Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.73
Peer-review started: August 3, 2018
First decision: October 19, 2018
Revised: November 23, 2018
Accepted: November 30, 2018
Article in press: December 1, 2018
Published online: January 6, 2019
Processing time: 155 Days and 6.2 Hours
Millard-Gubler syndrome (MGS) is caused by a lesion in the brainstem at the level of the facial nerve nucleus, and it is also a rare ventral pontine syndrome. Vertebrobasilar artery dissection (VAD) is an uncommon cause of ischemic stroke. To the best of our knowledge, this is the first case report on the coexistence of MGS and VAD in a young acute ischemic stroke patient.
We herein describe an unusual case of young acute ischemic stroke patient, presenting with acute right peripheral facial palsy, right abducens palsy, and contralateral hemihypesthesia, manifesting as MGS. After receiving dual antiplatelet therapy with aspirin and clopidogrel, as well as rosuvastatin, the patient recovered significantly. The high-resolution magnetic resonance imaging (MRI) indicated a diagnosis of VAD.
Our finding further demonstrated that high-resolution MRI is a useful technique to early detect underlying dissection in posterior circulation ischemic stroke.
Core tip: Millard-Gubler syndrome (MGS) is caused by a lesion in the brainstem at the level of facial nerve nucleus, and it is also a rare ventral pontine syndrome. We herein describe an unusual case of young acute ischemic stroke patient presenting with MGS. The high-resolution magnetic resonance imaging (MRI) indicated a diagnosis of vertebrobasilar artery dissection (VAD). This is the first case report on the coexistence of MGS and VAD in a young acute ischemic troke patient. Our finding further demonstrated that high-resolution MRI is a useful technique to early detect underlying dissection in posterior circulation ischemic stroke.
- Citation: Li XT, Yuan JL, Hu WL. Vertebrobasilar artery dissection manifesting as Millard-Gubler syndrome in a young ischemic stroke patient: A case report. World J Clin Cases 2019; 7(1): 73-78
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/73.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.73
Millard-Gubler syndrome (MGS) is one of uncommon pontine-crossed syndromes, and it involves the facial nerve nucleus, abducent nerve, and the corticospinal tract. It is characterized by paralysis affecting the face and the abducent nerve on the side of the lesion and central hemiplegia on the opposite side[1]. It has been reported that MGS could be found in patients with brainstem tumor such as cavernous angioma[2-4], rimary meningeal hemangiopericytoma[5], neurocysticercosis[6]. As far as we know, only five cases of MGS caused by cerebral infarction have been reported[1,7-10]. Vertebrobasilar artery dissection (VAD) is an uncommon cause of ischemic stroke, especially in posterior circulation ischemic stroke[11]. To the best of our knowledge, this is the first report on the coexistence of MGS and VAD in a young acute ischemic stroke patient, using the technique of high-resolution magnetic resonance imaging (MRI). Herein, we describe an unusual case of posterior circulation ischemic stroke manifesting as MGS caused by VAD.
A 49-year-old male presented with dizziness and slurred speech for two days.
Two days before admission, the patient experienced sudden dizziness and nausea, followed by slurred speech, dysphagia, and choking. Before the onset of the illness, he did not suffer from fever or cervical pain.
He suffered from hypertension for seven years. There was no other vascular risk factor or family history.
On admission, his neurological examination revealed right peripheral facial palsy, right abducens palsy, and left hemihypesthesia, suggesting the presence of MGS. Besides, dysarthria, absent gag reflex, and positive bilateral Babinski’s signs were also detected. The other cranial nerves and motor exam were normal. On admission, his blood pressure was 141/85 mmHg.
The laboratory tests showed elevated plasm cholesterol (7.83 mmol/L), glycosylated hemoglobin (9.0%), and homocysteine (15 μmol/L), and normal low density lipoprotein (1.4 mmol/L). For the routine blood test, his white blood cell was mildly elevated (10.63 × 109/L) and other items were normal.
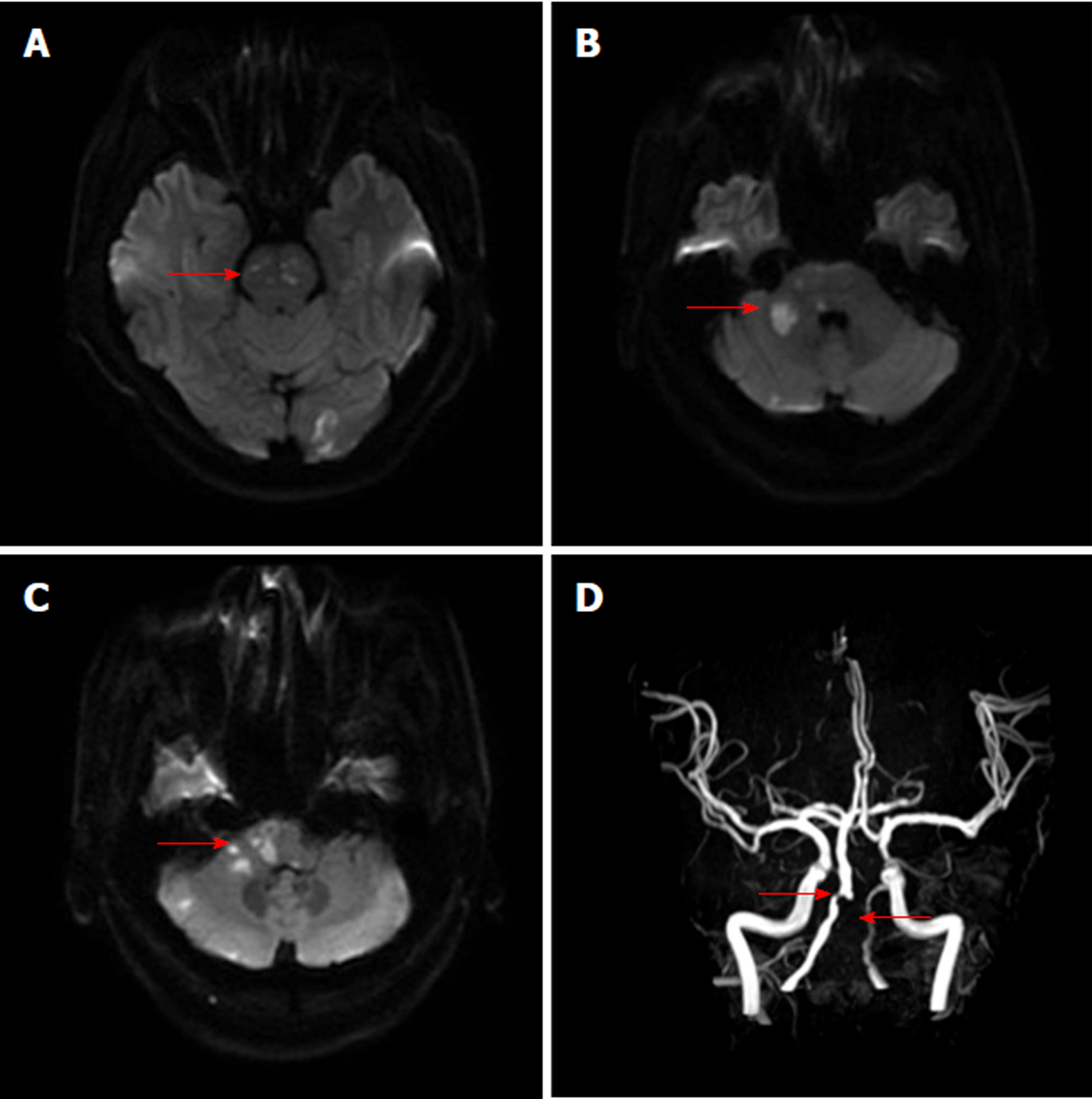
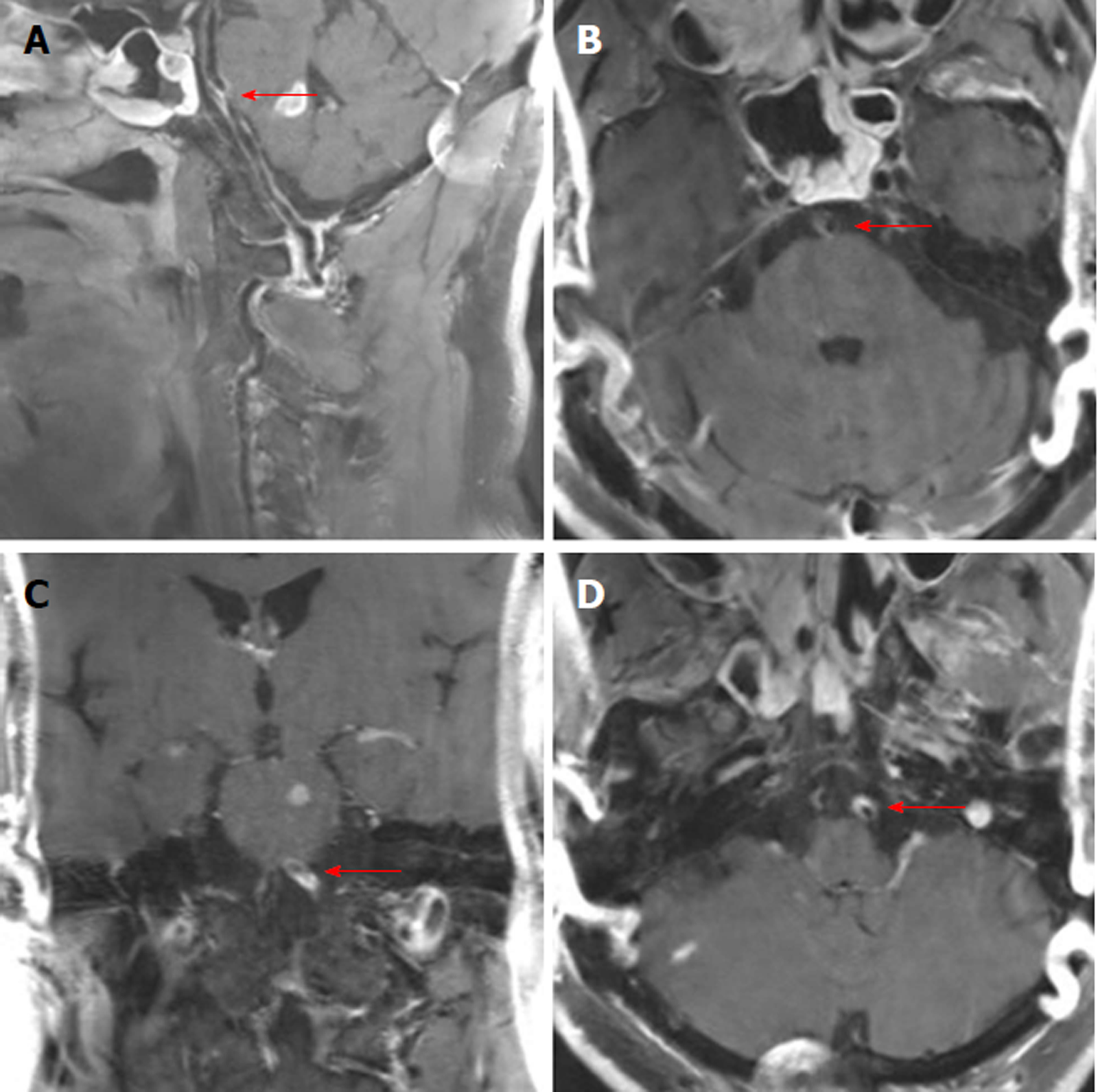
The chest X-ray film showed mild pneumonia. The parameters of MRI examination using a 3-Tesla system (Discovery MR750, GE Medical Systems, Milwaukee, Wis., United States) were as follows: MR angiography (MRA) (repetition time 21 ms; echo time 3.4 ms; slice thickness 0.9 mm), axial T2-weighted (repetition time 5838.7 ms; echo time 107.3 ms), axial T1-weighted imaging (repetition time 1800 ms; echo time 27.7 ms), axial diffusion weighted imaging (DWI) (repetition time 3000 ms; echo time 65.3 ms, b value 1000), and coronal fluid-attenuated inversion recovery sequences (repetition time 7500 ms; echo time 121.1 ms). Brain MRI revealed acute multifocal infarctions in the pons, ventral medulla oblongata, right middle cerebellar peduncle, and left occipital lobe (Figure 1A-C). Brain MRA without contrast agent indicated the occlusion of the left vertebral artery and severe stenosis of the proximal right vertebral artery (Figure 1D). The high-resolution MRI with contrast enhancement showed luminal irregularities with eccentric periluminal hematoma, indicating the dissection of the basilar artery and left vertebral artery (Figure 2).
According to the typical symptoms, physical examination, and imaging findings, this patient was diagnosed with acute ischemic stroke presenting as MGS caused by VAD.
The patient was given dual antiplatelet therapy with aspirin and clopidogrel, as well as rosuvastatin.
Nine days after his admission, he recovered significantly and was discharged from our department with mild residual right facial palsy and left hemihypesthesia.
MGS is caused by a lesion in the pons at the level of the facial nerve nucleus. This lesion involves the facial nerve nucleus, the abducent nerve, and the corticospinal tract. Clinical features include ipsilateral peripheral facial palsy, ipsilateral abducens paralysis, contralateral hemidysesthesia, and central hemiplegia caused by the lesion of the ventrolateral pons. To date, there are only five case reports of MGS due to cerebral infarction[1,7-10] (Table 1). One case was a 56-year-old male who presented with left lower facial paralysis and hemiparesthesia on the right side, and brain MRI revealed an acute infarct in the left ventral pons[1]. Another case was a 63-year-old man who presented with left hemiparesis and right facial paralysis involving the lower facial muscles and the orbicularis oculi but sparing the frontalis muscle. DWI indicated acute infarction in ventro-medial aspect of the medulla[7].
| Author | Time | Age, yr | Sex | Medical history | Physical examination | MRI | MRA | Others |
| Yasuda Y et al[9] | 1993 | 60 | Male | NA | Right peripheral facial nerve palsy, left hemiparesis, tongue deviated to the left, exaggerated deep tendon reflex, and equivocal left Babinski’s reflex | Cerebral infarction in the right ventral pons | Occlusion of both vertebral arteries | |
| Matlis A et al[8] | 1994 | 76 | Male | Hypertension, ischemic heart disease, and type II diabetes mellitus | Slight dysarthria, peripheral right facial palsy, flaccid left hemiparesis, brisk left deep tendon reflexes, and positive left Babinski’s reflex | Cerebral infarction in the right anteromedial pons | NA | |
| Onbas O et al[1] | 2005 | 56 | Male | NA | Left facial paralysis, right hemiparesthesia, and exaggerated deep tendon reflexes | Acute cerebral infarction in the left ventral part of the pons | Stenosis of the basilar artery | |
| Rose DZ et al[10] | 2010 | 45 | Male | HIV | Horizontal diplopia, left facial paralysis, and right hemiparesis | Acute cerebral infarction in the left pons | Unremarkable | MRSA meningo-vasculitis caused by the restricted diffusion of pus in the subarachnoid space |
| Ahdab R et al[7] | 2013 | 63 | Male | Diabetic and hypertensive | Right facial palsy involving the lower facial muscles and the orbicularis oculi but sparing the frontalis muscle and left hemiparesis | Acute cerebral infarction in the ventro-medial aspect of the medulla and limited to the right pyramid | Diffuse atherosclerotic changes of the basilar trunk with mild to moderate multisegmental narrowing, especially in the distal third |
VAD has been found more frequently in patients with posterior circulation ischemic stroke[12]. MRA, a non-invasive technique for dynamic assessment of the cranial circulation, is routinely used in stroke work-up to detect arterial occlusion. In addition, high-resolution MRI has been used to explore vascular diseases, with good advantages in the diagnosis of the dissection, and it can help to give more information about the etiology of cerebral infarction[13,14]. By using the combined high-resolution MRI, we speculated that the acute multifocal infarctions were caused by the dissection of the basilar artery and left vertebral artery.
To the best of our knowledge, this is the first report on the coexistence of MGS and VAD in a young acute ischemic stroke patient. Our case raises the importance that the utility of high-resolution MRI with fat saturation might be a useful tool to early detect the dissection in posterior circulation ischemic stroke, especially in young patients.
For acute ischemic stroke in a young patient, artery dissection should be considered in clinical work. High-resolution MRI with fat saturation is an important and useful tool to early detect the dissection, especially in posterior circulation ischemic stroke. Further studies are needed to warrant the potential findings and applications of high-resolution MRI, black blood T2-weighted MRI (angiitis, branch disease, etc.), and fat-saturation MRI (dissections) in stroke differential diagnosis and follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Sijens PE, Sergi C, Kai K, Gonzalez-Moreno EI, Elgafy H, Anand A S- Editor: Dou Y L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Onbas O, Kantarci M, Alper F, Karaca L, Okur A. Millard-Gubler syndrome: MR findings. Neuroradiology. 2005;47:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Dourado Leite R, Freitas C, Guimaraes S. Vertical muscle transposition with silicone band belting in VI nerve palsy. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kesikburun S, Safaz I, Alaca R. Pontine cavernoma hemorrhage leading to Millard-Gubler syndrome. Am J Phys Med Rehabil. 2011;90:263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kuhn J, Brümmendorf TH, Brassat U, Lehnhardt FG, Chung BD, Harnier S, Bewermeyer H, Harzheim A, Assheuer J, Netzer C. Novel KRIT1 mutation and no molecular evidence of anticipation in a family with cerebral and spinal cavernous malformations. Eur Neurol. 2009;61:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Ben Nsir A, Badri M, Kassar AZ, Hammouda KB, Jemel H. Hemangiopericytoma of the Cerebellopontine Angle: A Wolf in Sheep’s Clothing. Brain Tumor Res Treat. 2016;4:8-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Prasad R, Kapoor K, Srivastava A, Mishra O. Neurocysticercosis presenting as Millard Gubler syndrome. J Neurosci Rural Pract. 2012;3:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ahdab R, Saade HS, Kikano R, Ferzli J, Tarcha W, Riachi N. Pure ipsilateral central facial palsy and contralateral hemiparesis secondary to ventro-medial medullary stroke. J Neurol Sci. 2013;332:154-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Matlis A, Kleinman Y, Korn-Lubetzki I. Millard-Gubler syndrome. AJNR Am J Neuroradiol. 1994;15:179-181. [PubMed] |
| 9. | Yasuda Y, Matsuda I, Sakagami T, Kobayashi H, Kameyama M. Pontine infarction with pure Millard-Gubler syndrome: precise localization with magnetic resonance imaging. Eur Neurol. 1993;33:331-334. [PubMed] |
| 10. | Rose DZ, Parra-Herran C, Petito CK, Post MJ. Restricted Diffusion of Pus in the Subarachnoid Space: MRSA Meningo-Vasculitis and Progressive Brainstem Ischemic Strokes - A Case Report. Case Rep Neurol. 2010;2:101-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Ahn SS, Kim BM, Suh SH, Kim DJ, Kim DI, Shin YS, Ha SY, Kwon YS. Spontaneous symptomatic intracranial vertebrobasilar dissection: initial and follow-up imaging findings. Radiology. 2012;264:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Chien C, Chang FC, Huang HC, Tsai JY, Chung CP. Characteristics and Outcomes of Vertebrobasilar Artery Dissection with Accompanied Atherosclerosis. Cerebrovasc Dis Extra. 2017;7:165-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Zhang X, Jing J, Dong K, Wang Y, Wang Y. Teaching NeuroImages: Vertebrobasilar dolichoectasia with dissection manifested as infarct and subarachnoid hemorrhage. Neurology. 2018;90:e990-e991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Jung SC, Kim HS, Choi CG, Kim SJ, Lee DH, Suh DC, Kwon SU, Kang DW, Kim JS. Quantitative Analysis Using High-Resolution 3T MRI in Acute Intracranial Artery Dissection. J Neuroimaging. 2016;26:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |