Published online May 26, 2019. doi: 10.12998/wjcc.v7.i10.1155
Peer-review started: December 29, 2018
First decision: March 10, 2019
Revised: March 21, 2019
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: May 26, 2019
Processing time: 148 Days and 18.4 Hours
Mucormycosis is a very rare fungal infection, and its prognosis is poor. Most common sites of infection are the sinuses, lung, or skin, and gastric involvement is uncommon. The standard antifungal therapy is the treatment of choice for gastric mucormycosis. However, the symptoms of gastric mucormycosis are varied and the early diagnosis is not easy.
I report a 53-year-old alcoholic man, who was admitted due to epigastric pain. The upper gastrointestinal endoscopy revealed a huge ulcer lesion in the stomach, which was suspected to be gastric cancer. F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) showed diffusely intense FDG uptake at the ulcer lesion of the stomach, and several enlarged hypermetabolic lymph nodes were noted at the left gastric chain. Although, endoscopy and F-18 FDG PET/CT findings suggested advanced gastric cancer with regional lymph node metastases, there was no cancer cells in the biopsy results and multiple fungal hyphae were noted in the periodic acid-Schiff stained image.
He was diagnosed with gastric mucormycosis and successfully underwent amphotericin B and posaconazole treatment.
Core tip: Gastric mucormycosis is very rare, and there have been no reports on the F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) finding of gastric mucormycosis. Since, endoscopic findings of gastric mucormycosis reveal ulcer margin with inflammatory/necrotic debris and hemorrhagic clots, F-18 FDG PET/CT of gastric mucormycosis reveal increased FDG uptake in the infected site. Additionally, reactive enlarged hypermetabolic lymph nodes in the perigastric chain could be observed. In this regard, it was difficult to differentiate gastric mucormycosis and gastric cancer by endoscopic findings and radiologic examinations. Therefore, careful consideration of clinical manifestations and precise diagnosis are necessary for optimal treatment of gastric mucormycosis.
- Citation: Song BI. F-18 fluorodeoxyglucose positron emission tomography/computed tomography image of gastric mucormycosis mimicking advanced gastric cancer: A case report. World J Clin Cases 2019; 7(10): 1155-1160
- URL: https://www.wjgnet.com/2307-8960/full/v7/i10/1155.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i10.1155
Mucormycosis is a life-threatening angioinvasive infection caused by fungi in the order Mucorales. It has the characteristic of invading the blood vessels; thus, the prognosis is known to be very poor. Like other fungal infections, patients with mucormycosis may have several predisposing factors associated with immunosu-ppressive conditions such as diabetes or long-term corticosteroid use. Additionally, several studies reported that chronic alcoholism can be one of the important predisposing conditions[1].
The three common sites of mucormycosis are the sinuses (39%), lung (24%), and skin (19%). Mucormycosis less commonly occurs in the gastrointestinal (GI) tract (about 7%)[2]. In the GI tract, mucormycosis most commonly affects the stomach followed by the colon and ileum[2]. Pathogens are known to be acquired by ingestion of contaminated foods.
The treatment for gastric mucormycosis is amphotericin B, posaconazole, and adjuvant surgical removal of the “fungal ball”. The mortality rate is known to be extremely high, which is about 85%[3]. One of the major causes of gastric mucor-mycosis mortality is extremely delayed diagnoses; only 25% of patients with GI mucormycosis are diagnosed before death. Recently, several studies emphasized the importance of early diagnosis, which can affect the treatment direction and improve prognosis[4].
It is difficult to diagnose gastric mucormycosis because confirmation is possible only through histologic examination. In addition, some cases have been misdiagnosed as cancer during the initial screening due to their radiologic and endoscopic similarities. Several studies have reported the detection of mucormycosis in other organs using F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT); however, there have been no reports on the F-18 FDG PET/CT finding of gastric mucormycosis. We present an interesting case of a 53-year-old man who underwent F-18 FDG PET/CT and were initially suspected with advanced gastric cancer but finally confirmed as gastric mucormycosis.
A 53-year-old man presented with a 1-wk history of epigastric pain. He also had complaints of bloody vomitus and melena.
Three weeks prior to this hospital admission, abdominal discomfort and nausea had developed. He had visited the local hospital and received conservative treatment. However, the symptoms were not relieved after medication. A week ago, epigastric pain had developed, and he had vomited reddish blood on the day before admission. For further evaluation, the patient was transferred to this hospital.
He had no history of surgery and past medical history.
The patient had a history of severe alcohol abuse and a 20 pack-year history of smoking. Otherwise, there were no special circumstances in family history.
The physical examination revealed no palpable abdominal mass or signs of peritoneal irritation. His blood pressure was 153/120 mmHg, and pulse rate was 115/min. He appeared chronically ill.
The laboratory testing showed a normal white blood cell count, blood glucose, creatinine, coagulation profile, and serum electrolytes except low hemoglobin was present with a level of 10.5 g/dL (normal range: 13.5-17.5 g/dL).
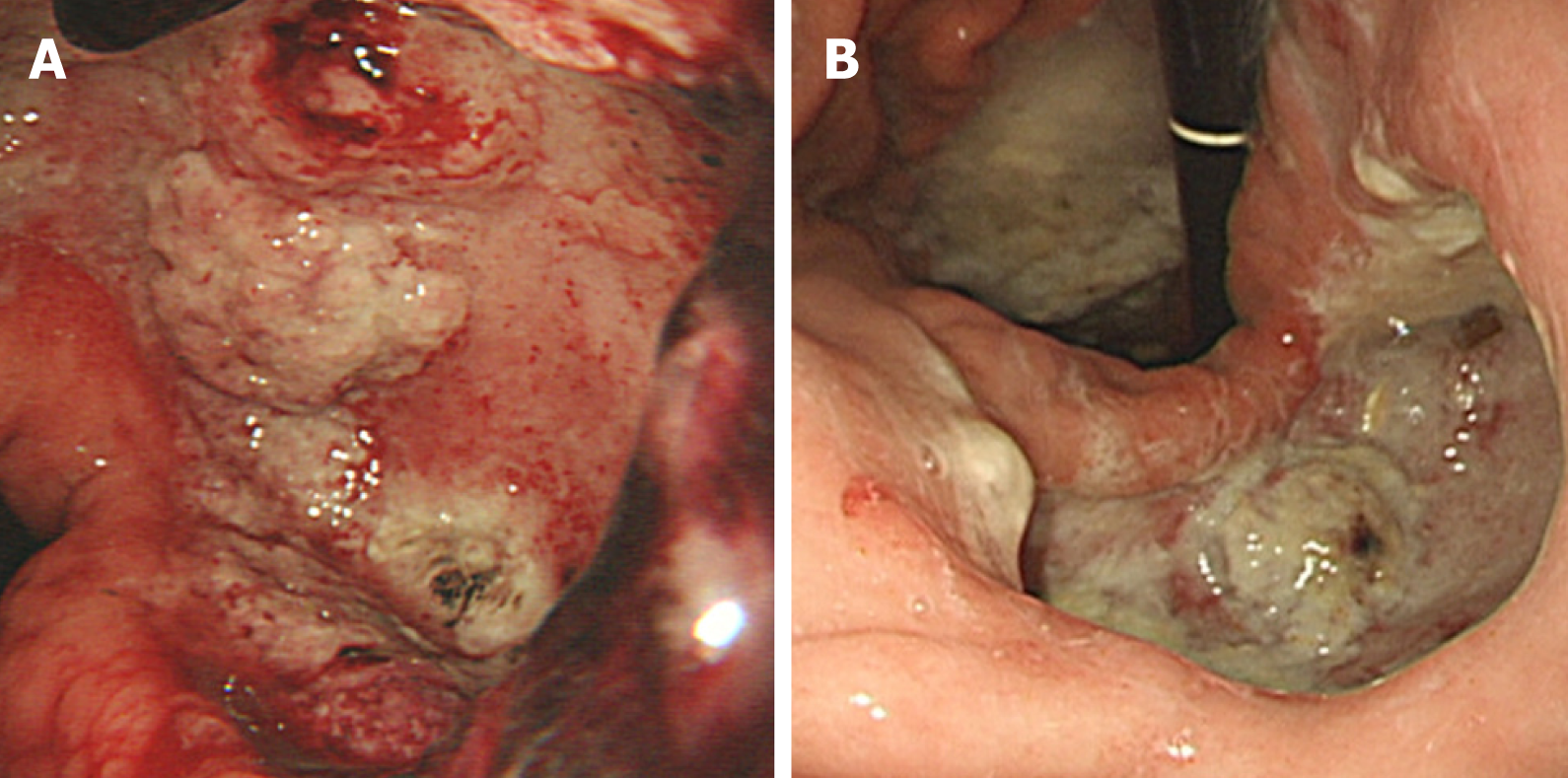
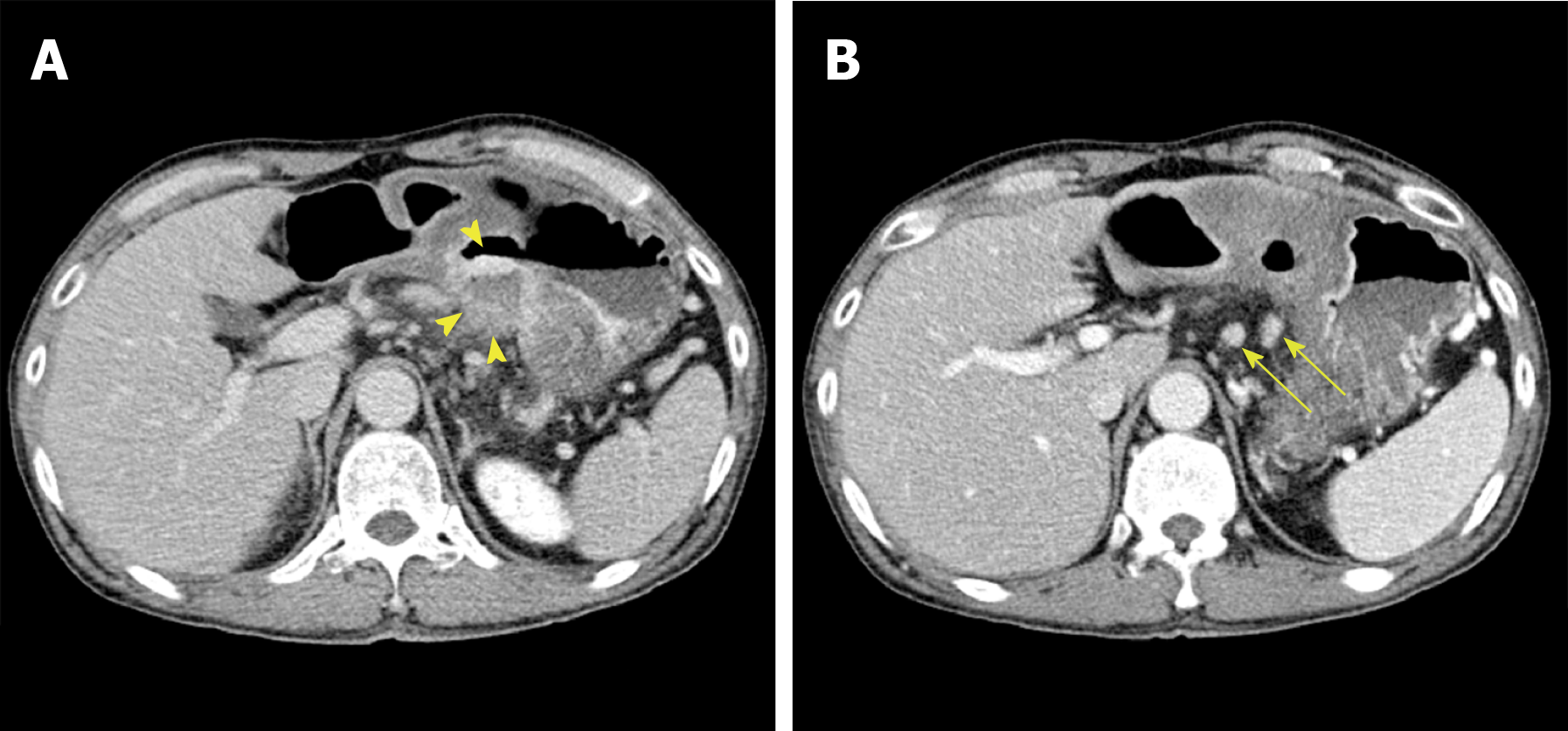
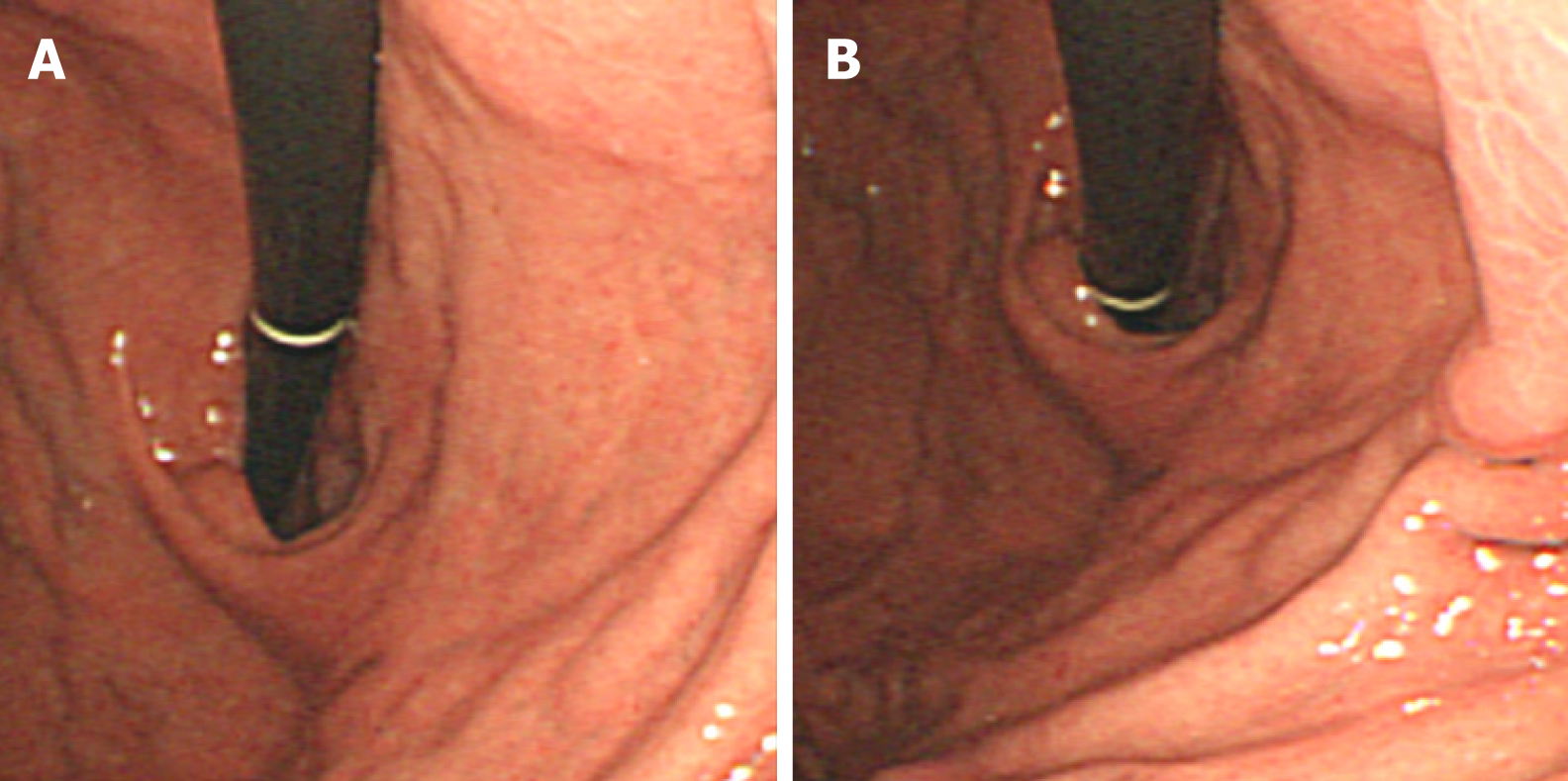
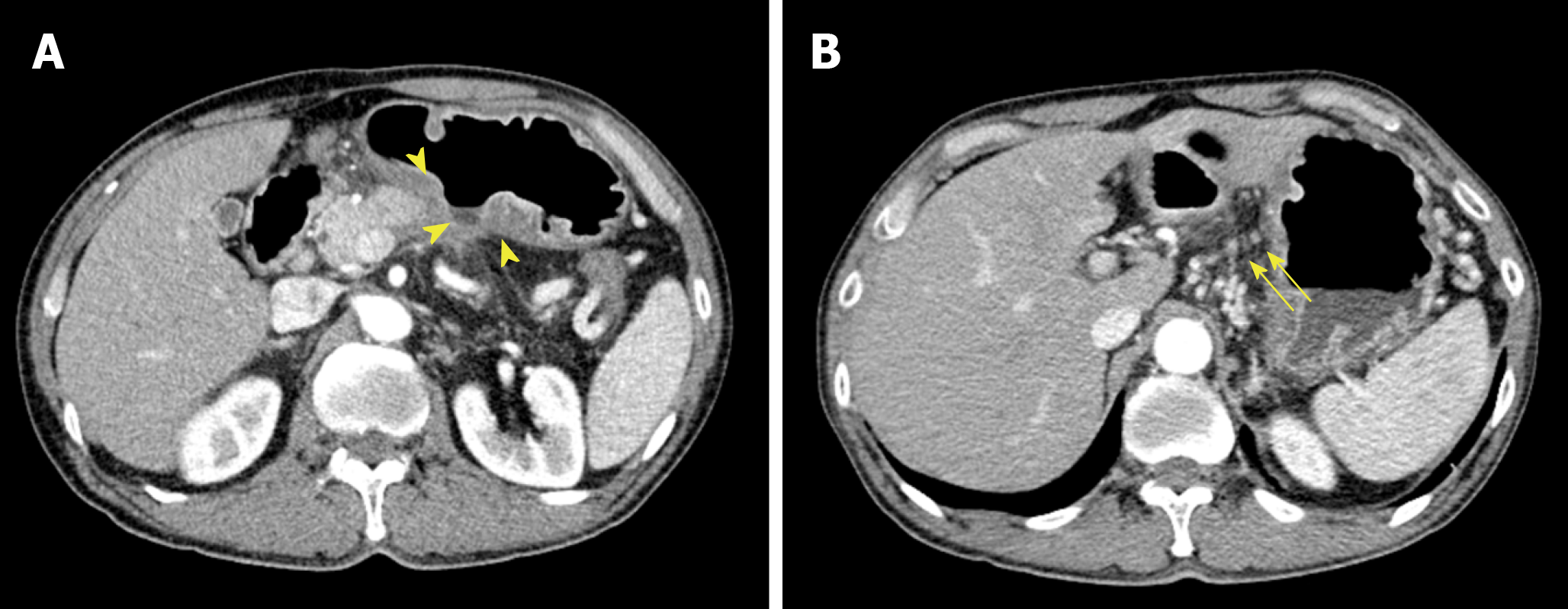
The patient’s upper GI endoscopy revealed diffuse erythema and edema of the gastric wall. Furthermore, a huge ulcer was detected in the posterior wall of the lower body (Figure 1), which was suspicious for Borrmann type II gastric cancer; therefore, patient underwent biopsy and additional studies for evaluation. Moreover, the contrast-enhanced abdominal CT showed a huge ulcer within the same lesion with diffuse wall thickening (Figure 2A). The margin of the pancreas was unclear, and multiple enlarged lymph nodes were observed in the left gastric and gastroepiploic chain (Figure 2B).
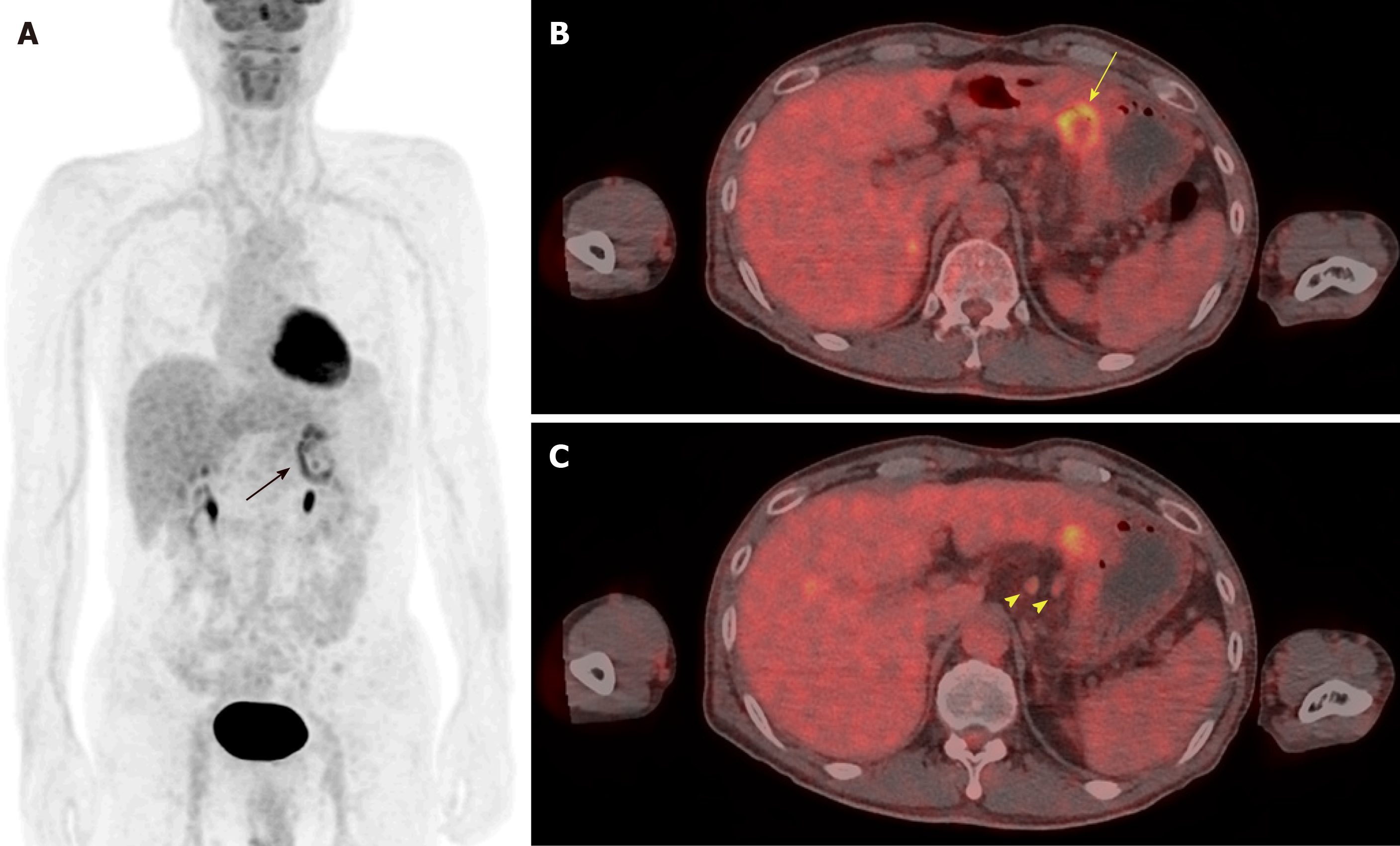
Because these findings strongly suggested gastric cancer at advanced stage, F-18 FDG PET/CT was performed for further evaluation. In the maximum intensity projection image, there was uneven FDG uptake on the stomach, but no other distant metastasis was seen (Figure 3A). Trans-axial F-18 FDG PET/CT image showed diffusely intense FDG uptake at the ulcer lesion (Figure 3B). In addition, several enlarged hypermetabolic lymph nodes were noted at the left gastric chain (Figure 3C). The clinical impression was gastric cancer with regional lymph node metastases.
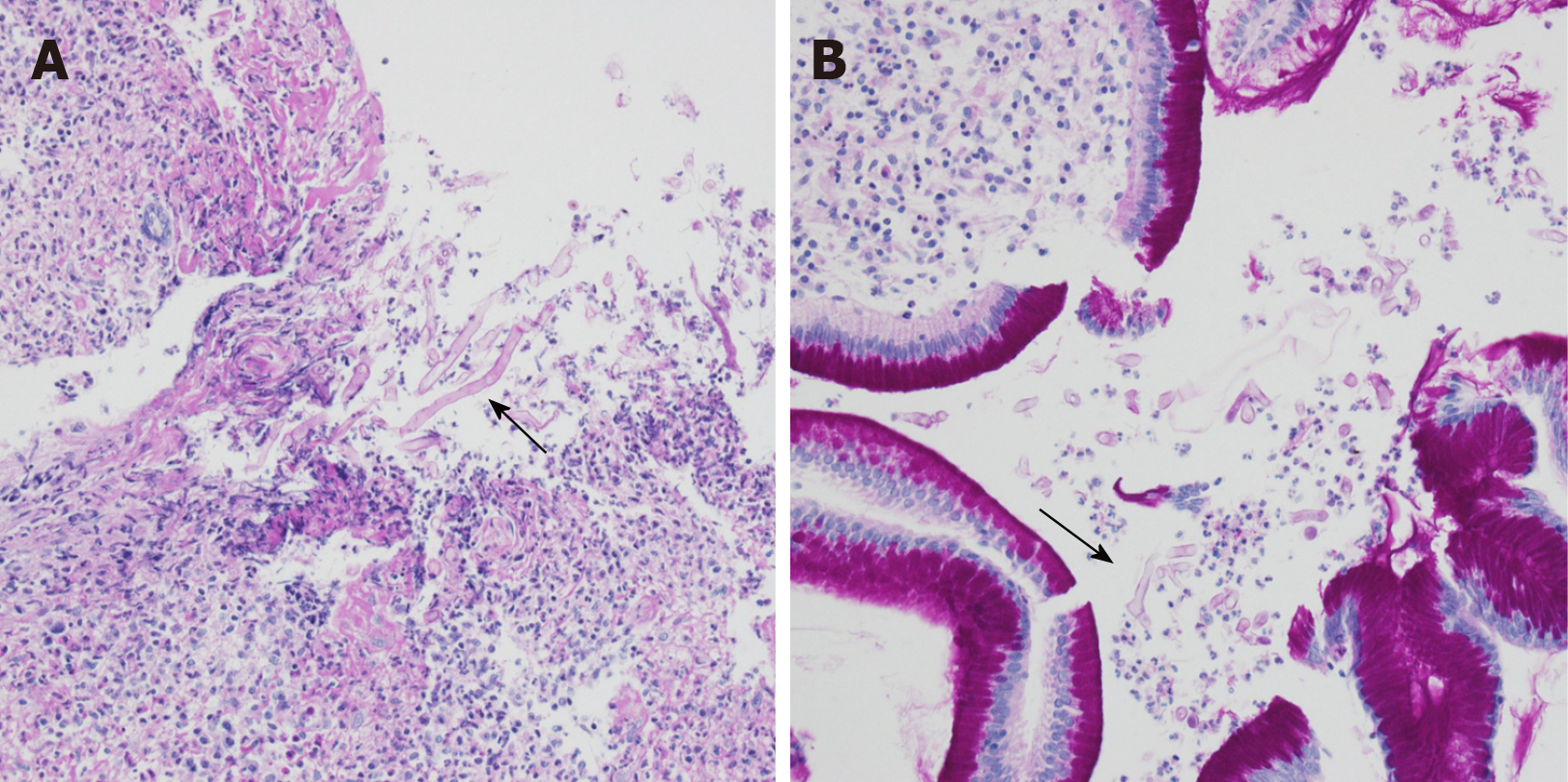
However, there were no malignant cells and only severe inflammation was seen in the first biopsy result. The patient underwent secondary endoscopic biopsy for a pathologic confirmation. However, no cancer cells were found, and multiple fungal hyphae were noted in the periodic acid-Schiff (PAS)-stained image. The results indicated mucormycosis based on the characteristics of the hyphae, which showed a 90-degree angle branching with no visible septa (Figure 4).
The final diagnosis of the presented case is gastric mucormycosis. Unfortunately, the type of mucormycosis remained undetermined.
The patient was treated with amphotericin B, given by injection, for 20 d and adjuvant posaconazole for 2 mo.
The follow-up endoscopy finding showed marked improvement of previous infected lesions and only mild redness of the gastric wall was observed (Figure 5). In addition, the follow-up CT showed much-improved state of previous gastric mucormycosis lesion and decreased size and number of previous enlarged lymph nodes (Figure 6). Thereafter, the therapy was terminated without further treatment.
With regard to the diagnosis, gastric mucormycosis has no specific finding on radiologic examination. In contrast-enhanced CT image, irregular thickening of the gastric folds can be seen with ulcer forming process[5]. In endoscopic examination, gastric mucormycosis appears as necrotizing ulcers combined with discoloration or blood clot. Because of the necrotizing tissues, gastric mucormycosis is often misdia-gnosed as gastric cancer[6,7].
Diagnostic confirmation of gastric mucormycosis can be made only through histo-logical examination. Fungal infections are best observed in Gomori methenamine silver or PAS stain. The pathologic finding is large and non-septa hyphae with 90-degree angle branching. Additionally, the three subtypes of gastric mucormycosis are detected through histological examination. The first type only exhibits the formation of a colony without invading the surrounding tissue. The second type involves infil-tration into the surrounding tissue as a presenting case. Lastly, the third type involves vascular invasion with presence of hyphae in the vessel lumen[8]. The vascular invasion type is frequently associated with poor prognosis.
Although several studies have reported on the F-18 FDG PET/CT findings of patients with mucormycosis, the F-18 FDG PET/CT findings of patients with gastric mucormycosis has not been reported. Increased FDG uptake at the infection focus was noted at the infection site, but there was no specific finding[9]. The F-18 FDG PET/CT finding of gastric mucormycosis in this patient also showed increased FDG uptake at the infection site. Therefore, it was difficult to differentiate gastric mucormycosis and gastric cancer by F-18 FDG PET/CT. However, as the importance of early diagnosis has been increasingly emphasized recently, some studies revealed that F-18 FDG PET/CT can be used to detect active metabolic changes reflecting inflammatory cell activity before the onset of anatomical abnormalities[10]. Furthermore, F-18 FDG PET/CT can give functional information, which can useful for the assessment of the overall therapeutic response[11].
The case report suggests that there is no definite method to completely differentiate mucormycosis and malignant tumors using F-18 FDG PET/CT. However, F-18 FDG PET/CT may contribute to the early diagnosis, assessment of disease extent, and evaluation of response to therapy in patients with gastric mucormycosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eleftheriadis NP, Tsolakis AV S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Ho YH, Wu BG, Chen YZ, Wang LS. Gastric Mucormycosis in an Alcoholic with Review of the Literature. Tzu Chi Med J. 2007;19:169–172. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S23-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 866] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 3. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1968] [Article Influence: 98.4] [Reference Citation Analysis (1)] |
| 4. | Walsh TJ, Skiada A, Cornely OA, Roilides E, Ibrahim A, Zaoutis T, Groll A, Lortholary O, Kontoyiannis DP, Petrikkos G. Development of new strategies for early diagnosis of mucormycosis from bench to bedside. Mycoses. 2014;57 Suppl 3:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Samet JD, Horton KM, Fishman EK. Invasive gastric mucormycosis: CT findings. Emerg Radiol. 2008;15:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Thomson SR, Bade PG, Taams M, Chrystal V. Gastrointestinal mucormycosis. Br J Surg. 1991;78:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | El Hachem G, Chamseddine N, Saidy G, Choueiry C, Afif C. Successful Nonsurgical Eradication of Invasive Gastric Mucormycosis. Clin Lymphoma Myeloma Leuk. 2016;16 Suppl:S145-S148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Feldman M, Friedman L, Brandt L. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 2016;875. |
| 9. | Sharma P, Mukherjee A, Karunanithi S, Bal C, Kumar R. Potential role of 18F-FDG PET/CT in patients with fungal infections. AJR Am J Roentgenol. 2014;203:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Altini C, Niccoli Asabella A, Ferrari C, Rubini D, Dicuonzo F, Rubini G. (18)F-FDG PET/CT contribution to diagnosis and treatment response of rhino-orbital-cerebral mucormycosis. Hell J Nucl Med. 2015;18:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Wu H, Huang F, Fan Z, Xu B. Utility of 18F-FDG PET/CT in diagnosis and management of mucormycosis. Clin Nucl Med. 2013;38:e370-e371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |