Published online Jul 26, 2019. doi: 10.12998/wjcc.v7.i14.1814
Peer-review started: March 15, 2019
First decision: April 18, 2019
Revised: May 2, 2019
Accepted: May 23, 2019
Article in press: May 23, 2019
Published online: July 26, 2019
Processing time: 136 Days and 13.7 Hours
Highly active antiretroviral therapy (HAART) is provided free of charge to all human immunodeficiency virus (HIV) positive residents in Italy. As fixed dose coformulations (FDCs) are often more expensive in comparison to the same drugs administered separately in a multi-tablet regimen (MTR), we considered a cost-effective strategy involving patients in the switch from their FDCs to corresponding MTRs including generic antiretrovirals.
To verify if this would affect the virological and immunological response in comparison to maintaining the FDC regimens.
From January 2012 to December 2013, we assessed the eligibility of all the HIV-1 positive adults on stable HAART being treated at our hospital-based outpatient clinic in Treviso, Italy. Participants who accepted to switch from their FDC regimen to the corresponding MTR joined the MTR group, while those who maintained a FDC regimen joined the FDC group. Clinical data, including changes in HAART regimens, respective reasons why and adverse effects, were recorded at baseline and at follow-up visits occurring at weeks 24, 48 and 96. All participants were assessed for virological and immunological responses at baseline and at weeks 24, 48 and 96.
Two hundred and forty-three eligible HIV-1 adults on HAART were enrolled: 163 (67%) accepted to switch to a MTR, joining the MTR group, while 80 (33%) maintained their FDCs, joining the FDC group. In a parallel analysis, there were no significant differences in linear trend of distribution of HIV-RNA levels between the two groups and there were no significant odds in favour of a higher level of HIV-RNA in either group at any follow-up and on the overall three strata analysis. In a before-after analysis, both FDC and MTR groups presented no significant differences in distribution of HIV-RNA levels at either weeks 48 vs 24 and weeks 96 vs 24 cross tabulations. A steady increase of mean CD4 count was observed in the MTR group only, while in the FDC group we observed a slight decrease (-23 cells per mmc) between weeks 24 and 48.
Involving patients in the switch from their FDC regimens to the corresponding MTRs for economic reasons did not affect the effectiveness of antiretroviral therapy in terms of virological response and immunological recovery
Core tip: In a cohort of adults with suppressed human immunodeficiency virus-type 1 (HIV-1) viremia, the switch from fixed-dose coformulations to the corresponding multitablet regimens (MTRs) did not affect the effectiveness of antiretroviral therapy in terms of virological response and immunological recovery. Our data came from a well-established study population that was demographically representative of the Italian HIV-infected population, with very high levels of adherence and complete virological suppression. By involving patients in the decision to switch to a MTR for economic reasons, the magnitude of the potential clinical benefits from fixed dose coformulation regimens appears to be cancelled by the efficacy and tolerability of the antiretroviral drugs currently available in MTRs, even in a generic formulation, despite a small risk of drug discontinuation in patients who switched to MTRs because of mere convenience issues due to the number of tablets.
- Citation: Rossi MC, Inojosa WO, Battistella G, Carniato A, Farina F, Giobbia M, Fuser R, Scotton PG. Desimplification to multi-tablet antiretroviral regimens in human immunodeficiency virus-type 1 infected adults: A cohort study. World J Clin Cases 2019; 7(14): 1814-1824
- URL: https://www.wjgnet.com/2307-8960/full/v7/i14/1814.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i14.1814
Highly active antiretroviral therapy (HAART) suppresses human immunodeficiency virus-type 1 (HIV-1) RNA plasma viral load below detectable limits, enabling immune reconstitution, reducing HIV drug resistance and preventing clinical disease progression, potentially as long as patients adhere to therapy. HAART is provided free of charge to all HIV positive residents in Italy. The pharmaceutical industry introduced the “fixed dose antiretroviral coformulations” (FDCs), with the purpose to reduce the number of daily pills. As FDCs are often more expensive in comparison to the same drugs administered separately in a multi-tablet regimen (MTR), we considered it a cost-effective strategy the switch from FDCs to corresponding MTRs including generic antiretrovirals. United States and European HIV treatment guidelines consider regimen simplification to FDCs to improve adherence to antiretroviral therapy[1,2]. However, the US Department of Health and Human Services states that there is limited data to support or refute the superiority of FDCs versus MTRs, particularly when considering the comparison between once-daily regimens of 1 pill versus 3 pills of individual drug products currently in use.
We conducted a prospective study among a cohort of adults with suppressed HIV-1 viremia who accepted to switch from FDCs to corresponding MTRs in order to verify if this would affect the virological and immunological response in comparison to maintaining the FDC regimens.
We assessed the eligibility of all the HIV-1 positive adults on stable HAART being treated at our hospital-based outpatient clinic in Treviso, Italy, from January 2012 to December 2013. To be eligible, patients had to be age 18 or older, on HAART regimen including the FDCs tenofovir/emtricitabine (Truvada® Gilead Sciences), or abacavir/lamivudine (Kivexa® ViiV Healthcare), or zidovudine/lamivudine (Combivir® ViiV Healthcare), or tenofovir/emtricitabine/efavirenz (Atripla® Gilead Sciences) for at least 6 months, and with an HIV-RNA level below 20 copies/mL for at least 6 months. We excluded patients with alcoholism, active intravenous drug use, dementia, and homelessness.
On follow up visits, all consecutive eligible patients who had signed the informed consent were enrolled in the cohort study and underwent a counselling focusing firstly on the adherence to HAART. An interview was conducted asking patients to recall missed doses (from yesterday to three days before) and through a visual analogue rating scale (VAS) assessing errors in the timetable or forgetfulness in taking HAART during the previous month, the supply of drugs during the previous three months and overall satisfaction to current HAART regimen[3]. Then, the counselling was focused on involving patients in reducing treatment costs by choosing to switch from their current FDCs to the corresponding MTR. Except for the switching from emtricitabine to lamivudine, all patients did not change their therapy in terms of active molecules or doses. According to WHO guidelines, we considered emtricitabine and lamivudine interchangeable[4]. In all cases the MTR increased the daily number of pills, but not dosing frequency.
Participants who accepted to switch from their FDC regimen to the corresponding MTR joined the MTR group, while those who maintained a FDC regimen joined the FDC group.
Clinical data, including changes in HAART regimens, respective reasons why and adverse effects, were recorded at baseline and at follow-up visits occurring at weeks 24, 48 and 96. All participants were assessed for virological and immunological responses at baseline and at week 24, 48 and 96. Plasma HIV RNA levels were quantified through COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 (Roche Diagnostics) with a lower limit of quantification at 20 copies/mL. According to the Italian HIV guidelines, virological failure was defined as two consecutive viral loads > 50 copies/mL[5]. CD4 cell count was assessed with FACSCanto II flow cytometer (BD Biosciences).
Patients were counselled on adherence to HAART at each visit, while formal interviews and VAS were repeated at week 24 only and results of the monitoring assessment were published elsewhere[6].
Data were analyzed using SPSS 13 and WinPEPI 11.43. Patients’ baseline characteristics were evaluated with N-1 chi-square test, or independent samples T test as appropriate. Plasma HIV-RNA levels were categorized in four ordinal categories (first: undetectable; second: < 20 copies/mL; third: ≥ 20 and ≤ 50; forth: > 50) and in two dichotomized categories (HIV-RNA < or ≥ 20 copies/mL) based on the lower limit of quantification by Taqman PCR. In the parallel analysis, differences in distribution were evaluated by Pearson’s chi-square (at baseline) and by Cochran-Armitage test or generalized odds ratio (GOR) with 95% confidence intervals (CI) at any follow-up, and by GOR with 95%CI, extended Mantel-Haenszel test for trend and a chi-square test for heterogeneity were performed for overall analysis. In the before-after analysis, cross tabulations of the four HIV-RNA levels at weeks 24, 48 and 96 were analyzed using the McNemar test with continuity correction, GOR with 95% CI, and a combination of the Mann-Whitney (weighting by sample sizes) in the pooled data analysis. Cross tabulations of the dichotomized HIV-RNA levels at weeks 24, 48 and 96 were analyzed by the McNemar test (two-tailed) and Fisher’s 95%CI after Higgins and Thompson’s I-squared test for heterogeneity. To test differences in CD4 count in a before-after analysis, means were compared by Fisher's LSD test. The procedure was repeated stratifying data for the three major ARV backbones Atripla, Truvada, and Kivexa. The 0.05 level of confidence was chosen. If not specified otherwise, all tests are two-tailed.
Ethical approval was obtained from the Research Ethics Boards of the Ca' Foncello Regional Hospital in Treviso, Italy (approval number EUDRACT 2011-004935-30) and all participants provided informed consent.
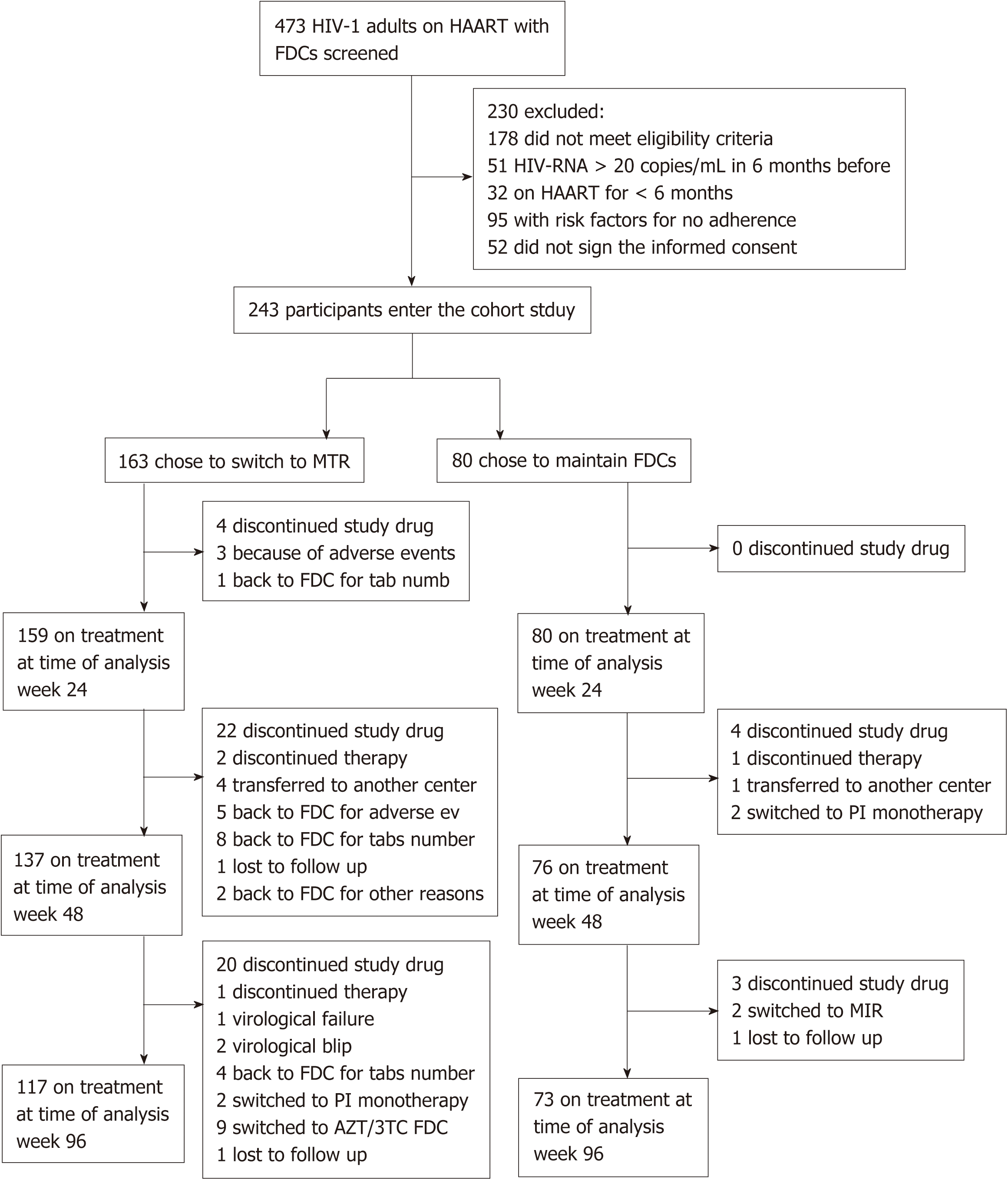
Between January and June 2012 we screened 473 HIV-1 adults on HAART including a FDC.
178 patients who did not meet eligibility criteria and 52 who did not sign the informed consent were excluded from the study (Figure 1). 243 participants were enrolled and allocated to a treatment group: 163 (67%) accepted to switch to a MTR, joining the MTR group, while 80 (33%) maintained their FDCs, joining the FDC group.
At baseline, the two groups did not show any significant difference except for a higher mean number of HAART tablets before enrollment in participants who entered in the MTR group (Table 1).
| FDC (n = 80) | MTR (n = 163) | ||
| Gender (female) | 32.5% (26) | 33.7% (55) | 0.037 P = 0.8471 |
| Italian origin | 73.8% (59) | 75.5% (123) | 0.083 P = 0.7731 |
| Comorbidities | |||
| HCV | 12.5% (10) | 8.6% (14) | 0.918 P = 0.3381 |
| HBV | 2.5% (2) | 1.2% (2) | 0.535 P = 0.4651 |
| Osteoporosis | 1 | 0 | |
| Injecting drugs addiction | 0 | 2 | |
| Diabetes | 1 | 2 | |
| Kidney failure | 1 | 0 | |
| Psychiatric disorders | 6.2% (5) | 4.9% (8) | 0.190 P = 0.6631 |
| Others diseases | 18.7% (15) | 27.0% (44) | 1.976 P = 0.1601 |
| Proportions of treated with | |||
| Atripla | 37.5% (30) | 28.2% (46) | 2.141 P = 0.1431 |
| Combivir | 7.5% (6) | 21.5% (35) | 7.439 P = 0.0061 |
| Kivexa | 30.0% (24) | 19.6% (32) | 3.239 P = 0.0721 |
| Truvada | 25.0% (20) | 30.7% (50) | 0.839 P = 0.3601 |
| Undetectable HIV-RNA at baseline | 75.0% (60) | 76.7% (125) | 0.084 P = 0.7721 |
| CD4 count at baseline | 698.5 (SD 295.27) | 671.6 (SD 268.87) | P = 0.4922 |
| Mean age (yr) | 47.3 (SD 10.77) | 47.06 (SD 9.47) | P = 0.8392 |
| Years of HAART | 8.7 (SD 4.26) | 8.7 (SD 4.85) | P = 0.9532 |
| HAART pills number | 2.4 (SD 1.35) | 2.9 (SD 1.56) | P = 0.0162 |
| Non HAART pills number | 1.4 (SD 2.71) | 1.1 (SD 1.95) | P = 0.4092 |
| Total pills number | 3.8 (SD 2.935) | 4.0 (SD 2.63) | P = 0.5722 |
A total of 190 participants completed the 96-wk follow-up; drug discontinuation rates were 8.7% (7/80) in the FDC group, and 28.2% (46/163) in the MTR group. The reason for and timing of premature drug discontinuation in the two groups are reported in Figure 1. In the parallel analysis, there were no significant differences in linear trend of distribution of HIV-RNA levels between the two groups (Table 2) and there were no significant odds in favour of a higher level of HIV-RNA in either group at any follow-up and on the overall three strata analysis. In the before-after analysis, both FDC and MTR groups presented no significant differences in distribution of HIV-RNA in the four levels and in the dichotomized levels at either weeks 48 vs 24 and weeks 96 vs 24 cross tabulations (Table 3). On the pooled data analysis of the dichotomized HIV-RNA levels, the McNemar test (two-tailed) showed a significant increase in patients with HIV-RNA levels < 20 copies/mL in the MTR group only. One patient in the MTR group presented a virological failure with 55 copies/mL at week 96 and 76 copies/mL after 3 mo.
| Weeks | HIV-RNA (copies/mL) | FDC | MTR | Total | Follow-up analysis | Overall analysis |
| Total | 80 | 163 | 243 | |||
| 0 | NR | 60 | 125 | 185 | 1P = 0.772 | |
| < 20 | 20 | 38 | 58 | |||
| 24 | NR | 60 | 104 | 164 | 2P = 0.354; GOR 0.704 (95%CI: 0.403 to 1.229) | 3P = 0.991; 4P = 0.366; GOR 0.956 (95%CI: 0.677 to 1.351); 5P = 0.298 |
| < 20 | 10 | 34 | 44 | |||
| 20-50 | 8 | 15 | 23 | |||
| > 50 | 2 | 5 | 7 | |||
| Discontinued study drug | 0 | 5 | 5 | |||
| 48 | NR | 56 | 100 | 156 | 2P = 0.828; GOR 0.996 (95%CI: 0.547 to 1.815) | |
| < 20 | 14 | 29 | 43 | |||
| 20-50 | 5 | 7 | 12 | |||
| > 50 | 1 | 1 | 2 | |||
| Discontinued study drug | 4 | 26 | 30 | |||
| 96 | NR | 53 | 92 | 145 | 2P = 0.310; GOR 1.385 (95%CI: 0.722 to 2.657) | |
| < 20 | 16 | 21 | 37 | |||
| 20-50 | 4 | 4 | 8 | |||
| > 50 | 01 | 01 | 0 | |||
| Discontinued study drug | 7 | 46 | 53 |
| Crosstabulation (Week 48 vs week 24) | Crosstabulation (Week 96 vs week 24) | Pooled data | |||||||||||
| FDC | |||||||||||||
| HIV-RNA (copies/mL) | NR | < 20 | 20-50 | > 50 | Discontinued study drug | Total | NR | < 20 | 20-50 | > 50 | Discontinued study drug | Total | 3P = 0.972; GOR 1.00 (95%CI: 0.66 to 2.06) |
| NR | 43 | 11 | 3 | 0 | 3 | 60 | 42 | 10 | 3 | 0 | 5 | 60 | |
| < 20 | 7 | 0 | 1 | 1 | 1 | 10 | 5 | 4 | 0 | 0 | 1 | 10 | |
| 20-50 | 5 | 2 | 1 | 0 | 0 | 8 | 4 | 2 | 1 | 0 | 1 | 8 | |
| > 50 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | |
| Total | 56 | 14 | 5 | 1 | 4 | 80 | 53 | 16 | 4 | 0 | 7 | 80 | |
| P = 0.8601; GOR = 1.00 (95%CI: 0.50 to 2.00) | P = 0.8451; GOR = 1.00 (95%CI: 0.46 to 2.16) | ||||||||||||
| HIV-RNA (copies/mL) | ≥ 20 | < 20 | Total | ≥ 20 | < 20 | Total | (I-squared = 0.0%); 2P = 0.072; Odds ratio (odds A: odds B) = 0.471; Fisher’s 95%CI: 0.176 to 1.151 | ||||||
| ≥ 20 | 1 | 9 | 10 | 1 | 8 | 9 | |||||||
| < 20 | 5 | 61 | 66 | 3 | 61 | 64 | |||||||
| Total | 6 | 70 | 76 | 4 | 69 | 73 | |||||||
| 2P = 0.285 | 2P = 0.132 | ||||||||||||
| MTR | |||||||||||||
| HIV-RNA (copies/mL) | NR | < 20 | 20-50 | > 50 | Discontinued study drug | Total | NR | < 20 | 20-50 | > 50 | Discontinued study drug | Total | 3P = 0.036; GOR 1.65 (95%CI: 0.97 to 5.52) |
| NR | 77 | 16 | 1 | 0 | 10 | 104 | 71 | 12 | 1 | 0 | 20 | 104 | |
| < 20 | 17 | 6 | 2 | 0 | 9 | 34 | 16 | 5 | 1 | 0 | 12 | 34 | |
| 20-50 | 4 | 6 | 3 | 1 | 1 | 15 | 4 | 4 | 1 | 0 | 6 | 15 | |
| > 50 | 2 | 1 | 0 | 0 | 2 | 5 | 1 | 0 | 1 | 0 | 3 | 5 | |
| Discontinued study drug | 0 | 0 | 1 | 0 | 4 | 5 | 0 | 0 | 0 | 0 | 5 | 5 | |
| Total | 100 | 29 | 7 | 1 | 26 | 163 | 92 | 21 | 4 | 0 | 46 | 163 | |
| P = 0.2031; GOR = 1.50 (95%CI: 0.85 to 2.64) | P = 0.0821; GOR = 1.86 (95%CI: 0.97 to 3.56) | ||||||||||||
| HIV-RNA (copies/mL) | ≥ 20 | < 20 | Total | ≥ 20 | < 20 | Total | (I-squared = 0.0%); 2P = 0.001; Odds ratio (odds A: odds B) = 0.227; Fisher’s 95%CI: 0.067 to 0.615 | ||||||
| ≥ 20 | 4 | 13 | 17 | 2 | 9 | 11 | |||||||
| < 20 | 3 | 116 | 119 | 2 | 104 | 106 | |||||||
| Total | 7 | 129 | 136 | 4 | 113 | 117 | |||||||
| 2P = 0.012 | 2P = 0.035 | ||||||||||||
There were no significant differences in mean CD4 count between the two groups at baseline. A steady increase of CD4 count was observed in the MTR group, while in the FDC group we observed a slight decrease (-23 cells per mmc) in mean CD4 count between weeks 24 and 48.
According to Fisher’s LSD test, there was a significant increase in mean CD4 count at any follow-up in the MTR group only (P < 0.001 each), while in the FDC group a significant increase was observed only at week 96. In the stratified analysis of the three major ARV backbones, after 96 weeks there was a significant increase in CD4 count in each MTR patient (Table 4), and in patients taking Atripla.
| Baseline | 24 wk | 48 wk | 96 wk | ||||||||
| n | mean CD4 (SD) | n | mean CD4 (SD) | P1 value | n | mean CD4 (SD) | P1 value | n | mean CD4 (SD) | P1 value | |
| FDC | 80 | 698.5 (295.3) | 80 | 730.3 (339.7) | 0.173 | 76 | 707.6 (281.9) | 0.717 | 73 | 751.7 (289.9) | 0.011 |
| Atripla | 30 | 649.7 (285.2) | 30 | 666.3 (278.3) | 0.549 | 29 | 709.9 (274.8) | 0.032 | 26 | 715.0 (286.2) | 0.02 |
| Truvada | 20 | 678.8 (261.3) | 20 | 658.9 (263.3) | 0.639 | 17 | 627.1 (224.8) | 0.227 | 17 | 741.4 (279.2) | 0.145 |
| Kivexa | 24 | 740.8 (356.4) | 24 | 823.0 (460.2) | 0.107 | 24 | 738.1 (341.0) | 0.957 | 24 | 773.5 (317.2) | 0.518 |
| MTR | 163 | 671.6 (268.9) | 159 | 730.4 (282.0) | < 0.001 | 137 | 745.2 (281.0) | < 0.001 | 117 | 769.0 (269.0) | < 0.001 |
| TDF/3TC/-EFV | 46 | 665.6 (251.3) | 43 | 742.9 (234.1) | 0.003 | 34 | 746.0 (261.7) | 0.002 | 30 | 759.9 (245.7) | < 0.001 |
| TDF/3TC | 50 | 665.7 (311.6) | 50 | 735.7 (341.6) | 0.003 | 44 | 751.8 (336.2) | < 0.001 | 40 | 766.8 (311.4) | < 0.001 |
| ABC/3TC | 32 | 675.4 (202.3) | 32 | 733.7 (225.5) | 0.06 | 29 | 740.7 (211.9) | 0.036 | 28 | 810.5 (247.2) | < 0.001 |
In our prospective study among a cohort of selected adults with suppressed HIV viremia and known to be adherent to HAART, we demonstrated that involving patients in the switch from their FDC regimens to the corresponding MTRs for economic reasons did not affect the effectiveness of antiretroviral therapy in terms of virological response and immunological recovery. In the parallel analysis, no statistically significant differences were observed in terms of virological response at weeks 24, 48 and 96 between the two groups. Only one patient in the MTR group (0.8%, 1/117 as treated population) presented a virological failure after 96 wk. This failure was detected as we adopted a very sensitive definition of virological failure and an extended follow-up period. As viremia was insufficient for RNA amplification and resistance testing, no emergent drug resistance mutations were detected. All other virological blips during the study period were of low intensity and were not detected in subsequent HIV-RNA determinations.
In the before-after analysis, virological response to both FDC and MTR regimens was highly maintained throughout the study. FDCs and MTRs increased CD4 count to similar levels by weeks 24, 48 and 96. A statistically significant increase in CD4 count was observed only in the MTR group probably as this group had a larger number of patients.
At baseline, patients who chose to switch to a MTR had significantly more como-rbidities in combined medical and psychiatric illnesses than the others, and they were already taking a significantly higher number of tablets for reasons other than HIV. This is an unexpected result, but we have no data to infer if patients already used to taking many drugs had less difficulty to increase their number of tablets. By contrast, patients on single tablet regimen (Atripla) were significantly more likely to refuse the switching to MTRs than patients on other FDC regimens.
We observed a high discontinuation rate of MTRs for reasons other than virological failure. A minimal proportion (4.9%) of patients discontinued a MTR because of adverse gastrointestinal symptoms considered a result of the switch. The average discomfort reported by patients was generally low to moderate, but enough to induce the prescribing doctor to return to the corresponding FDC. This fact is not a peculiarity of the switching from FDCs to a MTR, as a low proportion of mild to moderate adverse effects inducing drug discontinuations were registered also after switching to FDC regimens in several “simplification” trials[7-9].
Fifteen patients (9.2%) expressed a disappointment with the number of tablets in their MTR because of convenience issues, and returned to their previous FDC regimen. Finally, a number of patients were switched to a PI monotherapy to avoid ARV backbone toxicity or to generic FDC zidovudine/lamivudine when it was available during the last months of follow-up. All drug discontinuations occurred at scheduled visits except for 3 patients who requested unscheduled visits early after the switch to MTR because of adverse effects, therefore we can infer that healthcare costs for day ward services were not substantially affected. According to Walensky et al[10], compared with a slightly less effective generic-based regimen, the cost-effectiveness of first-line branded ART exceeds $ 100000/QALY. Formal cost-effectiveness analysis was beyond the scope of this study. However, based on 2011 HAART costs for the Veneto region, northern Italy, the switch from FDC to MTR represents a yearly savings of € 255, € 174, and € 142 per patient on Atripla, Truvada, and Kivexa treatment respectively. Therefore, the potential cost savings for the healthcare system for the 98 patients with suppressed HIV, who remained on MTR for 96 wk is about € 120000. Patients on Combivir who initially accepted to switch to a MTR (potentially with a cost savings of € 919 per patient per year), subsequently returned to a generic FDC with further cost savings.
It has been postulated that potential benefits of a single-tablet regimen would include improved adherence and quality of life, reduced risk of selective non-compliance, and a lower risk of prescription error, all of which might decrease the risk of treatment failure and drug resistance[11]. However, the pharmaceutical industry has made impressive progress over the past decades in simplifying dosing frequency and pill burden, and current MTRs are no longer those of the early times since the introduction of HAART, when patients were prescribed between 10 and 25 pills each day often to be divided in three daily doses, with specific food restrictions or requirements, and substantial toxicity[12,13]. Indeed, we observed very high rates of adherence to HAART 24 wk after the switch both in patients taking MTRs and patients taking FDCs, at an interim analysis published elsewhere[6].
Recent meta-analyses have shown only minimal or no benefits of FDC regimens in terms of adherence and treatment outcomes, and an independent study has shown that antiretroviral effectiveness was not hampered by a switch from Atripla STR to a triple tablet regimen (tenofovir/lamivudine/efavirenz) in a large cohort of Danish HIV patients[13-18].
Finally, a recent multicenter study in Canada showed that the simplification of Triumeq® ViiV Helthcare to a less expensive component of one generic pill of abacavir/lamivudine and one branded pill of Tivicay® ViiV Healthcare did not result in an additional risk of viral breakthrough, resistance, and side effects, and significantly reduced costs of therapy[19]. With the advent of the new generic drugs in Italy (abacavir/lamivudine, tenofovir/emtricitabine, darunavir, tenofovir, and in the near future rilpivirine), it will be of great interest to assess the effectiveness of the desimplification of Triumeq and other FDCs to their respective generic drugs in the context of our National Health System.
We studied a highly selected cohort of patients as all participants were enrolled after being fully instructed in the purpose of the study and those who freely chose to switch to MTR regimens were fully aware they had to increase the number of pills and take generic drugs for economic reasons. Therefore, potential biases could have occurred in the patient's choice of treatment, in reported symptoms after the switch and their reasons for treatment interruption. However, our data came from a well-established study population that was demographically representative of the national HIV-infected population, with very high levels of adherence and complete virological suppression consistent with the improvements observed over time in Western Europe[20] and results appear generalizable for clinical settings in which the antiretroviral drugs are dispensed free of charge and only from the hospital pharmacies of the National Health Service.
Our study shows that, by involving patients in the decision to switch to a MTR for economic reasons, the magnitude of the potential clinical benefits from FDC regimens appears to be cancelled by the efficacy of the antiretroviral drugs currently available in MTRs, even in a generic formulation. We noticed only a small risk of drug discontinuation, in patients who switched to MTRs because of mere convenience issues due to the number of tablets.
With the constant increase in the number of patients in HAART, innovative measures are increasingly needed to contain costs while maintaining the highest quality of care. The appropriate use of generic drugs and a good doctor-patient relationship must play a key role in balancing the needs of HIV patients with those of the Health System.
United States and European human immunodeficiency virus (HIV) treatment guidelines consider regimen simplification to fixed dose coformulations (FDCs) to improve adherence to antiretroviral therapy. However, the US Department of Health and Human Services states that there is limited data to support or refute the superiority of FDCs versus multi-tablet regimens (MTRs). With the constant increase in the number of patients in highly active antiretroviral therapy (HAART), innovative measures are increasingly needed to contain costs while maintaining the highest quality of care.
HAART is provided free of charge to all HIV positive residents in Italy. As FDCs are often more expensive in comparison to the same drugs administered separately in a MTR, we considered a cost-effective strategy involving patients in the switch from their FDCs to corresponding MTRs including generic antiretrovirals.
We conducted a prospective study among a cohort of adults with suppressed HIV-1 viremia who accepted to switch from FDCs to corresponding MTRs in order to verify if this would affect the virological and immunological response in comparison to maintaining the FDC regimens.
From January 2012 to December 2013, we assessed the eligibility of all the HIV-1 positive adults on stable HAART being treated at our hospital-based outpatient clinic in Treviso, Italy. Participants who accepted to switch from their FDC regimen to the corresponding MTR joined the MTR group, while those who maintained a FDC regimen joined the FDC group. Clinical data, including changes in HAART regimens, respective reasons why and adverse effects, were recorded at baseline and at follow-up visits occurring at weeks 24, 48 and 96. All participants were assessed for virological and immunological responses at baseline and at weeks 24, 48 and 96.
Two hundred and forty-three eligible HIV-1 adults on HAART were enrolled: 163 (67%) accepted to switch to a MTR, joining the MTR group, while 80 (33%) maintained their FDCs, joining the FDC group. In a parallel analysis, there were no significant differences in linear trend of distribution of HIV-RNA levels between the two groups and there were no significant odds in favour of a higher level of HIV-RNA in either group at any follow-up and on the overall three strata analysis. In a before-after analysis, both FDC and MTR groups presented no significant differences in distribution of HIV-RNA levels at either weeks 48 vs 24 and weeks 96 vs 24 cross tabulations. A steady increase of mean CD4 count was observed in the MTR group only, while in the FDC group we observed a slight decrease (-23 cells per mmc) between weeks 24 and 48.
Involving patients in the switch from their FDC regimens to the corresponding MTRs for economic reasons did not affect the effectiveness of antiretroviral therapy in terms of virological response and immunological recovery. The appropriate use of generic drugs and a good doctor-patient relationship must play a key role in balancing the needs of HIV patients with those of the Health System.
With the advent of the new generic drugs in Italy (abacavir/lamivudine, tenofovir/emtricita-bine, darunavir, tenofovir, and in the near future rilpivirine), it will be of great interest to assess the effectiveness of the desimplification of Triumeq and other new FDCs to their respective generic drugs in the context of our National Health System.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen C, Kamal SA S-Editor: Ji FF L-Editor: A E-Editor: Liu JH
| 1. | AIDSinfo. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. |
| 2. | European AIDS Clinical Society. EACS guidelines version 8.0. Available from: http://www.eacsociety.org/Portals/0/2015_eacsguidelines_8_0-english_rev-20160124.pdf. |
| 3. | Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysis. J Acquir Immune Defic Syndr. 2007;46:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Technical update on treatment optimization: pharmacological equivalence and clinical interchangeability of lamivudine and emtricitabine: a review of current literature: June 2012. Available from: http://www.who.int/iris/handle/10665/70936. |
| 5. | Antinori A, Marcotullio S, Ammassari A, Andreoni M, Angarano G, Carosi G, Cinque P, d'Arminio Monforte A, Di Perri G, Ensoli B, Ferrazzi E, Galli M, Mastroianni C, Matteelli A, Mazzotta F, Moroni M, Palù G, Puoti M, Puro V, Rizzardini G, Sagnelli E, Suter F, Vella S, Lazzarin A; Italian HIV Guidelines Working Group. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. New Microbiol. 2011;34:109-146. [PubMed] |
| 6. | Rossi MC, Battistella G, Farina F, Inojosa WO, Carniato A, Giobbia M, Fuser R, Scotton PG. Virological response and adherence in patients who changed from fixed dose antiretroviral coformulations to corresponding multitablet ART regimen. Proceedings of the 5th Italian Conference on AIDS and Retrovirus (ICAR); 2013, May 12-14; Turin, Italy: 116-117. . |
| 7. | Dejesus E, Young B, Morales-Ramirez JO, Sloan L, Ward DJ, Flaherty JF, Ebrahimi R, Maa JF, Reilly K, Ecker J, McColl D, Seekins D, Farajallah A; AI266073 Study Group. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr. 2009;51:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Arribas JR, Pialoux G, Gathe J, Di Perri G, Reynes J, Tebas P, Nguyen T, Ebrahimi R, White K, Piontkowsky D. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Pozniak A, Markowitz M, Mills A, Stellbrink HJ, Antela A, Domingo P, Girard PM, Henry K, Nguyen T, Piontkowsky D, Garner W, White K, Guyer B. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis. 2014;14:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Walensky RP, Sax PE, Nakamura YM, Weinstein MC, Pei PP, Freedberg KA, Paltiel AD, Schackman BR. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Llibre JM, Arribas JR, Domingo P, Gatell JM, Lozano F, Santos JR, Rivero A, Moreno S, Clotet B; Spanish Group for FDAC Evaluation. Clinical implications of fixed-dose coformulations of antiretrovirals on the outcome of HIV-1 therapy. AIDS. 2011;25:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1146] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 13. | Hernández Arroyo MJ, Cabrera Figueroa SE, Sepúlveda Correa R, Valverde Merino MP, Luna Rodrigo G, Domínguez-Gil Hurlé A; Tormes Team. Influence of the number of daily pills and doses on adherence to antiretroviral treatment: a 7-year study. J Clin Pharm Ther. 2016;41:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48:484-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, Gallant JE, Mugavero MJ, Mills EJ, Giordano TP. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, Ford N. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Hill A, Pozniak A, Simmons B. No difference in risk of virological failure between antiretroviral treatments using co-formulated versus individual drugs: Meta-analysis of 9 randomised trials in 2,568 patients. HIV Medicine. 2015;16:4. |
| 18. | Engsig FN, Gerstoft J, Helleberg M, Nielsen LN, Kronborg G, Mathiesen LR, Obel N. Effectiveness of antiretroviral therapy in individuals who for economic reasons were switched from a once-daily single-tablet regimen to a triple-tablet regimen. J Acquir Immune Defic Syndr. 2014;66:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Krentz H, Campbell S, Gill J. Desimplification of Single Tablet Antiretroviral (ART) Regimens-A Practical Cost-Savings Strategy? J Int Assoc Provid AIDS Care. 2019;18:2325958218822304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | De Luca A, Dunn D, Zazzi M, Camacho R, Torti C, Fanti I, Kaiser R, Sönnerborg A, Codoñer FM, Van Laethem K, Vandamme AM, Bansi L, Ghisetti V, van de Vijver DA, Asboe D, Prosperi MC, Di Giambenedetto S; SEHERE collaboration in Chain. Declining prevalence of HIV-1 drug resistance in antiretroviral treatment-exposed individuals in Western Europe. J Infect Dis. 2013;207:1216-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |