical staining was positive for progesterone and estrogen receptors (Figure 3).
Laparoscopic adrenalectomy was performed.
The postoperative course was uneventful and the patient was discharged 5 d after the surgical procedure. Two months later, 18-FDG positron emission tomography showed no pathological uptake, so no adjuvant therapy was administered. At the latest follow-up (December 2018), the patient was in good clinical condition with no recurrent disease.
Endometrial cancer is the most common gynecological cancer in developed countries. Most endometrial cancers are diagnosed at an early FIGO stage, and the most common histological type is EEC. These patients are generally considered at low risk, with 5-year survival rates of 95%; however, survival drop considerably to 69% and 13%, respectively, in the event of regional or distant metastases. Patients with endometrioid cancer in FIGO stages II to III, or with non-endometrioid cancer are at high risk of relapse[4]. The adrenal gland is a rare site of metastases from endometrial cancer. To our knowledge, only 11 previous cases have been reported in the English literature (Table 1), including: 8 cases of metachronous single-site metastases, 2 of metachronous multiple-site metastases, and one synchronous metastasis. Nakano et al[5] were the first to report a case of adrenal metastasis from endometrial cancer in 1975. On reviewing all the studies listed in Table 1: The patients’ median age was 62 years (range 39-77 years). There were two patients with FIGO stage I disease, two with FIGO stage II, two with FIGO stage III, and two with FIGO stage IV, while the stage was not stated in three cases. Six patients had an endometrioid histology, four had a non-endometrioid type, and no histology was available for one. When stratified according to the European Society of Medical Oncology 2016 Consensus Conference recommendations, nine patients were at high risk of recurrence or distant meta-stases[4].
| Case reports | Age (yr) | Histology of primary | Stage (FIGO) | Adjuvant treatment | DFS (mo) | Site | Treatment | F-U (mo) |
| Nakano et al[5], 1975 | 77 | Mixed | Na | Na | 26 | Metachronous multiple sites | Whole brain irradiation and supportive care | 28 |
| (clear cell-squamous) | ||||||||
| Lam et al[6], 2001 | Na | Na | Na | Na | Na | Metachronous single site | Laparotomic adrenalectomy | Na |
| Baron et al[7], 2008 | 76 | Endometrioid G1 | IV B | EBRT | 9 | Metachronous single site | Laparotomic partial adrenalectomy | 24 |
| Baron et al[7], 2008 | 62 | Endometrioid G1 | Na | VBT + 6 adriamycin- cisplatin cycles | 108 | Metachronous multiple site | Supportive treatment | 110 |
| Izaki et al[8], 2010 | 55 | Endometrioid | III C | 7 carboplatin paclitaxel cycles | 15 | Metachronous single site | Laparoscopic adrenalectomy + CT (3 carboplatin cycles) | 82 |
| Choi et al[9], 2011 | 62 | Squamous | III C | 6 cisplatin cycles | 10 | Metachronous single site | Laparoscopic adrenalectomy | 45 |
| Berretta et al[10], 2013 | 67 | Mixed (anaplastic-endometrioid) | IV B | Na | Na | Synchronous multiple sites | One-time laparotomic adrenalectomy + hysterectomy and salpingectomy + taxol and carboplatin chemotherapy | Na |
| Zaidi et al[11], 2013 | 75 | Endometrioid G3 | I B | Na | 6 | Metachronous single site | Supportive treatment | 9 |
| Singh Lubana et al[12], 2015 | 60 | Serous | II | EBRT + C + CT: 3 paclitaxel carboplatin cycles | 66 | Metachronous single sites | Laparotomic | 90 |
| adrenalectomy | ||||||||
| Rekhi et al[13], 2015 | 39 | Endometrioid G2 | II | VBT + EBRT | 24 | Metachronous single site | Robotic adrenalectomy + CT | Na |
| Mouka et al[14], 2016 | 58 | Endometrioid G3 | I B | 6 CT | 12 | Metachronous single site | Laparotomic | Na |
| adrenalectomy + CT | ||||||||
| Present case, 2019 | 53 | Endometrioid G2 | II B | No treatment | 36 | Metachronous single site | Laparoscopic adrenalectomy | 45 |
In most cases, the adrenal lesions were identified on CT scans. Laboratory tests revealed no hormone overproduction in any of the patients.
Gynecological and oncological societies have no shared approach to the adjuvant treatment of “high-risk” endometrial cancer. While the value of external beam radiotherapy and/or vaginal brachytherapy for local recurrence control is accepted almost worldwide, any use of chemotherapy to prevent distant relapses is at the clinician’s discretion[15]. Our review identified seven cases treated with adjuvant therapy after gynecological surgery: 4 patients were administered radiotherapy at least; 3 were treated with chemotherapy alone.
Disease-free survival after hysterectomy naturally varied considerably, given the marked differences between the cases reviewed in terms of disease stage and grade, histology, and adjuvant therapy. It ranged from 6 mo to 108 mo with a median disease-free survival in the series of 15 mo.
The surgical approach to adrenal gland metastases is controversial. Following the NCCN guidelines, many authors treat adrenal metastases directly with open surgery[16], but laparoscopic adrenalectomy has been used successfully for a variety of secondary tumors[17,18].
Our review found that only three of eight cases of single-site adrenal metastases from endometrial cancer (5.7-7.5 cm in diameter) were treated mini-invasively (laparoscopy or robotic surgery). The largest tumor (7.5 cm in diameter) was treated by Rekhi et al[13] with robotic adrenalectomy. Five patients underwent open adrenalectomy, and the largest tumor was reportedly 5 cm in diameter (range 3.5-5 cm). Our patient was successfully treated using a laparoscopic approach with no operative complications and with a good survival. A good outcome after mini-invasive surgery was reported by other Authors too. Izaki et al[8] and Choi et al[9] reported two similar cases, both high-risk patients with FIGO stage IIIC primary endometrial cancer and metachronous single-site adrenal metastases (5.7 and 6.0 cm in diameter, respectively); both patients were treated laparoscopically and had a long follow-up (82 and 45 mo, respectively).
So, despite the small number of patients considered, our review suggests that a laparoscopic approach is a valid alternative to open surgery for isolated adrenal metastases from endometrial cancer.
Finally, there is no general consensus regarding chemo-therapy after surgery for adrenal metastases. Izaki et al[8] described a patient who underwent laparoscopic adrenalectomy followed by three cycles of carboplatin-based chemotherapy, with a complete response at 67 mo. Other Authors[10,13,14] administered adjuvant chemo-therapy after surgery for adrenal secondary tumors, but provided no follow-up data.
Only one of the cases emerging from the literature review was managed with no surgery or oncological treatment. Zaidi et al[11] reported giving only palliative therapy to a patient with a low-stage uterine cancer and an early adrenal gland metastasis, and the outcome was very poor (she survived only 3 mo after her metastasis was diagnosed).
Adrenal metastasis from uterine cancer is rare, but should be suspected whenever an adrenal mass is detected in the follow-up after the resection of gynecological cancer. Although the number of cases reported in the literature is very small, laparoscopic resection seems to be feasible and safe, with good oncological results.
Manuscript source: Invited Manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Daniilidis A S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017;28:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 2. | National Comprehensive Cancer Network. Neuroendocrine and Adrenal Tumors; NCCN Clinical Practice Guidelines in Oncology. Version 4. 2018;. |
| 3. | Bradley CT, Strong VE. Surgical management of adrenal metastases. J Surg Oncol. 2014;109:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C; ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int J Gynecol Cancer. 2016;26:2-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 482] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 5. | Nakano KK, Schoene WC. Endometrial carcinoma with a predominant clear-cell pattern with metastases to the adrenal, posterior mediastinum, and brain. Am J Obstet Gynecol. 1975;122:529-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf). 2002;56:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Baron M, Hamou L, Laberge S, Callonnec F, Tielmans A, Dessogne P. Metastatic spread of gynaecological neoplasms to the adrenal gland: case reports with a review of the literature. Eur J Gynaecol Oncol. 2008;29:523-526. [PubMed] |
| 8. | Izaki H, Takahashi M, Shiirevnyamba A, Taue R, Furumoto H, Bando Y, Murakami Y, Fukawa T, Koizumi T, Yamamoto Y, Yamaguchi K, Nakatsuji H, Kishimoto T, Fukumori T, Kanayama HO. Long-term recurrence-free survivor after laparoscopic removal of solitary adrenal metastasis from endometrial adenocarcinoma. J Med Invest. 2010;57:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Choi JJ, Buttrick S, Zakashansky K, Nezhat F, Chin EH. Laparoscopic adrenalectomy for isolated adrenal metastasis from cervical squamous cell carcinoma and endometrial adenocarcinoma. Gynecol Oncol. 2011;122:684-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Berretta R, Patrelli TS, Faioli R, Mautone D, Gizzo S, Mezzogiorno A, Giordano G, Modena AB. Dedifferentiated endometrial cancer: an atypical case diagnosed from cerebellar and adrenal metastasis: case presentation and review of literature. Int J Clin Exp Pathol. 2013;6:1652-1657. [PubMed] |
| 11. | Zaidi SS, Lakhani VT, Fadare O, Khabele D. Adrenal gland metastasis is an unusual manifestation of endometrial cancer. Case Rep Surg. 2013;2013:428456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Singh Lubana S, Singh N, Tuli SS, Seligman B. Adrenal Metastasis from Uterine Papillary Serous Carcinoma. Am J Case Rep. 2016;17:289-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Rekhi B. Mismatch repair protein deficient endometrioid adenocarcinomas, metastasizing to adrenal gland and lymph nodes: Unusual cases with diagnostic implications. Indian J Pathol Microbiol. 2015;58:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Mouka V, Tsili AC, Messinis T, Papoudou-Bai A, Kamina S, Argyropoulou MI. Solitary adrenal metastasis from early-stage dedifferentiated endometrial carcinoma: CT findings and review of the literature. J Obstet Gynaecol. 2016;36:881-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A, Fyles A, Baron MH, Jürgenliemk-Schulz IM, Kitchener HC, Nijman HW, Wilson G, Brooks S, Carinelli S, Provencher D, Hanzen C, Lutgens LCHW, Smit VTHBM, Singh N, Do V, D'Amico R, Nout RA, Feeney A, Verhoeven-Adema KW, Putter H, Creutzberg CL; PORTEC study group. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 16. | Uberoi J, Munver R. Surgical management of metastases to the adrenal gland: open, laparoscopic, and ablative approaches. Curr Urol Rep. 2009;10:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Sarela AI, Murphy I, Coit DG, Conlon KC. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol. 2003;10:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Sebag F, Calzolari F, Harding J, Sierra M, Palazzo FF, Henry JF. Isolated adrenal metastasis: the role of laparoscopic surgery. World J Surg. 2006;30:888-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |