Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.95
Peer-review started: September 6, 2018
First decision: October 19, 2018
Revised: October 28, 2018
Accepted: December 7, 2018
Article in press: December 8, 2018
Published online: January 6, 2019
Processing time: 121 Days and 21.6 Hours
Pulmonary protozoal infections are rare. A 28-year-old woman was admitted to hospital with chief complains of cough, sputum, and dyspnea. The clinical laboratory tests for blood revealed an increased eosinophil percentage of 31.3% and significantly elevated total IgE. The chest computed tomography scan revealed that bilateral bronchial walls were thickening, accompanied with patchy spots scattered throughout bilateral lungs. A suspected multiflagellated protozoan was observed under a light microscope. But some different features were observed by electron microscopy, such as the orientation of flagella and nucleus. Besides, both bronchoalveolar lavage fluid and bronchoscopic brush smears underwent Gram staining and Pap staining, which revealed that numerous respiratory ciliated cells were scattered or accumulated in the sample. Finally, she was diagnosed with eosinophil pneumonia. Metronidazole, bronchodilators, and mucolytics were taken for 5 d and symptoms and pulmonary ventilation function improved. We herein report a case of chronic eosinophilic pneumonia, which was misdiagnosed as multiflagellated protozoan infection, and it is suggested that reliable diagnosis approaches are necessary, rather than clinical symptoms and morphological features.
Core tip: Lophomonas blattarum is a rare cause of respiratory infection. Nonspecific clinical symptoms and signs confuse diagnosis. On the other hand, it is easily misdiagnosed probably because of a set of common morphological features between multiflagellated protozoan and ciliated epithelial cells. Therefore, we reviewed the difficulties encountered during the diagnosis in order to improve the understanding of this disease and reduce the incidence of incorrect and missed diagnoses.
- Citation: Meng SS, Dai ZF, Wang HC, Li YX, Wei DD, Yang RL, Lin XH. Authenticity of pulmonary Lophomonas blattarum infection: A case report. World J Clin Cases 2019; 7(1): 95-101
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/95.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.95
Lophomonas blattarum (L. blattarum) is a protozoan that can cause infection in a variety of tissues and organs[1,2]. Clinically, the most common tissue infected is the respiratory tract, and the concomitant symptoms (including cough, sputum, and dyspnea) are similar to those of other respiratory conditions, such as bronchial asthma, pneumonia, bronchitis, or acute exacerbation of chronic obstructive pulmonary disease (AECOPD)[1,3-5,10,11]. Clinically significant pulmonary protozoal infections are rare but have been increasingly recognized in the past decades[2]. Predisposing factors contain immunosupression and aging process[1-2,6-9,11]. This endocommensal usually parasitizes the intestine of specific arthropods, such as termites and roaches[12-14]. Inhalation of aerosols containing L. blattarum cysts has been proposed to infect human beings[14-16], but this hypothesis has not been confirmed.
More than 100 cases of L. blattarum infection have been reported since the first case emerged in 1993. The vast majority of studies reported L. blattarum infection based on morphology under a light microscope. Since 2011, several reports have indicated that a few studies misidentified respiratory ciliated cells as L. blattarum or multi-flagellated protozoans[17-20]. The researchers assumed that despite some similar features between L. blattarum and respiratory ciliated cells, which are difficult to differentiate, a set of morphological features are unique to L. blattarum or respiratory ciliated cells under a light microscope. The morphological features observed by light microscopy are insufficient. Here, we present a case of chronic eosinophilic pneumonia that was initially misdiagnosed as a multi-flagellated protozoan infection.
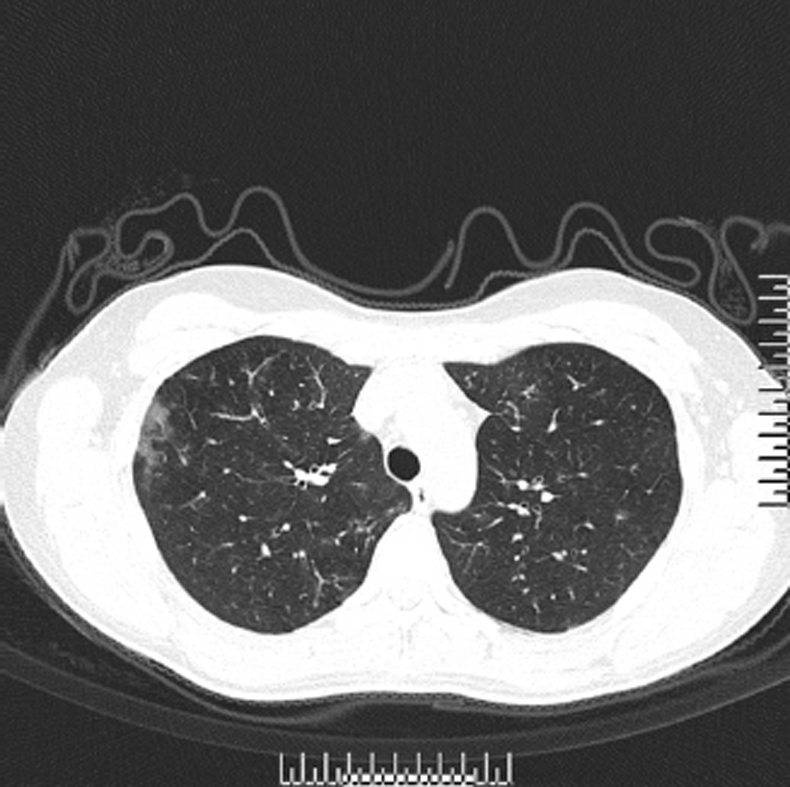
A 28-year-old female college teacher was admitted to Huaihe Hospital affiliated to Henan University on October 21, 2016, with chief complaints of cough, sputum, and dyspnea for 3 years without fever. Past medical history included allergic rhinitis for 5 years, and her father had a history of bronchial asthma. Physical examination of the patient showed that breath sounds from both lungs were rough, without rales or rhonchi, and other vital signs were as follows: blood pressure, 16.7/9.3 kPa, pulse rate, 84 bpm, respiratory rate, 21 breaths/min, and body temperature, 36.6 °C. The clinical laboratory blood tests revealed that the white blood cell count was 8.73 × 109/L, with 31.3% of eosinophils. The C-reactive protein was 0.1 mg/L (0-8.2mg/L), the erythrocyte sedimentation rate was 13 mm/hr (0-20mm/hr), and total IgE significantly increased. A chest computed tomography scan revealed that the bilateral bronchial walls showed thickening, accompanied with patchy spots scattered throughout the bilateral lungs (Figure 1), but enlarged mediastinal lymph nodes were not observed. Pulmonary function suggested a slightly weakened pulmonary ventilation function. The laboratory studies for tuberculosis and fungi were negative; however, the serum procalcitonin level was slightly increased. Meanwhile, the bronchoalveolar lavage fluid (BALF) was collected with a bronchofiberscope, and bronchoscopic brush smears were generated for cytologic and etiological diagnosis.
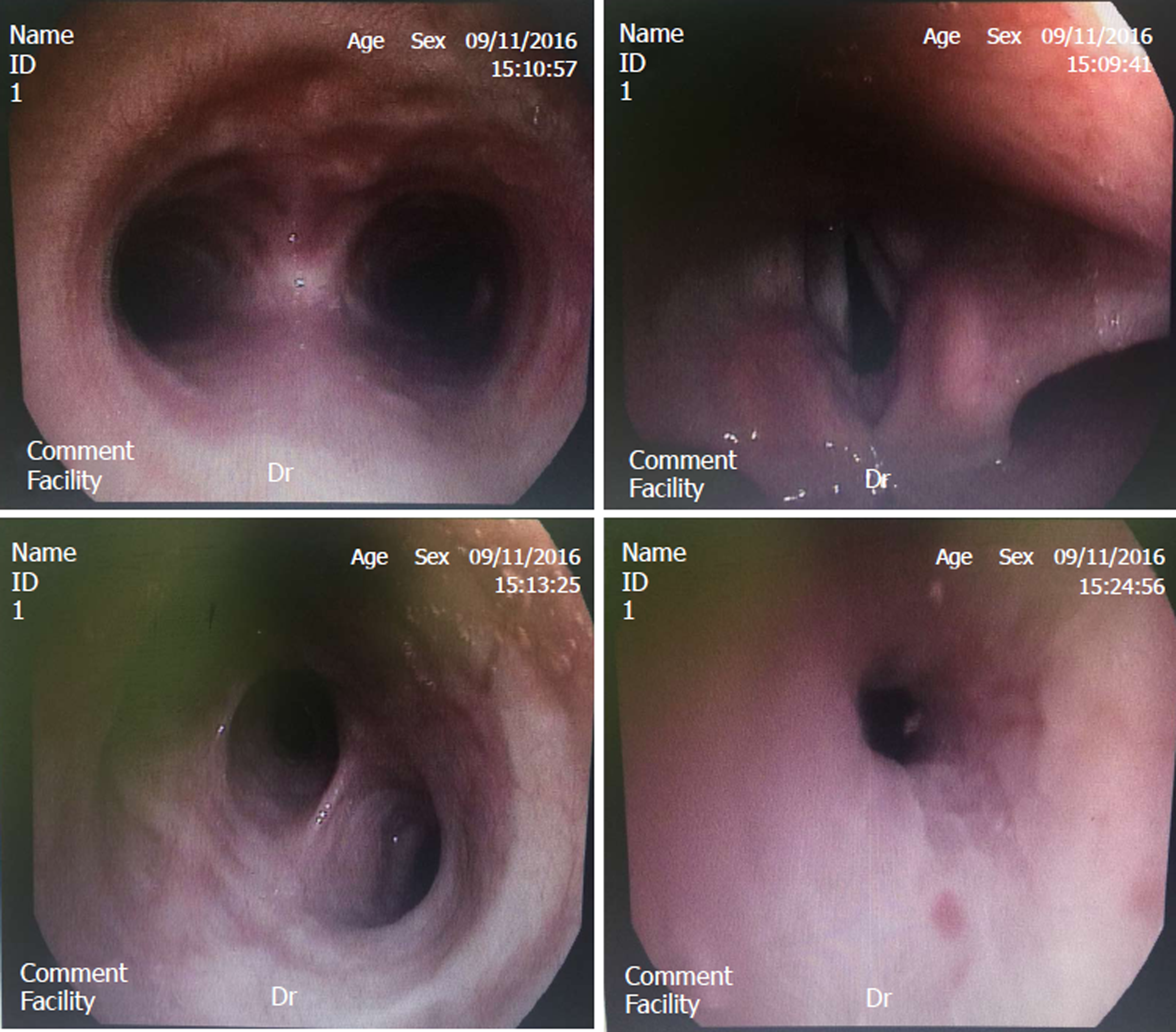
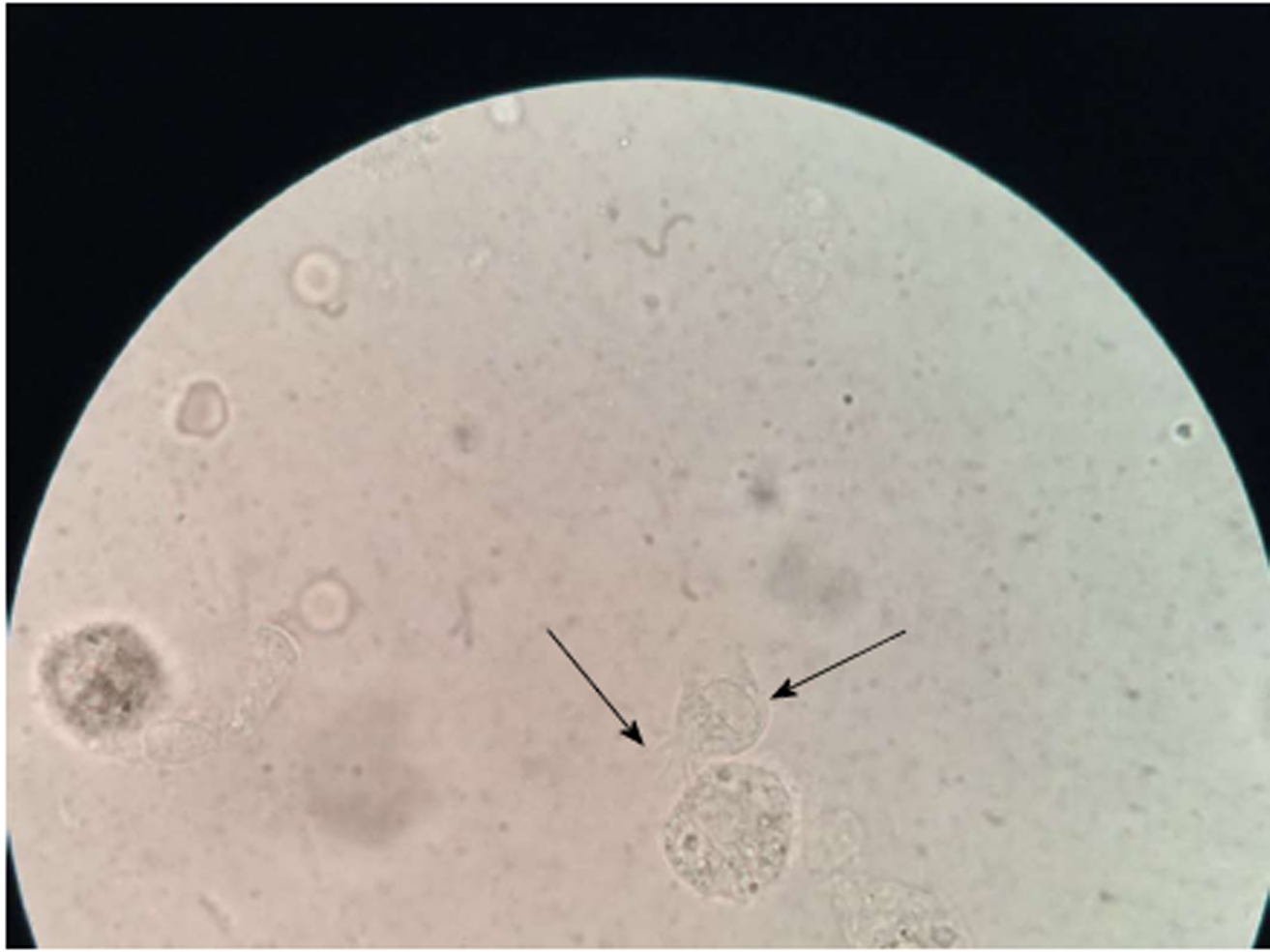
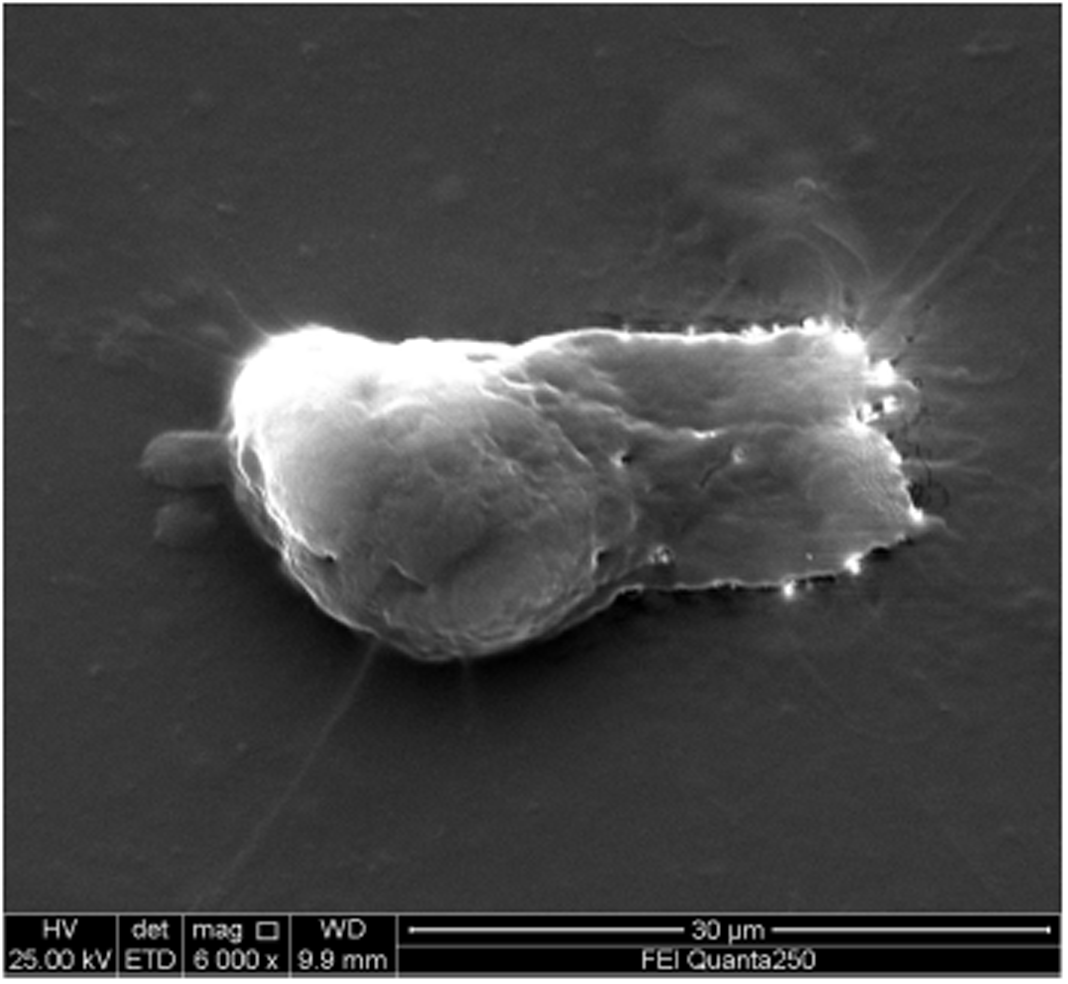
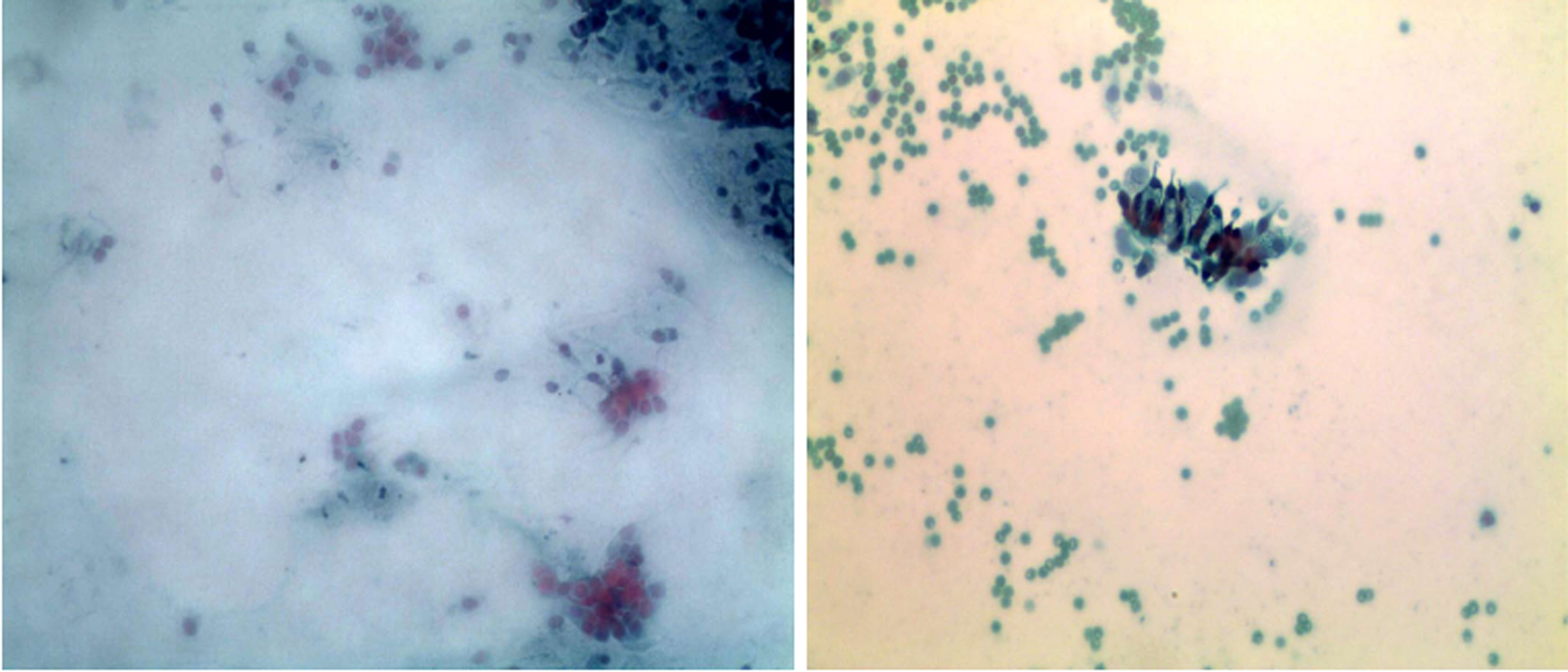
Bronchofiberscope analysis indicated that the bronchial mucosa showed hyperemia, edema, and congestion, especially in the right superior lobe (Figure 2). Under a light microscope, the cilia on the top of the cell oscillated rapidly to drive cell migration, and thus, observation of possible multi-flagellated protozoans in the BALF was reported (Figure 3). Subsequently, the patient received treatment with a bronchodilator agent and metronidazole. To investigate the ultrastructures of the potential multi-flagellated protozoans, we performed scanning electron microscopy (Figure 4), and columnar cells with a cluster of cilia at the top were found. A spherical nucleus was located in the basal region, with cilia at the apical end of the cell. Both BALF and bronchoscopic brush smears underwent Gram staining and Pap staining (Figure 5). Instead of multi-flagellated protozoan, numerous respiratory ciliated cells were scattered or accumulated in the sample.
Eventually, she was diagnosed with chronic eosinophilic pneumonia.
Intravenous metronidazole was administered for 5 d. Given the cytologic diagnosis, metronidazole was contraindicated, and bronchodilator, mucolytic, and nutritional therapies were used.
Intravenous metronidazole was administered for 5 d. The patient reported that the symptoms were improved. Metronidazole was contraindicated, and bronchodilator, mucolytic, and nutritional therapies were used. Three days later, pulmonary ventilation function improved substantially, and tests for tuberculosis, fungi, and Aspergillus fumigates were negative three times. However, the eosinophil number and percentage were highly increased to 2.57 × 109/L and 29.3%, respectively.
In recent decades, more than 100 cases of L. blattarum infection, most of which were observed in Chinese adults, have been reported. The possible links between L. blattarum infection and insects in the domestic environment have been explored[13-16,21,22]. L. blattarum is widely believed to parasitize the colon of cockroaches[23], and the parasite could be discharged through the secretions and excrement of the host, which are spread by contaminated clothing and food. Inhalation of L. blattarum cysts was suggested to be a route of transmission of this protozoan from cockroaches to human beings[1].
In this case, the patient had been abroad in Belarus from 2010 to 2012. Some cockroaches were found in garderobe, and insecticides were used. Due to the patient’s history of allergic rhinitis, long-term usage of corticosteroids could cause immunosuppression, which has been demonstrated to increase the risk of bacterial, viral, and fungal infections[2,6-9,24]. Based on these factors, we initially misidentified the respiratory ciliated cells as multi-flagellated protozoans. It is difficult to distinguish L. blattarum and ciliated epithelial cells based on morphology under a light microscope. There are several common morphological features. First, both are round and oval in shape, but the cells are sometimes columnar[1]. Second, both cell sizes are similar, with L. blattarum ranging from approximately 15 μm to 40 μm, comparable to that of the ciliated epithelial cells[25,26]. Third, movements of flagella and cilia, which are found in L. blattarum and ciliated epithelial cells, respectively, are observed by light microscopy in fresh samples. Fourth, due to the degeneration of ciliated cells in the bronchial secretion, the nuclei become misty and may even disappear, and the nuclei of L. blattarum are not always present. Due to these morphological similarities, ciliated bronchial cells may have been erroneously identified as multi-flagellated protozoans. In addition, nonspecific symptoms, such as cough, sputum, and dyspnea, can not help with the identification, although specific pathogens, such as bacteria and fungi, were not detected. All of these symptoms could be attributed to AECOPD or interstitial pneumonia. Specific diagnosis cannot be made based on laboratory studies and imaging analyses (only subsidiary diagnoses).
Judgements based on light microscopic structures and clinical symptoms without the confirmation of electron microscopy or molecular evidence are inadvisable. By electron microscopy, the morphological features of flagellated protozoa and ciliated epithelial cells are clear[27]. The nuclei of ciliated epithelial cells are located at the basal end, with cilia at the apical end of the cell, while flagellated protozoa show granular cytoplasm, phagocytic particles, and a tuft of flagella. In addition to these characteristics, the cilia are regularly oriented, whereas the flagella have an irregular arrangement. Significantly, electron microscopy showed some ultrastructures of flagellated protozoa that are not found in ciliated epithelial cells, such as the calyx, axial filament, and parabasal body[27,28]. These peculiar structures could promote temporary motility, while the ciliated epithelial cells could show similar mobility under a light microscope, even if detached from the airway epithelium for a long period[29]. Thus, movement detected by light microscopy is not a distinguishing feature.
Most reported cases in recent decades showed a positive response after metronidazole treatment, as in this case. The improvement was not only attributed to the broad spectrum property of this antibiotic, especially against anaerobic bacteria, but also to the administration of bronchodilator agents and physicotherapeutics, such as noninvasive ventilation. Although eosinophilia and increased total IgE indicated parasite or protozoan infection, direct evidence, such as isolation and culture, was inadequate, and images of light microscopy were not sufficient for a conclusive identification. Approximately 30 cases had eosinophilia[29], accounting for one-third of the total cases. Protozoan infection in these cases, which would explain the eosinophil counts, could be re-examined. Another possible issue is that the source of samples, sputum and throat swabs, could be contaminated with oral flora and food materials. Finally, except for 10 cases from abroad[1], the majority of cases were reported in China; however, there is no evidence that flagellated protozoan infection is an endemic disease.
In this case, we used approaches including light microscopy, scanning electron microscopy, BALF and bronchoscopic brush smears staining for diagnosis. Although multi-flagellated protozoan morphological features were distinguished from bronchial ciliated epithelia cells by scanning electron microscopy, transmission electron microscopy is essential for exploring internal structures.
In conclusion, this case illustrated the misdiagnosis of multi-flagellated protozoan infection, although predisposing factors were compatible with those of published cases, including eosinophilia, immunocompromised respiratory symptoms, and suspected protozoa under a light microscope. It is difficult to distinguish these protozoa from normal bronchial ciliated epithelia cells only via light microscopy, electron microscopy, or molecular evidence, but isolation and culture may be worth pursuing. In contrast to these characteristics, clinical responses may confuse the diagnosis. Metronidazole is used to treat a wide range of anaerobic bacteria, which are poorly detected by routine testing. However, a combination with multiple agents improved clinical manifestations. Furthermore, the route of transmission, pathogenesis, and molecular techniques should be further investigated.
L. blattarum is a rare cause of respiratory infection. Nonspecific clinical symptoms and signs, even laboratory routine test and positive treatment, could not help for identification. Microscopic examination of sputum smear is fast for initial and presumptive diagnosis, but it is not credible due to morphological similarities of ciliated epithelial cells. Combination of smear staining, electron microscopy, and even molecular techniques should be applied for diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
of interest.
P- Reviewer: Chiu KW, García-Elorriaga G S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Martinez-Girón R, van Woerden HC. Lophomonas blattarum and bronchopulmonary disease. J Med Microbiol. 2013;62:1641-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Vijayan VK. Is the incidence of parasitic lung diseases increasing, and how may this affect modern respiratory medicine? Expert Rev Respir Med. 2009;3:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Zeng H, Kong X, Chen X, Luo H, Chen P, Chen Y. Lophomonas blattarum infection presented as acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2014;6:E73-E76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Martínez-Girón R. Potential role of protozoa and tight junctions in the airway epithelium disruption. Clin Respir J. 2011;5:e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Martínez-Girón R, Doganci L. Lophomonas blattarum: a bronchopulmonary pathogen. Acta Cytol. 2010;54:1050-1051. [PubMed] |
| 6. | Ribas A, Martínez-Girón R, Sánchez-Del-Río J, González-Alonso D. Protozoal forms in the sputum of immunocompromized patients. Scand J Infect Dis. 2005;37:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Duboucher C, Noël C, Durand-Joly I, Gerbod D, Delgado-Viscogliosi P, Jouveshomme S, Leclerc C, Cartolano GL, Dei-Cas E, Capron M, Viscogliosi E. Pulmonary coinfection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum Pathol. 2003;34:508-511. [PubMed] |
| 8. | Wang Y, Tang Z, Ji S, Zhang Z, Chen J, Cheng Z, Cheng D, Liu Z, Li L. Pulmonary Lophomonas blattarum infection in patients with kidney allograft transplantation. Transpl Int. 2006;19:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | He Q, Chen X, Lin B, Qu L, Wu J, Chen J. Late onset pulmonary Lophomonas blattarum infection in renal transplantation: a report of two cases. Intern Med. 2011;50:1039-1043. [PubMed] |
| 10. | Martínez-Girón R, Ribas A, Astudillo-González A. Flagellated protozoa in cockroaches and sputum: the unhygienic connection? Allergy Asthma Proc. 2007;28:608-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Saldaña NG, Mendoza FJO, Larrauri FR, Trujillo DMG, Montoya EV, De La Garza EA, Olguín HJ. Bronchopulmonary infection by Lophomonas blattarum in a pediatric patient after hematopoietic progenitor cell transplantation: first report in Mexico. J Thorac Dis. 2017;9:E899-E902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 12. | Strand MA, Brooks MA. Pathogens of Blattidae (cockroaches). Bull World Health Organ. 1977;55 Suppl 1:289-296. [PubMed] |
| 13. | Silvanose CD, Bailey TA, Samour JH, Naldo JL. Intestinal protozoa and associated bacteria in captive houbara bustards (Chlamydotis undulata) in the United Arab Emirates. Avian Pathol. 1999;28:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Graczyk TK, Knight R, Tamang L. Mechanical transmission of human protozoan parasites by insects. Clin Microbiol Rev. 2005;18:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Bittencourt-Silvestre J, Lemgruber L, de Souza W. Encystation process of Giardia lamblia: morphological and regulatory aspects. Arch Microbiol. 2010;192:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Zaragatzki E, Hess M, Grabensteiner E, Abdel-Ghaffar F, Al-Rasheid KA, Mehlhorn H. Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitol Res. 2010;106:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Martínez-Girón R, van Woerden HC. The burden of Lophomonas blattarum under the light microscope. J Thorac Dis. 2014;6:E191-E192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Martínez-Girón R, van Woerden HC, Doganci L. Lophomonas misidentification in bronchoalveolar lavages. Intern Med. 2011;50:2721; author reply 2723. [PubMed] |
| 19. | Martínez-Girón R, van Woerden HC. Bronchopulmonary lophomoniasis: emerging disease or unsubstantiated legend? Parasit Vectors. 2014;7:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Mu XL, Shang Y, Zheng SY, Zhou B, Yu B, Dong XS, Cao ZL, Jiang N, Sun KK, Chen YC, Xi W, Gao ZC. [A study on the differential diagnosis of ciliated epithelial cells from Lophomonas blattarum in bronchoalveolar lavage fluid]. Zhonghua Jie He He Hu Xi Za Zhi. 2013;36:646-650. [PubMed] |
| 21. | Xue J, Li YL, Yu XM, Li DK, Liu MF, Qiu JF, Xue JJ. Bronchopulmonary infection of Lophomonas blattarum: a case and literature review. Korean J Parasitol. 2014;52:521-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Ohkuma M, Noda S, Hongoh Y, Nalepa CA, Inoue T. Inheritance and diversification of symbiotic trichonymphid flagellates from a common ancestor of termites and the cockroach Cryptocercus. Proc Biol Sci. 2009;276:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Leschine SB. Cellulose degradation in anaerobic environments. Annu Rev Microbiol. 1995;49:399-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 217] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Duboucher C, Gerbod D, Noël C, Durand-Joly I, Delgado-Viscogliosi P, Leclerc C, Pham S, Capron M, Dei-Cas E, Viscogliosi E. Frequency of trichomonads as coinfecting agents in Pneumocystis pneumonia. Acta Cytol. 2005;49:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Mitchell DR. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv Exp Med Biol. 2007;607:130-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Lindemann CB, Lesich KA. Flagellar and ciliary beating: the proven and the possible. J Cell Sci. 2010;123:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Beams HW, Sekhon SS. Further studies on the fine structure of Lophomonas blattarum with special reference to the so-called calyx, axial filament, and parabasal body. J Ultrastruct Res. 1969;26:296-315. [PubMed] |
| 28. | Kessel RG, Beams HW. Freeze fracture and scanning electron microscope studies on the nuclear envelope and perinuclear cytomembranes (parabasal apparatus) in the protozoan, Lophomonas blattarum. J Submicrosc Cytol Pathol. 1990;22:367-378. [PubMed] |
| 29. | Li R, Gao ZC. Lophomonas blattarum Infection or Just the Movement of Ciliated Epithelial Cells? Chin Med J (Engl). 2016;129:739-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |