Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1554
Peer-review started: January 7, 2019
First decision: February 13, 2019
Revised: April 4, 2019
Accepted: May 2, 2019
Article in press: May 2, 2019
Published online: July 6, 2019
Processing time: 180 Days and 23.2 Hours
Several studies have largely focused on the significant role of the nervous and immune systems in the process of tumorigenesis, including tumor growth, proliferation, apoptosis, and metastasis. The brain-gut-axis is a new paradigm in neuroscience, which describes the biochemical signaling between the gastrointestinal (GI) tract and the central nervous system. This axis may play a critical role in the tumorigenesis and development of GI cancers. Mechanistically, the bidirectional signal transmission of the brain-gut-axis is complex and remains to be elucidated. In this article, we review the current findings concerning the relationship between the brain-gut axis and GI cancer cells, focusing on the significant role of the brain-gut axis in the processes of tumor proliferation, invasion, apoptosis, autophagy, and metastasis. It appears that the brain might modulate GI cancer by two pathways: the anatomical nerve pathway and the neuroendocrine route. The simulation and inactivation of the central nervous, sympathetic, and parasympathetic nervous systems, or changes in the innervation of the GI tract might contribute to a higher incidence of GI cancers. In addition, neurotransmitters and neurotrophic factors can produce stimulatory or inhibitory effects in the progression of GI cancers. Insights into these mechanisms may lead to the discovery of potential prognostic and therapeutic targets.
Core tip: Although studies have revealed the role of the brain-gut axis in cancer, the bidirectional signal transmission of the brain-gut axis remains unclear. This review summarizes current findings concerning the relationship between the brain-gut axis and gastrointestinal (GI) cancer and focuses on the significant role of the brain-gut axis in tumorigenesis and cancer progression, including tumor proliferation, invasion, apoptosis, autophagy, and metastasis. The central nervous, sympathetic, and parasympathetic nervous systems, neurotransmitters, and neuropeptides may regulate the malignant tumor phenotype in GI cancers. An insight into these mechanisms may lead to the discovery of potential prognostic markers and new targets for GI cancer therapy.
- Citation: Di YZ, Han BS, Di JM, Liu WY, Tang Q. Role of the brain-gut axis in gastrointestinal cancer. World J Clin Cases 2019; 7(13): 1554-1570
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1554.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1554
Gastrointestinal (GI) cancer is one of the most common malignancies worldwide[1]. In the last few decades, the incidence and mortality of GI cancers have markedly increa-sed, with a recent trend toward younger individuals. Although advances in clinical diagnosis and comprehensive therapy have partly prolonged survival and improved quality of life, the progression-free survival and overall survival rates of GI cancers have not improved[2,3]. Many researchers have focused on the immune system and related cellular signaling pathways in the recognition and elimination of cancer cells[4-6]. Like the immune system, the nervous system has emerged as a new paradi-gm. The “brain-gut axis” is thought to have an important role in the progression and metastasis of GI cancers[7].
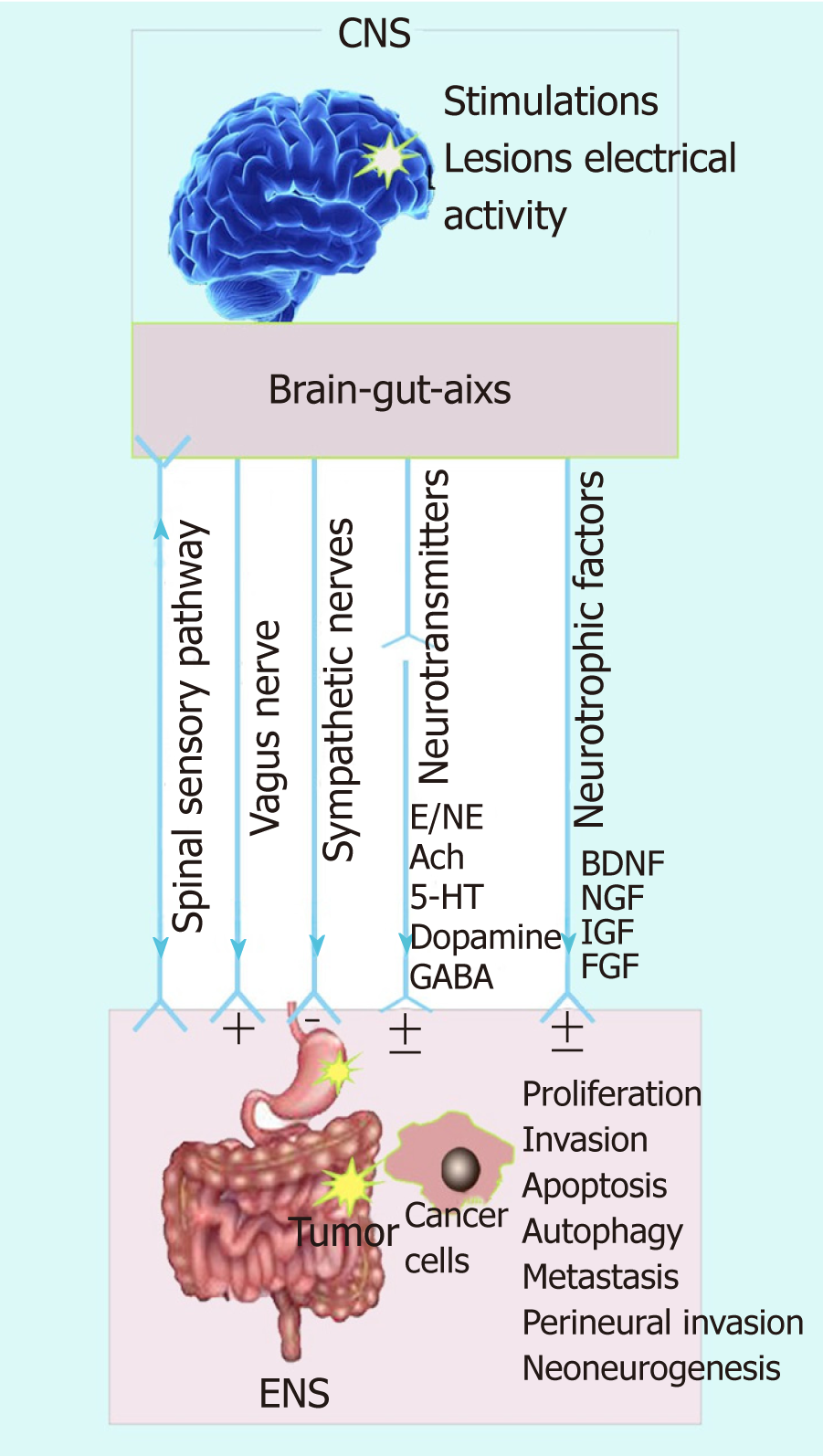
Broadly defined, the brain-gut axis includes the central nervous system (CNS), the autonomic nervous system comprised of the sympathetic and parasympathetic systems, and the gastric or gut microbiota. The concept of brain-gut axis, which describes the interaction between the brain and the gut, was proposed early in the twentieth century. The brain-gut axis represents a bidirectional relationship of the brain-to-gut modulation of GI function, and a gut-to-brain pathway. The gut can synthesize and secrete a range of neuroactive molecules that cross the blood-brain barrier and influence the function of the CNS[8]. Likewise, some neuroactive molecules can be conveyed from the brain to the gut by the sympathetic and parasympathetic systems, or the humoral pathway[9]. These findings indicate that the nervous and humoral pathways could convey signals from tumor cells to the brain, followed by brain-mediated modulation of tumor growth in the peripheral tissues. Furthermore, recent studies have suggested that neural signaling molecules are involved in the tumorigenesis and progression of GI cancers[7,10]. However, the mechanism of modula-tion from the brain to the gut remains unclear. Several studies in the past few decades have suggested that the nervous system might modulate tumor cell proliferation and metastasis via several pathways, including the parasympathetic pathway and the sensory nerves[11]. This neurobiological conclusion of cancer etiopathogenesis was based on several findings, including nerve infiltration of tumor tissues, the effects of stimulation or lesions of the CNS, and the effects of neurotransmitters or neuropep-tides on tumor incidence and progression.
In this review, we summarize the latest studies on the mechanisms by which the brain-gut axis regulates oncogene activation and tumor promotion or suppression, including the anatomical nerve pathway and the neuroendocrine-immune system. These findings may inform studies involving the search for new therapeutic targets for GI cancer treatment.
Tumor occurrence and development can be affected by the tumor microenvironment and signaling molecules. Recent studies have indicated that nerves can infiltrate and innervate tumors in many malignancies, including pancreatic cancer[12], colorectal cancer (CRC)[13], biliary tract cancer[14], and gastric cancer[15]. These findings suggest that tumor innervations are closely associated with poor clinical outcomes[16]. Con-sistent with this, approximately 60% of gastric cancer tissues are capable of perineural invasion, and the overall survival of these patients is significantly worse than that of patients without perineural invasion. In addition, neural invasion was positively related to vascular invasion and lymph node metastasis[17]. A study involving CRC reported an overall survival rate of 25% in patients with neural invasion compared to 72% for patients without neural invasion[18-21]. Almost all cases of pancreatic ductal adenocarcinoma feature neural invasion. The median overall survival for patients without neural invasion is longer than that for patients with neural invasion (56 mo vs 28 mo)[22-24]. Recent experimental data suggest that this process may be regulated by neurotrophic factors that promote regeneration of axons of the adjacent nerve cells, followed by gradual invasion into the tumor[25]. The nerve cells also synthesize and secrete neurotransmitters or neuropeptides, which may constitute a positive microenvironment for cancer survival and proliferation. Besides, the infiltrative nerve fibers may act as a physical support for cell migration and metastasis. These hypoth-eses are supported by the observations that pancreatic cancer cells are more aggre-ssive and have a lower apoptotic ratio when located in close proximity to a nerve space[26].
Many studies have shown that several stimulations and lesions of the CNS can promote proliferation and metastasis of cancer cells by regulating specific immune functions and electrical activity. Electrical impulses from the lateral hypothalamus can significantly enhance the cytotoxicity of natural killer (NK) cells, which may be positively correlated with the prognosis of cancer patients. Cytotoxicity can be signifi-cantly suppressed when the lateral hypothalamus is injured. However, some studies have shown an opposite reaction to chronic electrical stimulation from the ventrome-dial hypothalamic nucleus. Other studies have suggested a close relationship between pinealectomy and the higher incidence of breast cancer in mice, and the lower inci-dence when melatonin is administered.
Furthermore, some studies have suggested that the activation of the hypothalamic-pituitary-adrenal axis in patients with depression could induce DNA repair deficiency and angiogenesis, which may promote cell survival and eventually tumorigenesis. The inactivation of sensory neurons induced by capsaicin might promote the invasion and metastasis of tumor cells. When the brain-gut axis is activated, the brain might react to the cancer cells with neuroendocrine-immune and behavioral responses, including the involvement of neuropeptides, neurotransmitter metabolism, regional brain activity, and behavior changes[27]. Researchers have found that some areas of the CNS are in a depressive state in patients with GI carcinoma[28,29]. A neuroendocrine-immune perspective of cancer might offer a better explanation for the age-related increased incidence in stomach cancer. In addition, the effects of aging on DNA damage and repair in the CNS might also partly explain the increasing cancer inci-dence rate in older people[30]. Age-related changes in brain innervation of the GI tract might increase inflammatory reaction in the gut and lead to a higher incidence of GI cancers[31]. Therefore, drugs that can modulate the CNS or signal transmission between nerves and tumor cells may provide a new avenue for cancer treatment.
The sympathetic and parasympathetic nervous systems mainly comprise peripheral nerves involved in the regulation of basic electric rhythm, neural conduction, and signal integration. The sympathetic nervous system, especially the activation of the sympathoadrenal axis, promotes tumorigenesis in the GI tract[32,33]. A recent study demonstrated that chemical sympathectomy with 6-hydoxydopamine (6-OHDA) evidently reduced the incidence of colon cancer in Wistar rats[34]. They speculated that three mechanisms could be responsible. First, 6-OHDA may exert an immunomo-dulatory effect on humoral immune responsiveness. Second, chemical sympathe-ctomy might decrease the activity of acetylcholinesterase in both the pre- and post-ganglionic synapses of the parasympathetic system. Third, the adminis-tration of 6-OHDA could result in selective damage to catecholaminergic neurons in the periphe-ral nervous system and CNS. Moreover, the activation of the sympathetic nerves might also promote proliferation and metastasis of pancreatic ductal adenocarcinoma cells[35].
The role of the parasympathetic nervous system in GI cancers has been extensively investigated. Surgical therapy, such as vagotomy for gastric ulcers, has provided an opportunity for researchers to understand the effect of vagotomy on cancer incidence in humans. Some retrospective studies have showed that vagotomy could increase the risk of gastric cancer[36,37]. Several other studies revealed that the inactivation of the vagus nerve substantially promoted liver, kidney, and lung cancer metastasis by upregulating the levels of substance P (SP). Therefore, the vagus nerve might protect against metastasis[38]. Nevertheless, another study demonstrated that the activation of the vagal nerve could contribute to gastric tumorigenesis by M3 receptor mediated Wnt signaling in stem cells, suggesting that the vagal denervation might be a feasible strategy for the management of gastric cancer[39]. The contentious results from the above studies reflect that the risk of cancer in patients after vagotomy may be due to other factors that are important in tumorigenesis, such as Helicobacter pylori infection, hypochlorhydria, smoking, and bile reflux[36,37,40,41]. The chemotherapy drug vincristine can induce vagal neuropathy and disrupt the potential modulatory effects on tumor metastasis[42].
The homeostasis in mammalian bodies is regulated by the nervous, endocrine, and immune systems[43]. The brain-gut axis is a complicated means of communication in the CNS, enteric nervous system, and endocrine-immune system, which is important in tumorigenesis and development. The widely distributed neuropeptides and neuro-transmitters are responsible for information exchange between the brain and gut[44]. Growing evidence suggests that neurotransmitters and neuro-peptides are involved in the modulation of tumor proliferation, migration, invasion, and angiogenesis. Here, we discuss recent advances in the understanding of neural signaling in cancer and its potential implications in cancer therapy.
Epinephrine and norepinephrine are crucial mediators of the stress response due to their rapidly increased concentration in response to stress events. Both of them exert their effects by binding to α or β-adrenergic receptors (ARs)[44]. Recent studies have shown that chronic stress can result in angiogenesis and tumor growth in a mouse model of CRC through the catecholamine and adrenoceptor system. Epinephrine is involved in stress-induced tumorigenesis and progression in GI cancer by activation of ARs.
A variety of studies have confirmed that epinephrine promotes growth, invasion, and angiogenesis of malignant cells by upregulating the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9, which are critical proteins related to carcinogenesis[45-47]. The activation of β-adrenoceptors may promote cell growth and proliferation in gastric cancer[48]. This effect can be prevented by the beta-blocker propranolol[49]. Additionally, a previous study demonstrated that epinephrine promotes proliferation of esophageal squamous cell carcinoma cells via beta-adrenoceptor-dependent transactivation of the extracellular signal-regulated kinase (ERK)/cyclooxygenase-2 pathway. Inhibition of the postganglionic parasym-pathetic fiber can significantly decrease the proliferation and metastasis of HT-29 colon cancer cells[50]. In an in vitro study, epidermal growth factor (EGF) promoted esophageal cancer cell proliferation by enhancing the expression of tyrosine hydroxy-lase and the cellular secretion of epinephrine; these effects could be attenuated by beta-adrenoceptor antagonists[51].
Mechanistically, certain classical signaling pathways have been implicated in esophageal cancer cell proliferation[52]. A study involving CRC suggested that adre-naline could promote cell proliferation and migration via activation of the β-adrenergic receptor (β-AR)–cyclic AMP (cAMP)–protein kinase A (PKA) pathway[53]. Another study confirmed that the β-AR-camp-PKA signaling pathway might be involved in the vascularization and metastasis in colon cancer. There is substantial evidence confirming that MMP-3, -7, and -9 play key roles in the invasion and metastasis of gastric cancer cells[54]. Epinephrine can bind to the promoter region of MMP-7 and upregulate its expression depending on the activation of signal transdu-cer and activator of transcription 3 and activator protein 1 (AP1), thereby mediating the invasion and metastasis of gastric cancer cells. Conversely, the classical β-blocker propranolol can inhibit the migration and invasion via down-regulation of MMP-7 expression[55], indicating that beta-adrenoceptor antagonists might be useful phar-macological tools for the treatment of metastatic cancer. Taken together with the anti-proliferative action of beta-adrenoceptor antagonists on tumor cells, it seems that adrenoceptor antagonists might be capable of inhibiting primary cancer cell growth and counteracting the migration and metastasis of primary tumors. The data provide solid evidence of the potential value of β-adrenoceptor antagonists as pharmaco-logical tools for the therapy of metastatic cancer.
Acetylcholine is a neuron neurotransmitter that produces excitatory and inhibitory outputs in the neural system. Acetylcholine function is mediated by nicotinic receptor (n-AchR) and muscarinic acetylcholine receptor (mAchR). Nicotine, the main harmful ingredient of cigarettes, promotes proliferation and invasion of various cancer cells in vitro and tumor growth and metastasis in vivo by activating nicotinic nAchRs.
Schaal et al[56] first postulated the potential activating role of nAchRs in cancer cells. The authors demonstrated the binding of the tobacco-specific nitrosamine, 4-(me-thylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), to n-AchR with higher affinity than the natural ligand, acetylcholine. Furthermore, NNK exhibited a great carcinogenic potential by promoting the differentiation and proliferation of lung, colorectal, and stomach cancer cells[56]. Multiple pathways appear to be involved in the activation of nAchR, which can enhance colorectal tumor promoting events. Nicotine treatment increased the stemness property of colon cells by upregulating the expres-sion of sex determining region Y-box 2 and aldehyde dehydrogenase, and enhancing cancer stem cell populations. These effects were exerted mainly through activation of α7 n-AchR/AKT and mitogen-activated protein kinase (MAPK) signaling pathways. Other studies found that nicotine activated ERK1/2 and upregulated the levels of VEGF and MMP-9, molecules belonging to recognized proliferation and survival-related signaling pathways in pancreatic cancer cells. However, in gastric cancer, nicotine and NNK enhanced cell proliferation significantly through a different mecha-nism. The former activated the ERK1/2 pathway, while the latter was dependent on p38 MAPK[57].
Nicotinic can regulate the synthesis and release of catecholamines from sympa-thetic nerve endings and the adrenal medulla. Therefore, it is not surprising that nicotine and NNK extracts promote cell proliferation in a variety of human cancer cells, from the upper to the lower GI tract, through n-AchR and beta-adrenoceptors. Nicotine reportedly induces epinephrine production in colon cancer cells, and beta-adrenoceptor antagonists can abrogate the nicotine-stimulated tumor growth in a dose-dependent manner in mice. Other authors suggested that nicotine decreases the production of gamma-aminobutyric acid (GABA)[53] by reducing the activity of GAB A-synthesizing enzymes in pancreatic ductal adenocarcinoma.
The other receptor, mAChR, is also overexpressed in colon carcinoma and stomach cancer[58]. It has been demonstrated that mAChR can increase the migration and invasion of CRC cells by transactivation of the EGFR-ERK pathway. The demon-stration that acetylcholine is involved in epithelial mesenchymal transition (EMT) and increases the expression of several markers via the M3 muscarinic receptor (M3R) has provided an insight into the mechanisms of gastric cancer growth and metastasis and will inform the discovery of new targets for gastric cancer treatment[59].
Serotonin, which is chemically termed 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter that is synthesized by serotonergic neurons and enterochromaffin cells of the gut mucosa. The gut is the main source of 5-HT in the body, especially in the enterochromaffin cells of the GI tract. Recent evidence has implicated serotonin as a regulator of proliferation, regeneration, and repair in tumor biology[60]. Foregut and midgut carcinoids usually contain higher levels of serotonin than the hindgut carcin-oids[61]. 5-HT receptors are overexpressed in the small intestinal neuroendocrine tumor tissues, especially in high-grade tumor cells. 5-HT is a potential mitogen for many types of tumor cells, including bladder, pancreas, lung, and especially, colon cancer cells. The mitogenic effect of 5HT in small cell lung cancer cells involves both 5HT1A and 5HT1D receptor types[62]. The blockade of serotonin receptors by 2-bromolysergic acid diethylamide reputedly results in a decreased mitotic rate in tumor cells[63].
Serotonin has been demonstrated to be upregulated in colon carcinoma tissues, and exogenous serotonin can promote the proliferation of CRC cells. Indirect evidence suggests that the effect of this amine on colonic tumors involves a cellular uptake mechanism. Furthermore, citalopram and fluoxetine, which are two specific inhibitors of serotonin uptake, significantly suppressed cell division in dimethylhydrazine-induced colonic tumors in rats, and retarded the growth of CRC cells of human colonic tumors that had been propagated as xenografts in immune-deprived mice[64]. 5-HT also promoted angiogenesis, but not proliferation, in a colorectal tumor animal model[53]. The authors maintained that the specific mechanism involved 5-HT, which significantly down-regulated the expression of MMP-12, resulting in the secretion of an endogenous inhibitor of angiostatin[53]. The collective research data indicate that 5-HT receptor antagonists may be potential anticancer agents for the effective treatment of GI cancers.
Interestingly, Vicaut et al[65] reported a direct mitogenic effect of high doses of 5-HT on tumor cells in athymic nude mice, whereas low doses of 5-HT inhibited tumor growth by decreasing the oxygen tension and blood supply to the tumors. Another study revealed that serotonin was involved in the induction of tumor ischemia by constricting tumor-feeding arterioles and selective reduction of tumor blood flow, which produced an anticancer effect[66]. However, El-Salhy et al[67] observed that serotonin did not directly significantly affect tumor volume or weight, and did not directly reduce the tumor blood vessel related volume density. Furthermore, the combination of serotonin, octreotide, and galanin had an antitumor effect, as eviden-ced by the marked effects on tumor size and apoptotic index[67].
DA is the precursor of adrenaline and norepinephrine, yet it has an opposite effect on tumor growth[68]. Contrary to adrenaline and norepinephrine, which display a tumor promoting activity in many stress-induced cancers, dopamine may exert negative or positive effects on tumorigenesis. Peripheral DA is involved in tumor progression and has anticancer effects on immunomodulation, including inflammasomes in cancer, immune effector cells like T lymphocytes, myeloid-derived suppressor cells, tumor-associated macrophages, and NK cells. Dopamine receptors (DRs) can be classified into two types: D1-like (D1 and D5) and D2-like receptors (D2–D4), based on their opposite functions on the accumulation of cyclic adenosine monophosphate (cAM-P)[69].
A clinical research study reported that DA is significantly decreased in GI cancer, especially in cancers of advanced stages[70]. Evaluation of the expression of D2 receptors in stomach tissue revealed a significant decrease in the concentration of DRs in malignant stomach tissue compared to normal and benign controls, while the affinity of the receptors was similar. This alteration may be of significance in under-standing the etiopathogenesis of gastric cancer on the basis of peripheral neurotrans-mitters[70]. Accumulating evidence suggests that D1-like receptors (DRD1 and DRD5) may have an inhibitory effect on cancer growth, including breast, colon, and gastric cancers. For example, Kou et al[71] reported that DRD1 significantly suppressed the expression and phosphorylation of insulin-like growth factor 1 (IGF-1) and thus inhibited IGF-1 induced vascular smooth muscle cell proliferation. Another study revealed that activation of DRD5 inhibited phosphorylation of both mTOR and its downstream targets[72].
DRD2 was reported to be negatively correlated with survival durations of patients with gastric cancer; DA inhibited the EGFR/AKT pathway and MMP-13 production via D2R, and thus suppressed the invasion and migration of gastric cancer cells[73]. An elegant study demonstrated that exogenous DA depressed tumor angiogenesis and growth by activating its specific DR2 in several animal models, including ovarian, gastric, breast, and colon cancer models[74]. The effect of DA in retarding angiogenesis has been investigated in CRC. In these studies, DA reduced the tumor microvessel density by suppressing the expression of VEGF and vascular permeability factor (VPF). Both are prime cytokines that reportedly induce tumor angiogenesis by stimulating proliferation and migration of endothelial cells and recruitment of bone marrow–derived endothelial progenitor cells[75,76]. Moreover, DA is able to inhibit proliferation and migration of tumor endothelial cells by suppressing the expression of VEGFR2, MAPK, and focal adhesion kinase phosphorylation (FAK). Exogenous DA was demonstrated to significantly inhibit tumor growth and increase the life span in combination with anticancer drugs, indicating that DA could enhance the sensitivity to the anticancer drugs[77]. DA and its receptor agonists could be the foundations of novel cancer therapies in the future.
GABA is the major inhibitory neurotransmitter of the CNS. In the brain, it counteracts the stimulatory effect of epinephrine and norepinephrine, which are physiological agonists of β-ARs. GABA involvement in the pathological progression of cancer has been described[78]. There are three types of GABA receptors: GABAA and GABAC are ionotropic receptors, and GABAB is a metabotrophic receptor. GABAB is related to chemokine and catecholaminergic receptors, which are involved in the regulation of leukocyte and tumor cell migration[79,80]. The GABAergic system may be significant in cancer cell proliferation, migration, and survival[81,82]. In most cases, the levels of GABA receptors are significantly changed in cancer cells. Glutamate decarboxylase activity and GABA content were reported to be significantly increased in colon cancer[83]. GABA is an inhibitory regulator for the migration and invasion of SW480 colon carcinoma cells. A study using a three-dimensional collagen matrix indicated that GABA reduced the norepinephrine-induced activity of cell migration to spontan-eous migration levels; the reduction was mediated by the GABAB receptor and intracellularly transduced by a significantly decreased cAMP concentration[84]. In addition, GABA and baclofen decreased the 5-bromo-2’-deoxyuridine labeling index of the gastric antral mucosa[85]. GABA had no effect on the protein tyrosine phospha-tase-mediated phospholipase C pathway that mediates the ATP-independent calcium release and which may trigger migratory activity. In the pancreas in the setting of CRC, GABAB receptor was reported to inhibit isoproterenol-induced cAMP signaling, prevent ERK1/2 activation, and promote cell proliferation and migration[86]. GABAA receptors were reportedly overexpressed in the KATO III gastric cancer cell line. However, the expression of GABAB receptors was not detected in these cells. Further studies by the same authors demonstrated that the binding of GABA to the GABAA receptor significantly promoted the proliferation of KATOIII cells by activating the ERK-1/2/cyclin D1 pathway[87]. The same results were obtained in pancreatic ductal adenocarcinoma, ovarian, prostate, and breast cancers[88]. In another study, GABA was shown to stimulate the growth of human hepatocellular carcinoma cells through overexpressed GABAA receptor subunit in a dose-dependent manner[89]. GABA also reportedly stimulated pancreatic cancer growth through increased intracellular Ca2+ levels and the MAPK/Erk cascade by the overexpression of GABAA[90]. Therefore, GABAergic systems, especially GABAA and GABAB, may have different effects in different cancers. These findings implicate GABA as a promising molecular target for the development of new therapeutic strategies for GI cancer.
It has been proposed that cancer cells can invade peripheral tissues to promote their own migration and to stimulate their own innervation. Cancer cells secrete neuronal growth factors and axon guidance molecules, which can promote the growth of nerves (axonogenesis) and in return exert autocrine or paracrine effects in the cancer cells. Recently, it was reported that nerve growth factor (NGF), brain-derived nerve growth factor (BDNF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF) stimulate neurogenesis, and are positively correlated with a poor prognosis in various cancers[91]. Neurotrophic factors can be subdivided into various types, which include neurotrophins, neuropoietins, IGFs, and transforming growth factors. Most are involved in cancer cell progression. Neurotrophin activities are mediated through two classes of cell surface receptors: Trk tyrosine kinase receptors and neurotrophin receptor p75NTR. NGF preferentially binds TrkA, whereas BDNF and NT-4/5 bind TrkB. NT-3 primarily binds TrkC, but also TrkA and TrkB to a lesser extent[92]. Here, we discuss the effects of some of the most closely linked neurotrophic factors in GI cancer.
BDNF is a member of the neurotrophin family. It is widely expressed in the hippoca-mpus, cortex, and synapses of the basal forebrain[70]. BDNF was recently associated with tumor progression in some malignancies, such as lung cancer[93], breast cancer, and colon cancer[94,95]. These studies revealed that BDNF and its high-affinity receptor (tropomyosin receptor kinase B, TrkB) are overexpressed in human colon and gastric cancer tissue, compared to the undetectable levels in normal tissue[96], and are signifi-cantly associated with the cancer stage at diagnosis. These findings suggest that BDNF is a potential prognostic marker in GI cancer. BDNF was also found to be elevated in SW480, LoVo, Caco-2, and HRT18 colon cancer cell lines. The BDNF/TrkB pathway reportedly has a profound effect on cell proliferation, differentiation, invasiveness, and migration, and chemotherapeutic response in CRC. BDNF and TrkB knockdown significantly decreased the rates of growth and proliferation, and increa-sed the apoptosis rate of colon cancer cells[94]. Interestingly, the authors described that BDNF exerted its antiapoptotic effect via the BDNF-Akt-Bcl2 signaling pathway[94]. Another study revealed that BDNF increased the migration of colon cancer cells by regulating the activity of VEGF/heme oxygenase-1 (HO-1) through the ERK, p38, and PI3K/Akt signaling pathways[79,97]. Another study observed the involvement of BDNF and NT-4/5 in an autocrine loop that mediated cell resistance to apoptosis. It was further observed that the use of antibodies blocking BDNF/TrkB resulted in tumor growth inhibition, characterized by an increase in cell apoptosis, suggesting that BDNF could contribute to cancer cell survival and serve as a prospective target mole-cule to inhibit tumor growth[98].
A recent study revealed that the BDNF/TrkB axis was activated in advanced gastric cancer tissues with a bone metastatic potential and overexpressed in bone metastatic gastric cancer cell lines[99]. In addition, the elevated levels of TrkB correlated with disease severity in patients. Additionally, the authors observed that BDNF upregulated the expression of long pentraxin 3, thereby promoting interactions between bone metastatic gastric cancer cells and osteoblasts, and osteoclastogenesis[99]. Another study reported that the blockade of gastric-releasing peptide receptor by RC-3095 can significantly increase BDNF secretion via an EGFR dependent mechanism, indicating that this protein may be used as a marker to predict adverse pathological and clinical outcomes[100]. Others described that BDNF is crucial for tumor angio-genesis and growth; it could promote endothelial cell proliferation, migration, and invasion by upregulating RhoA, VEGF, and caspase-9[101]. Exogenous HGF can stimulate angiogenesis by promoting TrkB expression and phosphorylation[102]. The collective findings indicate that BDNF is a potential therapeutic target in GI cancer, although the effectiveness of such a therapy in humans requires further investigation.
NGF exerts its actions through the binding to two types of NGF receptors: High-affinity tropomyosin receptor kinase A (TrkA) and low-affinity p75 neurotrophin receptor (p75NTR)[103,104]. In addition to the repair and maintenance of post-mitotic neurons, NGF also plays a significant role in the process of tumorigenesis.
Interestingly, the high expression of TrkA was observed in esophageal, thyroid, and colorectal carcinomas, whereas p75NTR expression was significantly lower in gastric and colon carcinomas. The difference in expression of NGF/Trk/p75NTR in tumors suggests that the NGF family and their receptors may play different roles or have directly opposite effects on various carcinomas originating from different tissues. The involvement of NGF in cancer cells was first demonstrated by its ability to promote proliferation of breast tumor-derived cell lines. This mitogenic effect is mediated through the activation of MAPKs[105]. Recent studies have shown that NGF/TrkA are overexpressed in colon cancer. Furthermore, serological analysis revealed that NGF is secreted by CRC cells, but not by normal colon epithelial cells. The activation of NGF/TrkA can promote cell survival, proliferation, and differentia-tion via phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) and Ras/MAPK signaling pathways[106,107]. The observation that NGF promoted gastric tumorigenesis via a feedback-loop ACh-NGF-yes-associated protein axis indicated that inhibition of ACh, M3R, or NGF may be an important future therapeutic strategy in the treatment of GI cancers[108]. In another study, NGF and HO1 were found to be coexpressed in gastric carcinoma tissues, and were significantly associated with shorter overall survival and regression-free survival, suggesting that NGF-HO1-related pathways might have a role in the progression of gastric carcinomas[109]. It has been reported that NGF promotes angiogenesis and induces the expression of proangiogenic molecules (VEGF/FGFs) in some tumors. Thus, the ability of NGF and TrkA to modulate biological behavior (including metastasis, migration, and angiogenesis) of many cancers could potentially be exploited as an innovative approach for cancer therapy. However, some studies have shown that the precursor of NGF (proNGF) to p75NTR can decrease cell survival and promote apoptosis. In one study, NGF significantly increased the anti-proliferative effect of 5-fluorouracil[110]. Therefore, the role of NGF-related signaling in human malignant tumors remains contentious.
Cancer-induced bone pain (CIBP) is a common and challenging problem for metastatic patients, which reduces quality of life. NGF has been shown to play an important role in modulating inflammatory and CIBP states[111]. In humans, NGF levels are elevated in a variety of chronic CIBP patients and these levels correlate with pain intensity, and with persistent mechanisms of allodynia and hyperalgesia[112]. Recently, mouse studies have demonstrated that NGF can significantly induce sprouting of sensory and sympathetic nerve fibers in bone metastases[113,114]. This nerve sprouting is associated with bone pain and has not been observed in a normal healthy bone. Therefore, novel anti-NGF and anti-TrkA drugs are under investigation for treatment of CIBP[115]. Studies have demonstrated that tanezumab, a humanized monoclonal antibody, blocks the binding of NGF to TrkA, which can significantly reduce pain and improve physical function and global scores in patients with CIBP[116,117].
IGF peptides include two structurally similar ligands (IGF-I and IGF-II), two high-affinity membrane receptors (IGF-1R and IGF-2R), and their binding proteins (IGFBP-1to IGFBP-6)[118,119]. Both IGF-I and IGF-II exert their biological effects through the activation of IGF-1R. Binding of ligands activates downstream cascades resulting in proliferative, differentiative, and anti-apoptotic effects that include cellular growth and proliferation, cell cycle control, differentiation, extracellular matrix formation, and angiogenesis.
IGFs are reported to play a significant role in cancer progression and increased levels of circulating IGF-I constitute a risk factor for the development of breast, prostate, lung, and GI tract cancers[120,121]. Recently, increased expression of IGF-I, IGF-II, and IGF-IR was detected in CRC and they have been widely accepted as markers for CRC risk[122,123]. Tricoli et al[124] described that approximately 60% of the colon cancer specimens contained mildly or markedly elevated levels of IGF1 and IGF2 mRNA or protein, compared to normal colonic tissue. 1,2-dimethylhydrazine was reported to promote the growth of colonic aberrant crypt foci and increase colonic tumor volume without affecting tumor numbers in IGF-II overexpressing mice, compared to the wild-type mice. Moreover, the authors found that transgenic overexpression of IGFBP-2, which displays high affinity for IGF-II, reduced the appearance of dysplastic aberrant crypt foci and inhibited tumor growth in the same model[125]. A number of studies have investigated the correlation between IGFR-related markers and gastric cancer risk. IGF-1 expression was markedly elevated from benign proliferative lesions to malignant lesions, compared to normal gastric mucous membrane, indicating that the increased expression of IGF-1 may be positively related to gastric carcino-genesis[126,127]. Gryko et al[128] found that the expression of IGF-1R was associated with lymph node metastasis and high histological malignancy grade, implicating IGF-1R as an independent predictor of survival in patients with gastric cancer. Tumor cells commonly express and secrete IGF-I and IGF-II, which act as autocrine growth factors to promote the initiation and development of tumors. The IGF signaling pathways involving multiple interaction components are extremely complex and form networks. It has been reported that the promotion of tumor growth and inhibition of apoptosis may be mediated by IGFR-activated pathways, such as the MAPK and PI3K/AKT pathways[129].
Phosphorylated IGF1R can stimulate the Ras-Raf-MEK-MAPK pathway, which regulates gene transcription and cellular metabolism, resulting in cell growth and survival. IGF1R can reportedly recruit PI3K to the cell membrane, leading to activation of the PI3K/AKT pathway, which ultimately plays a regulatory role in apoptosis and cellular proliferation[130]. Other studies have revealed that overexpre-ssed IGF-II can promote cell proliferation via the up-regulated expression of cyclooxygenase-2 and prostaglandins[131]. Theoretically, molecules that target the IGF pathway can act as a single therapeutic agent by itself, or can also enhance the activity of other cytotoxic chemotherapeutic drugs. Currently, there are many inhibitors of the IGF pathway that are under investigation.
FGFs and their receptors (FGFRs) have diverse functions that include cell prolifera-tion, migration, survival, regulation of angiogenesis, and tissue repair. Dysregulation of the FGF/FGFR signaling pathway has been associated with many developmental disorders and with cancer. Recent evidence suggests that any abnormalities in FGFs or FGFRs may promote multiple steps of cancer progression (proliferation, survival, tumor angiogenesis, and epithelial to mesenchymal transition) by gene amplification, chromosomal translocation, and mutations.
It has long been recognized that FGFRs are overexpressed in many cancer cell types, including hepatocellular carcinoma, breast cancer, gastric cancer, and CRC[132-134]. Several studies have identified that FGF18, FGF20, and SPRY4 are potent targets of the canonical WNT signaling pathway in GI tract cancer. The overexpre-ssion of FGFR has recently been shown to be associated with a poor prognosis and resistance to chemotherapeutic drugs. FGFR1/2 amplification remained a significant independent risk factor for poor disease-free survival and overall survival in poorly differentiated adenocarcinoma of the stomach[132,135,136]. However, others observed that expression of FGFR1–4 had different impacts on clinical outcomes in diffuse-type gastric cancer (DGC) and intestinal-type gastric cancer (IGC). High FGFR4 expression was positively correlated with the depth of invasion, lymph node metastasis, pathological stage, and distant metastasis in both DGC and IGC, while the elevated expression of FGFR1 and 2 was correlated with a poor outcome and survival in DGC[137,138]. FGF2 upregulation as well as CagA-dependent SHP2 activation promote pro-oncogenic factors, inducing healing of the gastric mucosal damage associated with H. pylori infection, which is a causative pathogen in gastric cancer[132]. The activation of FGF7-FGFR2 signaling cascade was reported to play a significant role in the development and progression of DGC[136]. The largest genomic analysis carried out to date revealed that FGFR2 amplification is always accompanied by deletion of the coding exon located proximal to the C-terminus[139]. In vitro studies revealed that GC cell lines overexpressing FGFR2 are highly sensitive to tyrosine kinase inhibitors and monoclonal antibodies directed at FGFR signaling[140]. FGFR2 has thus attracted considerable attention as a novel therapeutic candidate for the development of targeted anticancer agents. FGFR2 silencing significantly inhibited the growth and survival of Snu-16 (gastric cancer cell line) and resulted in tumor growth regression in vivo[141]. A recent study revealed that FGF8 was overexpressed in the majority of CRC tissues and was significantly correlated with lymph node metastasis and poor outcome[142]. It was further demonstrated that FGF8 could promote the proliferation and metastasis of CRC cells by activating YAP1. Serum basic FGF levels were associated with lymph node metastasis in cases of CRC[143]. A similar study showed that the overexpression of the FGFR-1 may promote liver metastasis in advanced CRC, suggesting that FGFR-1 may be a potential predictor of liver metastasis in CRC[144]. However, due to the complexity of CRC pathogenesis, the mechanism remains unclear. Moreover, a growing body of preclinical data shows that the inhibition of FGFR signaling can result in anti-proliferative and/or proapoptotic effects, both in vitro and in vivo, thus confirming the validity of the FGF/FGFR axis as a potential therapeutic target.
Growing evidence supports the view that the brain-gut axis is involved in tumor development, including tumor cell proliferation, invasion, and metastasis. Tumor cells could synthesize and release various kinds of neurotransmitters, which produce different biological effects by binding to their receptors on tumor cells. Mechanis-tically, the bidirectional signal transmission of the brain-gut-axis is complex and remains to be elucidated. Therefore, the neurobiological view of cancer might enable a better understanding of oncogenesis and open new prospects for cancer diagnosis and treatment. In this review, we have discussed the current findings and summarized the influences of anatomical nerve pathways (CNS and the sympathetic and parasympathetic nervous systems) and the neuroendocrine-immune system (several neurotransmitters and factors) on GI cancer growth. The simulation and inactivation of the CNS and the sympathetic and parasympathetic nervous systems, or changes in the innervation of the GI tract might alter the immunomodulatory influences of the vagal nerve, which could promote inflammatory processes and contribute to a higher incidence of cancers of the GI tract. Modulation of the neurotransmitters and the stimulation of signal transmission between nerves and tumor cells (e.g., electrical stimulation of nerves innervating tumors and β-blockers) might promote or inhibit GI cancer progression[145,146]. In addition, neurotransmitters and neurotrophic factors can exert either stimulatory or inhibitory effects on the progression of GI cancer (Figure 1). These effects may dependent on different types of tissues and neurotransmitters, as well as the subtypes of receptors of the neurotransmitters or neurotrophic factors involved (Table 1). Therefore, a better understanding of neurotransmitter or neuropeptide receptor activators, inhibitors, or antagonists for the treatment of cancer, together with modifications of corresponding receptors, may represent a promising therapeutic strategy for cancer in the future.
| Family | Member | Signaling receptor | Role in GI cancer |
| Neurotransmitters | Epinephrine (Adrenaline) | α/β adrenergic receptors | Promote proliferation, invasion, and angiogenesis |
| Acetylcholine | Muscarinic and nicotinic acetylcholine receptor | Increase stemness activity | |
| Promote migration and invasion | |||
| 5-hydroxytryptamine | 5-HT1-7 receptors | Promote mitogen and angiogenesis | |
| Dopamine | Dopamine 1–5 receptors | Suppress invasion and migration | |
| Reduce tumor microvessel density | |||
| Gamma-aminobutyric acid | GABAB GABAA, GABAA-ρ receptor | Reduce migration (GABAA) | |
| Promote proliferation (GABAB) | |||
| Neurotrophic factors | Brain-derived neurotrophic factor | TrkB | Promote proliferation and migration |
| Decrease apoptosis | |||
| Nerve growth factor | TrkA | Promote survival, proliferation, differentiation, and angiogenesis | |
| Involved in cancer-induced bone pain | |||
| Insulin-like growth factor | IGF type I/II receptor | Promote proliferation | |
| Fibroblast growth factors | FGFR-1–4 | Promote growth, survival, and metastasis |
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abadi ATB, Ali I, Herbella F, Tanida S S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Wang J
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21372] [Article Influence: 2137.2] [Reference Citation Analysis (3)] |
| 2. | Bhargava A, Bunkar N, Khare NK, Mishra D, Mishra PK. Nanoengineered strategies to optimize dendritic cells for gastrointestinal tumor immunotherapy: from biology to translational medicine. Nanomedicine (Lond). 2014;9:2187-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Haddad P, Mir MR, Jamali M, Abdirad A, Alikhasi A, Farhan F, Memari F, Sadighi S, Shahi F. Gastrointestinal tumor board: an evolving experience in Tehran Cancer Institute. Acta Med Iran. 2013;51:270-273. [PubMed] |
| 4. | Greaves M. Cancer causation: the Darwinian downside of past success? Lancet Oncol. 2002;3:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Dyer MA, Abramson DH. Mutations and cancer: one or two historical perspectives? Lancet Oncol. 2009;10:834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lang K, Entschladen F, Weidt C, Zaenker KS. Tumor immune escape mechanisms: impact of the neuroendocrine system. Cancer Immunol Immunother. 2006;55:749-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ondicova K, Mravec B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010;11:596-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin Ther. 2015;37:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 9. | Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 10. | Tsang SW, Auyeung KK, Bian ZX, Ko JK. Pathogenesis, Experimental Models and Contemporary Pharmacotherapy of Irritable Bowel Syndrome: Story About the Brain-Gut Axis. Curr Neuropharmacol. 2016;14:842-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Ondicova K, Pecenák J, Mravec B. The role of the vagus nerve in depression. Neuro Endocrinol Lett. 2010;31:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Wang X, Zhang H, Wang T, Lau WY, Wang X, Sun J, Yuan Z, Zhang Y. The concept and controversy of retroperitoneal nerve dissection in pancreatic head carcinoma (Review). Int J Oncol. 2015;47:2017-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Nomura A, Majumder K, Giri B, Dauer P, Dudeja V, Roy S, Banerjee S, Saluja AK. Inhibition of NF-kappa B pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab Invest. 2016;96:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Shirai K, Ebata T, Oda K, Nishio H, Nagasaka T, Nimura Y, Nagino M. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2008;32:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Rabben HL, Zhao CM, Hayakawa Y, Wang TC, Chen D. Vagotomy and Gastric Tumorigenesis. Curr Neuropharmacol. 2016;14:967-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Amit M, Na'ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016;16:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 17. | Duraker N, Sişman S, Can G. The significance of perineural invasion as a prognostic factor in patients with gastric carcinoma. Surg Today. 2003;33:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Matsushima T, Mori M, Kido A, Adachi Y, Sugimachi K. Preoperative estimation of neural invasion in rectal carcinoma. Oncol Rep. 1998;5:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, Berger DH, Albo D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131-5137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 21. | Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, Fuse A. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Chatterjee D, Katz MH, Rashid A, Wang H, Iuga AC, Varadhachary GR, Wolff RA, Lee JE, Pisters PW, Crane CH, Gomez HF, Abbruzzese JL, Fleming JB, Wang H. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, Katoh H. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Entschladen F, Palm D, Drell TL 4th, Lang K, Zaenker KS. Connecting a tumor to the environment. Curr Pharm Des. 2007;13:3440-3444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082-6090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 436] [Article Influence: 25.6] [Reference Citation Analysis (1)] |
| 28. | Tashiro M, Kubota K, Itoh M, Yoshioka T, Yoshida M, Nakagawa Y, Bereczki D, Sasaki H. Hypometabolism in the limbic system of cancer patients observed by positron emission tomography. Psychooncology. 1999;8:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Kergozien S, Delcros JG, Jouan H, Moulinoux JP. Induction of Fos protein expression in spinal cord neurons of tumour-bearing rats. Br J Cancer. 1999;80:1512-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Anisimov VN. Biology of aging and cancer. Cancer Control. 2007;14:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Phillips RJ, Walter GC, Powley TL. Age-related changes in vagal afferents innervating the gastrointestinal tract. Auton Neurosci. 2010;153:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Makale MT, Kesari S, Wrasidlo W. The autonomic nervous system and cancer. Biocybern Biomed Eng. 2017;37:443-452. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Tatsuta M, Iishi H, Baba M, Yano H, Sakai N, Uehara H, Hirasawa R, Nakaizumi A. Alpha1-adrenoceptor stimulation enhances experimental gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Int J Cancer. 1998;77:467-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Tatsuta M, Iishi H, Baba M, Taniguchi H. Inhibition of azoxymethane-induced experimental colon carcinogenesis in Wistar rats by 6-hydroxydopamine. Int J Cancer. 1992;50:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Kiba T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: recent developments. Pancreas. 2004;29:e51-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Caygill CP, Knowles RL, Hall R. Increased risk of cancer mortality after vagotomy for peptic ulcer: a preliminary analysis. Eur J Cancer Prev. 1991;1:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Ekbom A, Lundegårdh G, McLaughlin JK, Nyrén O. Relation of vagotomy to subsequent risk of lung cancer: population based cohort study. BMJ. 1998;316:518-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Erin N, Akdas Barkan G, Harms JF, Clawson GA. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters substance P level. Regul Pept. 2008;151:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 458] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 40. | Jenkins JT, Duncan JR, Hole D, O'Dwyer PJ, McGregor JR. Malignant disease in peptic ulcer surgery patients after long term follow-up: a cohort study of 1992 patients. Eur J Surg Oncol. 2007;33:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Abadi AT, Kusters JG. Management of Helicobacter pylori infections. BMC Gastroenterol. 2016;16:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Weissman-Fogel I, Dashkovsky A, Rogowski Z, Yarnitsky D. An animal model of chemotherapy-induced vagal neuropathy. Muscle Nerve. 2008;38:1634-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Mravec B, Gidron Y, Kukanova B, Bizik J, Kiss A, Hulin I. Neural-endocrine-immune complex in the central modulation of tumorigenesis: facts, assumptions, and hypotheses. J Neuroimmunol. 2006;180:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Heffner KL, Loving TJ, Robles TF, Kiecolt-Glaser JK. Examining psychosocial factors related to cancer incidence and progression: in search of the silver lining. Brain Behav Immun. 2003;17 Suppl 1:S109-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695-2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 47. | Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357-10364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 48. | Shin VY, Wu WK, Chu KM, Koo MW, Wong HP, Lam EK, Tai EK, Cho CH. Functional role of beta-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol Sci. 2007;96:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs. 2009;20:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Coelho M, Moz M, Correia G, Teixeira A, Medeiros R, Ribeiro L. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep. 2015;33:2513-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 51. | Liu X, Wu WK, Yu L, Sung JJ, Srivastava G, Zhang ST, Cho CH. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J Cell Biochem. 2008;105:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Mancino M, Ametller E, Gascón P, Almendro V. The neuronal influence on tumor progression. Biochim Biophys Acta. 2011;1816:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Al-Wadei HA, Schuller HM. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. 2009;218:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Otani Y, Kubota T, Sakurai Y, Igarashi N, Yokoyama T, Kimata M, Wada N, Kameyama K, Kumai K, Okada Y, Kitajima M. Expression of matrix metalloproteinases in gastric carcinoma and possibility of clinical application of matrix metalloproteinase inhibitor in vivo. Ann N Y Acad Sci. 1999;878:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Shi M, Liu D, Duan H, Han C, Wei B, Qian L, Chen C, Guo L, Hu M, Yu M, Song L, Shen B, Guo N. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer. 2010;9:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Schaal C, Padmanabhan J, Chellappan S. The Role of nAChR and Calcium Signaling in Pancreatic Cancer Initiation and Progression. Cancers (Basel). 2015;7:1447-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | DeVore NM, Scott EE. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes. J Biol Chem. 2012;287:26576-26585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Yang T, He W, Cui F, Xia J, Zhou R, Wu Z, Zhao Y, Shi M. MACC1 mediates acetylcholine-induced invasion and migration by human gastric cancer cells. Oncotarget. 2016;7:18085-18094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Xiang T, Fei R, Wang Z, Shen Z, Qian J, Chen W. Nicotine enhances invasion and metastasis of human colorectal cancer cells through the nicotinic acetylcholine receptor downstream p38 MAPK signaling pathway. Oncol Rep. 2016;35:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004;53:1520-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 61. | Grin A, Streutker CJ. Neuroendocrine tumors of the luminal gastrointestinal tract. Arch Pathol Lab Med. 2015;139:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Cattaneo MG, Fesce R, Vicentini LM. Mitogenic effect of serotonin in human small cell lung carcinoma cells via both 5-HT1A and 5-HT1D receptors. Eur J Pharmacol. 1995;291:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Tutton PJ, Barkla DH. The influence of serotonin on the mitotic rate in the colonic crypt epithelium and in colonic adenocarcinoma in rats. Clin Exp Pharmacol Physiol. 1978;5:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Tutton PJ, Barkla DH. Influence of inhibitors of serotonin uptake on intestinal epithelium and colorectal carcinomas. Br J Cancer. 1982;46:260-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Vicaut E, Laemmel E, Stücker O. Impact of serotonin on tumour growth. Ann Med. 2000;32:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Baguley BC, Cole G, Thomsen LL, Li Z. Serotonin involvement in the antitumour and host effects of flavone-8-acetic acid and 5,6-dimethylxanthenone-4-acetic acid. Cancer Chemother Pharmacol. 1993;33:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | El-Salhy M, Sitohy B, Norrgård O. Triple therapy with octreotide, galanin, and serotonin reduces the size and blood vessel density and increases apoptosis of a rat colon carcinoma. Regul Pept. 2003;111:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Rubí B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance. Endocrinology. 2010;151:5570-5581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 69. | Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2113] [Cited by in RCA: 1914] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 70. | Basu S, Dasgupta PS. Alteration of dopamine D2 receptors in human malignant stomach tissue. Dig Dis Sci. 1997;42:1260-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Kou X, Han Y, Yang D, Liu Y, Fu J, Zheng S, He D, Zhou L, Zeng C. Dopamine d(1)-like receptors suppress proliferation of vascular smooth muscle cell induced by insulin-like growth factor-1. Clin Exp Hypertens. 2014;36:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Leng ZG, Lin SJ, Wu ZR, Guo YH, Cai L, Shang HB, Tang H, Xue YJ, Lou MQ, Zhao W, Le WD, Zhao WG, Zhang X, Wu ZB. Activation of DRD5 (dopamine receptor D5) inhibits tumor growth by autophagic cell death. Autophagy. 2017;13:1404-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Huang H, Wu K, Ma J, Du Y, Cao C, Nie Y. Dopamine D2 receptor suppresses gastric cancer cell invasion and migration via inhibition of EGFR/AKT/MMP-13 pathway. Int Immunopharmacol. 2016;39:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Moreno-Smith M, Lee SJ, Lu C, Nagaraja AS, He G, Rupaimoole R, Han HD, Jennings NB, Roh JW, Nishimura M, Kang Y, Allen JK, Armaiz GN, Matsuo K, Shahzad MM, Bottsford-Miller J, Langley RR, Cole SW, Lutgendorf SK, Siddik ZH, Sood AK. Biologic effects of dopamine on tumor vasculature in ovarian carcinoma. Neoplasia. 2013;15:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2188] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 76. | Cooke EJ, Zhou JY, Wyseure T, Joshi S, Bhat V, Durden DL, Mosnier LO, Drygalski AV. Vascular Permeability and Remodelling Coincide with Inflammatory and Reparative Processes after Joint Bleeding in Factor VIII-Deficient Mice. Thromb Haemost. 2018;118:1036-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002;22:5321-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Jansen A, Hoepfner M, Herzig KH, Riecken EO, Scherübl H. GABA(C) receptors in neuroendocrine gut cells: a new GABA-binding site in the gut. Pflugers Arch. 2000;441:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Glassmeier G, Herzig KH, Höpfner M, Lemmer K, Jansen A, Scherubl H. Expression of functional GABAA receptors in cholecystokinin-secreting gut neuroendocrine murine STC-1 cells. J Physiol. 1998;510:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Szczaurska K, Mazurkiewicz M, Opolski A. [The role of GABA-ergic system in carcinogenesis]. Postepy Hig Med Dosw. 2003;57:485-500. [PubMed] |
| 82. | Watanabe M, Maemura K, Oki K, Shiraishi N, Shibayama Y, Katsu K. Gamma-aminobutyric acid (GABA) and cell proliferation: focus on cancer cells. Histol Histopathol. 2006;21:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 83. | Moon MS, Cho EW, Byun HS, Jung IL, Kim IG. GAD 67KD antisense in colon cancer cells inhibits cell growth and sensitizes to butyrate and pH reduction and H2O2 and gamma-radiation. Arch Biochem Biophys. 2004;430:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Zomot E, Kanner BI. The interaction of the gamma-aminobutyric acid transporter GAT-1 with the neurotransmitter is selectively impaired by sulfhydryl modification of a conformationally sensitive cysteine residue engineered into extracellular loop IV. J Biol Chem. 2003;278:42950-42958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Tatsuta M, Iishi H, Baba M, Nakaizumi A, Uehara H, Taniguchi H. Effect of gamma-butyrolactone on baclofen inhibition of gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Oncology. 1992;49:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Maemura K, Shiraishi N, Sakagami K, Kawakami K, Inoue T, Murano M, Watanabe M, Otsuki Y. Proliferative effects of gamma-aminobutyric acid on the gastric cancer cell line are associated with extracellular signal-regulated kinase 1/2 activation. J Gastroenterol Hepatol. 2009;24:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Mazurkiewicz M, Opolski A, Wietrzyk J, Radzikowski C, Kleinrok Z. GABA level and GAD activity in human and mouse normal and neoplastic mammary gland. J Exp Clin Cancer Res. 1999;18:247-253. [PubMed] |
| 89. | Li YH, Liu Y, Li YD, Liu YH, Li F, Ju Q, Xie PL, Li GC. GABA stimulates human hepatocellular carcinoma growth through overexpressed GABAA receptor theta subunit. World J Gastroenterol. 2012;18:2704-2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, Nakagawa H. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67:9704-9712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Bixby JL, Harris WA. Molecular mechanisms of axon growth and guidance. Annu Rev Cell Biol. 1991;7:117-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 204] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 983] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 93. | Okamura K, Harada T, Wang S, Ijichi K, Furuyama K, Koga T, Okamoto T, Takayama K, Yano T, Nakanishi Y. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer. 2012;78:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | Yang X, Martin TA, Jiang WG. Biological influence of brain-derived neurotrophic factor (BDNF) on colon cancer cells. Exp Ther Med. 2013;6:1475-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Yang X, Martin TA, Jiang WG. Biological influence of brain-derived neurotrophic factor on breast cancer cells. Int J Oncol. 2012;41:1541-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Okugawa Y, Tanaka K, Inoue Y, Kawamura M, Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, Mohri Y, Kusunoki M. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108:121-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 97. | Huang SM, Lin C, Lin HY, Chiu CM, Fang CW, Liao KF, Chen DR, Yeh WL. Brain-derived neurotrophic factor regulates cell motility in human colon cancer. Endocr Relat Cancer. 2015;22:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | Vanhecke E, Adriaenssens E, Verbeke S, Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X, Hondermarck H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin Cancer Res. 2011;17:1741-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 99. | Choi B, Lee EJ, Shin MK, Park YS, Ryu MH, Kim SM, Kim EY, Lee HK, Chang EJ. Upregulation of brain-derived neurotrophic factor in advanced gastric cancer contributes to bone metastatic osteolysis by inducing long pentraxin 3. Oncotarget. 2016;7:55506-55517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Brunetto de Farias C, Rosemberg DB, Heinen TE, Koehler-Santos P, Abujamra AL, Kapczinski F, Brunetto AL, Ashton-Prolla P, Meurer L, Reis Bogo M, Damin DC, Schwartsmann G, Roesler R. BDNF/TrkB content and interaction with gastrin-releasing peptide receptor blockade in colorectal cancer. Oncology. 2010;79:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, Poon RT. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: implication in hepatocellular carcinoma. Clin Cancer Res. 2011;17:3123-3133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | Au CW, Siu MK, Liao X, Wong ES, Ngan HY, Tam KF, Chan DC, Chan QK, Cheung AN. Tyrosine kinase B receptor and BDNF expression in ovarian cancers - Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009;281:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 103. | Demir IE, Tieftrunk E, Schorn S, Friess H, Ceyhan GO. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim Biophys Acta. 2016;1866:37-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 104. | Krüttgen A, Schneider I, Weis J. The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol. 2006;16:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Descamps S, Lebourhis X, Delehedde M, Boilly B, Hondermarck H. Nerve growth factor is mitogenic for cancerous but not normal human breast epithelial cells. J Biol Chem. 1998;273:16659-16662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864-17870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 107. | Uchida N, Kanazawa M, Suzuki Y, Takeda M. Expression of BDNF and TrkB in mouse taste buds after denervation and in circumvallate papillae during development. Arch Histol Cytol. 2003;66:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin CS, Gershon MD, Hara A, Zhao CM, Chen D, Worthley DL, Koike K, Wang TC. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 109. | Noh SJ, Kim KM, Jang KY. Individual and co-expression patterns of nerve growth factor and heme oxygenase-1 predict shorter survival of gastric carcinoma patients. Diagn Pathol. 2017;12:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Wang CZ, Luo X, Zhang B, Song WX, Ni M, Mehendale S, Xie JT, Aung HH, He TC, Yuan CS. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol. 2007;60:69-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 112. | McKelvey L, Shorten GD, O'Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem. 2013;124:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 113. | Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14:R101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Shinoda M, Honda T, Ozaki N, Hattori H, Mizutani H, Ueda M, Sugiura Y. Nerve terminals extend into the temporomandibular joint of adjuvant arthritic rats. Eur J Pain. 2003;7:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Lucchesi M, Lanzetta G, Antonuzzo A, Rozzi A, Sardi I, Favre C, Ripamonti CI, Santini D, Armento G. Developing drugs in cancer-related bone pain. Crit Rev Oncol Hematol. 2017;119:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Ekman EF, Gimbel JS, Bello AE, Smith MD, Keller DS, Annis KM, Brown MT, West CR, Verburg KM. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol. 2014;41:2249-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 117. | Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 118. | Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Pollak MN. Insulin-like growth factors and neoplasia. Novartis Found Symp. 2004;262:84-98; discussion 98-107, 265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | LeRoith D, Werner H, Beitner-Johnson D, Roberts CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 914] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 121. | Ewing GP, Goff LW. The insulin-like growth factor signaling pathway as a target for treatment of colorectal carcinoma. Clin Colorectal Cancer. 2010;9:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Nosho K, Yamamoto H, Taniguchi H, Adachi Y, Yoshida Y, Arimura Y, Endo T, Hinoda Y, Imai K. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin Cancer Res. 2004;10:7950-7957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 124. | Tricoli JV, Rall LB, Karakousis CP, Herrera L, Petrelli NJ, Bell GI, Shows TB. Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986;46:6169-6173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 125. | Diehl D, Hessel E, Oesterle D, Renner-Müller I, Elmlinger M, Langhammer M, Göttlicher M, Wolf E, Lahm H, Hoeflich A. IGFBP-2 overexpression reduces the appearance of dysplastic aberrant crypt foci and inhibits growth of adenomas in chemically induced colorectal carcinogenesis. Int J Cancer. 2009;124:2220-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Ennishi D, Shitara K, Ito H, Hosono S, Watanabe M, Ito S, Sawaki A, Yatabe Y, Yamao K, Tajima K, Tanimoto M, Tanaka H, Hamajima N, Matsuo K. Association between insulin-like growth factor-1 polymorphisms and stomach cancer risk in a Japanese population. Cancer Sci. 2011;102:2231-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 127. | Wang HB, Zhou CJ, Song SZ, Chen P, Xu WH, Liu B, Zhu KX, Yu WH, Wu HL, Wang HJ, Lin S, Guo JQ, Qin CY. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol Res Pract. 2011;207:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Gryko M, Kiśluk J, Cepowicz D, Zińczuk J, Kamocki Z, Guzińska-Ustymowicz K, Pryczynicz A, Czyżewska J, Kemona A, Kędra B. Expression of insulin-like growth factor receptor type 1 correlate with lymphatic metastases in human gastric cancer. Pol J Pathol. 2014;65:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 129. | Rosenthal SM, Cheng ZQ. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci U S A. 1995;92:10307-10311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 130. | Sekharam M, Zhao H, Sun M, Fang Q, Zhang Q, Yuan Z, Dan HC, Boulware D, Cheng JQ, Coppola D. Insulin-like growth factor 1 receptor enhances invasion and induces resistance to apoptosis of colon cancer cells through the Akt/Bcl-x(L) pathway. Cancer Res. 2003;63:7708-7716. [PubMed] |
| 131. | Di Popolo A, Memoli A, Apicella A, Tuccillo C, di Palma A, Ricchi P, Acquaviva AM, Zarrilli R. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene. 2000;19:5517-5524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Katoh M, Katoh M. FGF signaling network in the gastrointestinal tract (review). Int J Oncol. 2006;29:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 133. | Sandhu DS, Baichoo E, Roberts LR. Fibroblast growth factor signaling in liver carcinogenesis. Hepatology. 2014;59:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 134. | Bedussi F, Bottini A, Memo M, Fox SB, Sigala S, Generali D. Targeting fibroblast growth factor receptor in breast cancer: a promise or a pitfall? Expert Opin Ther Targets. 2014;18:665-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 135. | Stemmermann G, Heffelfinger SC, Noffsinger A, Hui YZ, Miller MA, Fenoglio-Preiser CM. The molecular biology of esophageal and gastric cancer and their precursors: oncogenes, tumor suppressor genes, and growth factors. Hum Pathol. 1994;25:968-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 136. | Yoshida N, Ishikawa T, Ichiishi E, Yoshida Y, Hanashiro K, Kuchide M, Uchiyama K, Kokura S, Ichikawa H, Naito Y, Yamamura Y, Okanoue T, Yoshikawa T. The effect of rebamipide on Helicobacter pylori extract-mediated changes of gene expression in gastric epithelial cells. Aliment Pharmacol Ther. 2003;18 Suppl 1:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 137. | Inokuchi M, Murase H, Otsuki S, Kawano T, Kojima K. Different clinical significance of FGFR1-4 expression between diffuse-type and intestinal-type gastric cancer. World J Surg Oncol. 2017;15:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 138. | Inokuchi M, Fujimori Y, Otsuki S, Sato Y, Nakagawa M, Kojima K. Therapeutic targeting of fibroblast growth factor receptors in gastric cancer. Gastroenterol Res Pract. 2015;2015:796380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 139. | Ueda T, Sasaki H, Kuwahara Y, Nezu M, Shibuya T, Sakamoto H, Ishii H, Yanagihara K, Mafune K, Makuuchi M, Terada M. Deletion of the carboxyl-terminal exons of K-sam/FGFR2 by short homology-mediated recombination, generating preferential expression of specific messenger RNAs. Cancer Res. 1999;59:6080-6086. [PubMed] |
| 140. | Zhao WM, Wang L, Park H, Chhim S, Tanphanich M, Yashiro M, Kim KJ. Monoclonal antibodies to fibroblast growth factor receptor 2 effectively inhibit growth of gastric tumor xenografts. Clin Cancer Res. 2010;16:5750-5758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 141. | Lai WT, Krishnappa V, Phinney DG. Fibroblast growth factor 2 (Fgf2) inhibits differentiation of mesenchymal stem cells by inducing Twist2 and Spry4, blocking extracellular regulated kinase activation, and altering Fgf receptor expression levels. Stem Cells. 2011;29:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 142. | Liu R, Huang S, Lei Y, Zhang T, Wang K, Liu B, Nice EC, Xiang R, Xie K, Li J, Huang C. FGF8 promotes colorectal cancer growth and metastasis by activating YAP1. Oncotarget. 2015;6:935-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 143. | Jibiki N, Saito N, Kameoka S, Kobayashi M. Clinical significance of fibroblast growth factor (FGF) expression in colorectal cancer. Int Surg. 2014;99:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 144. | Sato T, Oshima T, Yoshihara K, Yamamoto N, Yamada R, Nagano Y, Fujii S, Kunisaki C, Shiozawa M, Akaike M, Rino Y, Tanaka K, Masuda M, Imada T. Overexpression of the fibroblast growth factor receptor-1 gene correlates with liver metastasis in colorectal cancer. Oncol Rep. 2009;21:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 145. | Siddiqui EJ, Thompson CS, Mikhailidis DP, Mumtaz FH. The role of serotonin in tumour growth (review). Oncol Rep. 2005;14:1593-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Mu J, Huang W, Tan Z, Li M, Zhang L, Ding Q, Wu X, Lu J, Liu Y, Dong Q, Xu H. Dopamine receptor D2 is correlated with gastric cancer prognosis. Oncol Lett. 2017;13:1223-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |