Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.49
Peer-review started: November 12, 2018
First decision: November 28, 2018
Revised: December 1, 2018
Accepted: December 12, 2018
Article in press: December 12, 2018
Published online: January 6, 2019
Processing time: 57 Days and 1.6 Hours
Cervical lymph node metastasis in papillary thyroid carcinoma (PTC) affects the treatment and prognosis of patients. Ultrasound is a common imaging method for detecting cervical lymph nodes in PTC patients; however, it is not accurate in determining lymph node metastasis.
To evaluate the value of contrast-enhanced ultrasound combined with elastography in evaluating cervical lymph node metastasis in PTC.
A total of 94 patients with PTC were recruited. According to pathological results, lymph nodes were divided into two groups: metastatic group (n = 50) and reactive group (n = 63). The routine ultrasound findings, contrast-enhanced ultrasound and elastography data were recorded and compared. Logistic regression was used to generate predictive probability distributions for the diagnosis of lymph node metastasis with different indicators. Receiver operating characteristic curve analysis was used to test the efficacy of contrast-enhanced ultrasound combined with elastography based on routine ultrasound in evaluating PTC cervical lymph node metastasis.
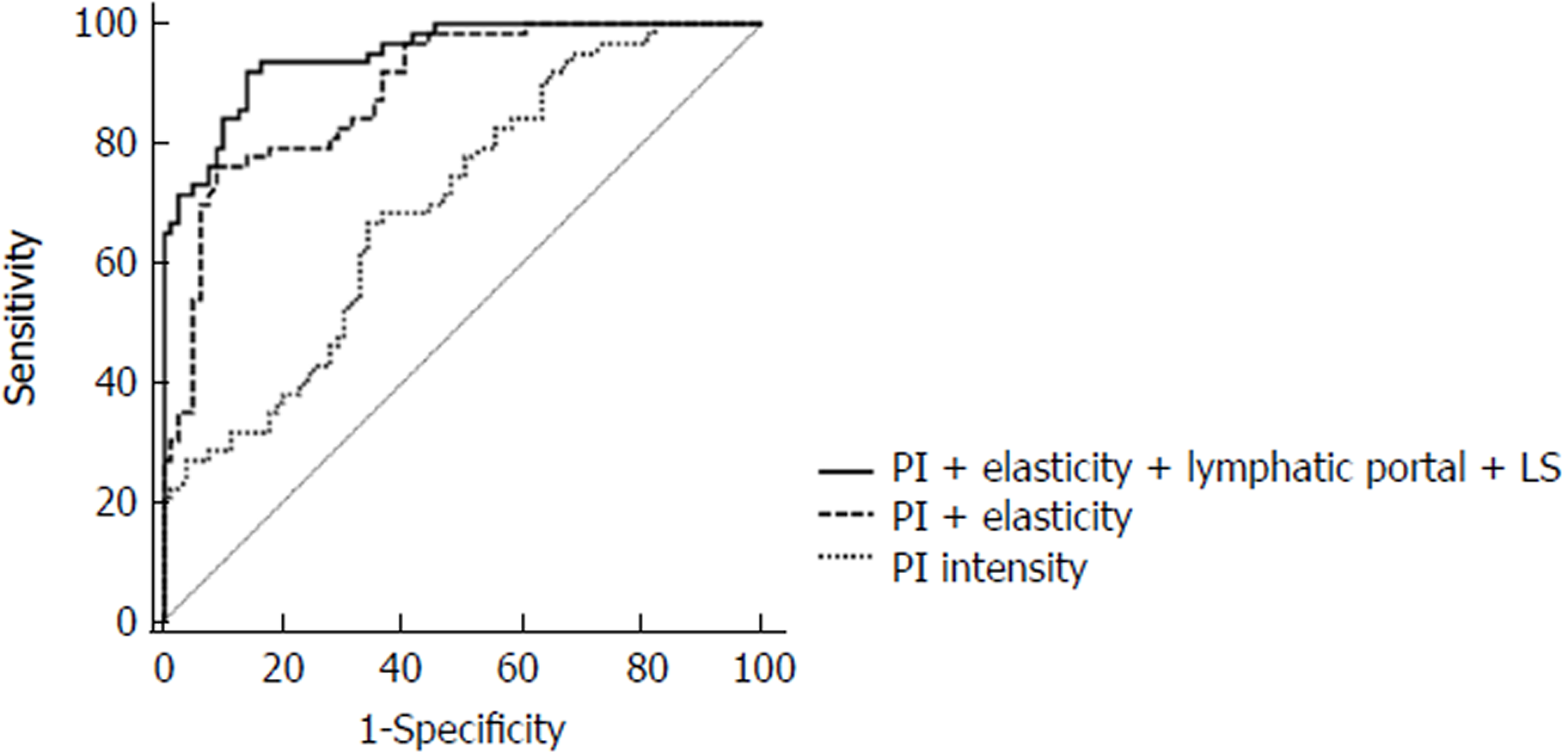
The ratio of long diameter/short diameter (L/S) ≤ 2, irregular marginal morphology, missing lymphatic portal, peripheral or mixed blood flow distribution, peak intensity (PI), non-uniform contrast distribution and elasticity score in the metastatic group were significantly higher than those in the reactive group (P < 0.05). L/S ratio, missing lymphatic portal, PI and elasticity score had a significant influence on the occurrence of PTC cervical lymph node metastasis (P < 0.05). Furthermore, the area under the curve (AUC) for lymph node metastasis diagnosed using the combination of PI ratio, elasticity score, missing lymphatic portal and LS was 0.936, which was significantly higher than the AUC for PI ratio alone. The difference was statistically significant (P < 0.05). The fitting equation for the combined diagnosis was logit(P) = -12.341 + 1.482 × L/S ratio + 3.529 × missing lymphatic portal + 0.392 × PI + 3.288 × elasticity score.
Based on the gray-scale ultrasound, the combination of contrast-enhanced ultrasound and elastography can accurately assess PTC cervical lymph node metastasis.
Core tip: Cervical lymph node metastasis of papillary thyroid carcinoma affects the prognosis and treatment of patients. Currently, contrast-enhanced ultrasound and ultrasound elastography have been gradually applied to the diagnosis of cervical lymph node metastasis. However, it is not clear whether contrast-enhanced ultrasound and elastography combined with conventional ultrasound improve the accuracy of lymph node metastasis diagnosis. In this study, we analyzed the differences among conventional ultrasound, contrast-enhanced ultrasound and elastography in metastatic and reactive lymph nodes, and used a logistic regression model to fit the probability equation of lymph node metastasis to improve the diagnostic accuracy of metastatic lymph nodes.
- Citation: Jiang W, Wei HY, Zhang HY, Zhuo QL. Value of contrast-enhanced ultrasound combined with elastography in evaluating cervical lymph node metastasis in papillary thyroid carcinoma. World J Clin Cases 2019; 7(1): 49-57
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/49.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.49
Cervical lymph node metastasis in papillary thyroid carcinoma (PTC) often affects the treatment and prognosis of patients. Accurate preoperative assessments is of great importance[1-3]. Ultrasound is currently the most commonly used diagnostic method for cervical lymph node metastasis of PTC. However, ultrasound is subjective and easily leads to missed diagnosis or misdiagnosis[4,5]. Recently, contrast-enhanced ultrasound and ultrasound elastography have gradually been applied for diagnosing cervical lymph node metastasis of PTC. Xiang et al[6] found that contrast-enhanced ultrasound could predict lymph node metastasis of PTC. Park et al[7] reported the value of elastography in diagnosing lymph node metastasis of PTC. However, there has been controversy over whether contrast ultrasound and elastography combined with conventional ultrasound can improve the diagnostic accuracy for lymph node metastasis. Accordingly, the present study explored the optimal diagnosis of cervical lymph node metastasis in PTC by analyzing the features of conventional ultrasound, contrast ultrasound and elastography.
We enrolled 94 patients with PTC at Shenzhen Nanshan District People’s Hospital from May 2016 to May 2018. According to the pathological results of postoperative lymph nodes, the patients were divided into a metastatic group and a reactive group. There were 43 patients with a total of 50 nodules in the metastatic group (10 males and 33 females, mean age 49.27 ± 13.85 years). There were 51 patients with a total of 63 nodules in the response group (16 males and 47 females, mean age 48.51 ± 15.30 years). The inclusion criteria were as follows: (1) patients diagnosed with PTC by puncture biopsy and planned for an operation; (2) no treatment with anti-thyroid medicine before operation; and (3) patients underwent preoperative contrast-enhanced ultrasound and elastography examinations. The exclusion criteria included patients with severe liver failure, renal failure, hematopoietic dysfunction, or other serious medical diseases. All subjects underwent total or subtotal resection and central lymph node dissection. Some underwent neck dissection. The present study was approved by the ethics committee of Shenzhen Nanshan District People’s Hospital, and written informed consent was obtained from all patients.
An Esaote MyLab 90X (Italy) Ultrasound System with a linear probe was used. The subjects lied in a supine position, with their neck and shoulders raised and their head pulled back to fully expose the neck. Ultrasound examination was performed on each subject. The anteroposterior diameter (S) and transverse diameter (L) of suspected lymph nodes were measured. L/S ratio was calculated and recorded as > 2 or ≤ 2. Other indexes such as marginal morphology of suspected lymph nodes (regular/irregular), lymphatic portal (existing/missing), cortical echo of lymph node (low/equal/high echo), calcification (no/yes), liquefaction (no/yes), blood flow distribution (central/peripheral/mixed blood flow), and blood flow classification (0-2) were recorded.
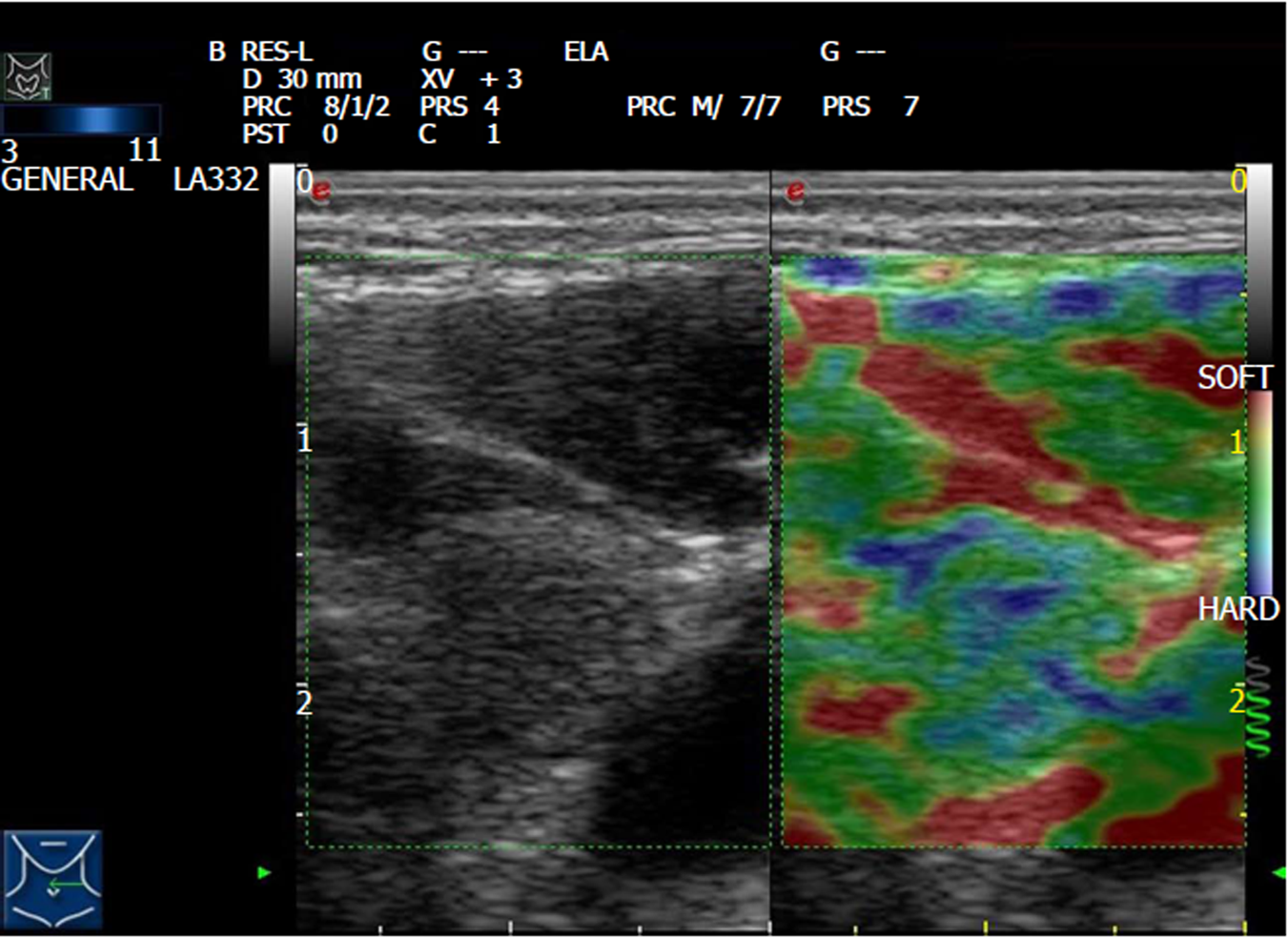
SonoVue (Bracco SpA, Milan, Italy) was used as an ultrasound contrast agent, which was a lyophilized powder added to 5 mL of 0.9% saline, and gently shaken into a uniform microbubble suspension. Subsequently, 1.2 mL of SonoVue was injected via the anterior antecubital vein, and washed with 5 mL of saline. The timer was turned on, and the dynamic contrast was observed and collected for approximately two minutes. Peak intensity (PI), time to peak (TP), area under the curve (AUC), contrast distribution (uniform/non-uniform), perfusion area (no/yes), and boundary condition (unclear/clear) were recorded. Finally, the ultrasound real-time tissue elastic imaging (RTE) function was turned on. RTE is an elastographic technique that converts the change in the amplitude of the echo signal before and after compression into a real-time color image. The probe was placed in a vertical position on the lesion and was slightly vibrated. When the pressure indicator was constant green (representing good RTE image quality), the image was frozen and stored. The color distribution of RTE was distributed according to the following rules: the softer part is mainly red, and the harder part is blue. According to the different colors of the lesion area, the elasticity score of 5 points was used for evaluation: 1 point, the lesion and the surrounding tissue are equally green; 2 points, the lesion area is a mixture of blue-green and green (mainly green); 3 points, the lesion area is a mixture of blue-green and green (mainly blue); 4 points, the lesion area is equally blue; and 5 points, the lesion area is all covered with blue, and a small part of the surrounding tissue is also blue[8]. All examinations were performed by the same experienced doctor.
SPSS 19.0 statistical software was used in the present study. Measurement data are expressed as the mean ± standard deviation (SD), and count data are expressed as numbers. Comparison of two sets of measurement data were performed by the t-test, while the X2-test was used to compare the count data. Logistic regression was used for the multivariate analysis of different factors to further screen for potential independent factors in the diagnosis of lymph node metastasis, and it was used to generate a combination of predictive probability distributions for different indicators. Receiver operating characteristic (ROC) curves were used to test the efficacy of different indicator combinations. The value of the combined use of different indicators in the diagnosis of lymph node metastasis in PTC was evaluated.
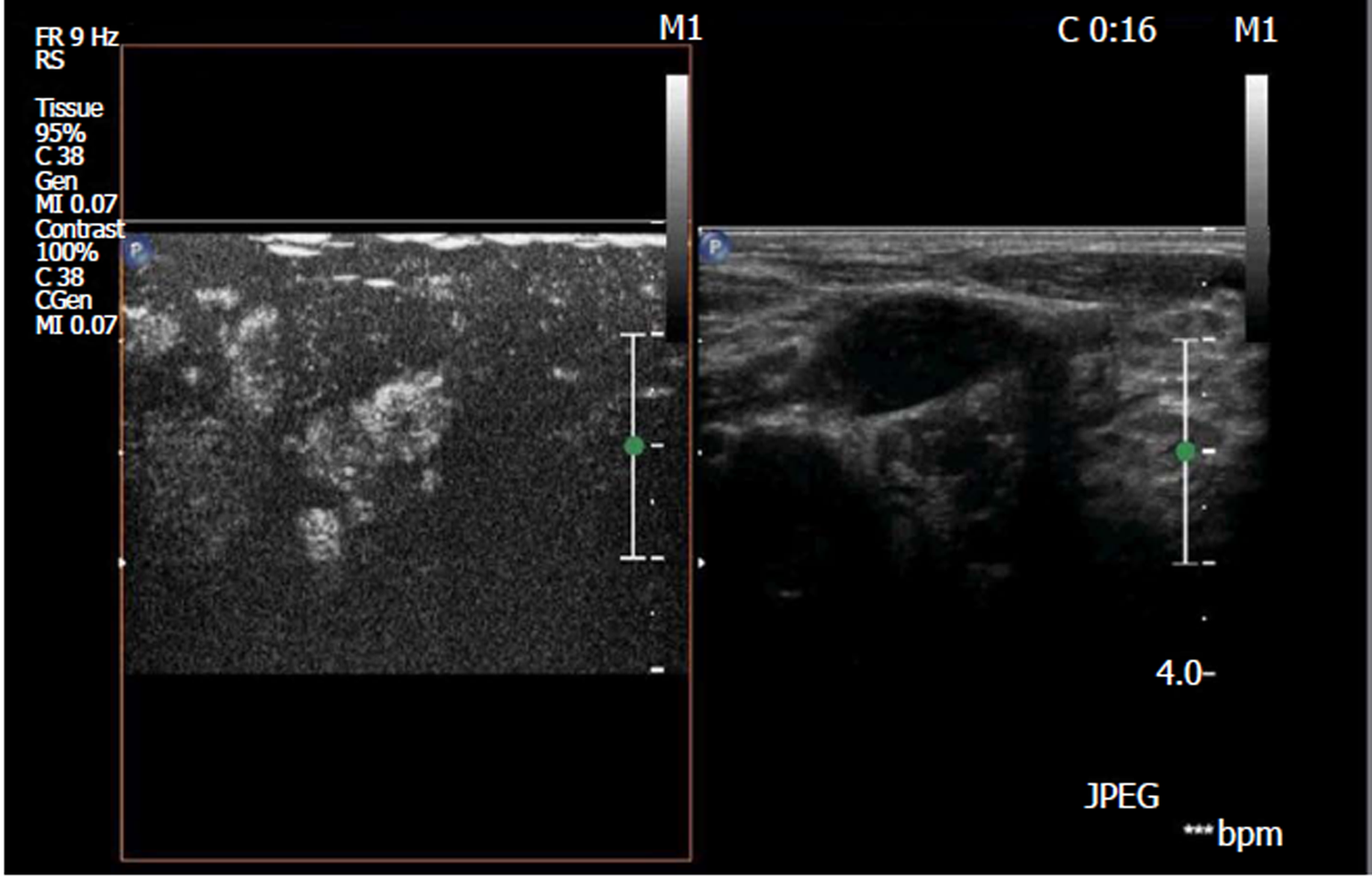
According to the pathological results of postoperative lymph nodes, 50 lymph nodes were assigned to the metastatic group, while 63 lymph nodes were assigned to the reactive group. Ultrasound revealed that compared with reactive lymph nodes, metastatic lymph nodes mostly had a round or round-like shape and L/S ratio ≤ 2. These metastatic lymph nodes had unclear boundaries, irregular margins, and internal non-uniform low echo. The partial cortices were in high or equal echo, and most of these lacked high echo. Some lymph nodes had small calcification and liquefaction. Doppler ultrasound revealed that blood flow signals were abundant in metastatic lymph nodes, and peripheral or mixed blood flow patterns were more common. Contrast-enhanced ultrasound revealed that the PI of metastatic lymph nodes was higher and more non-uniformed distributed (Figure 1). RTE revealed that the metastatic lymph nodes showed a blue-green distribution, and the surrounding tissues were mostly green (Figure 2).
The comparison of ultrasound examination results in these two groups is presented in Table 1. L/S ratio ≤ 2, irregular marginal morphology, missing lymphatic portal, peripheral or mixed blood flow distribution, PI, non-uniform contrast distribution and elasticity score in the metastatic group were significantly higher than those in the reactive group (P < 0.05).
| Group | Metastatic group (n = 50) | Reactive group (n = 63) | t/χ2 | P | |
| L/S ratio | > 2 | 24 | 60 | 32.606 | 0 |
| ≤ 2 | 26 | 3 | |||
| Boundary morphology | Irregular | 36 | 7 | 43.13 | 0 |
| Regular | 14 | 55 | |||
| Liquefaction | Yes | 2 | 0 | - | 0.113* |
| No | 48 | 63 | |||
| Calcification | Yes | 5 | 6 | 0.007 | 0.932 |
| No | 45 | 57 | |||
| Lymphatic portal | Yes | 23 | 59 | 31.795 | 0 |
| No | 27 | 4 | |||
| Cortical echo | Low | 35 | 53 | 3.229 | 0.072 |
| Qual/high | 15 | 10 | |||
| Blood flow distribution | Central type | 26 | 53 | 13.678 | 0 |
| Peripheral or hybrid | 24 | 10 | |||
| Blood flow classification | 0 | 6 | 6 | 2.914 | 0.233 |
| 1 | 33 | 50 | |||
| 2 | 11 | 7 | |||
| PI | 26.42 ± 6.79 | 21.82 ± 7.91 | 5.237 | 0 | |
| TP (s) | 24.19 ± 4.37 | 24.32 ± 5.16 | 1.133 | 0.259 | |
| Area under curve | 5.88 ± 1.57 | 5.73 ± 1.72 | 1.631 | 0.105 | |
| Contrast distribution | Non-uniform | 24 | 10 | 13.678 | 0 |
| Uniform | 26 | 53 | |||
| No perfusion zone | Yes | 4 | 2 | - | 0.140* |
| No | 46 | 61 | |||
| Boundary | Unclear | 8 | 9 | 0.064 | 0.8 |
| Clear | 42 | 54 | |||
| Elasticity score | 1 | 0 | 33 | 31.795 | 0 |
| 2 | 14 | 22 | |||
| 3 | 23 | 8 | |||
| 4 | 13 | 0 | |||
| 5 | 0 | 0 |
The multivariate logistic regression analysis of L/S ratio, marginal morphology, missing lymphatic portal, blood flow distribution, PI, contrast distribution and elasticity score revealed that L/S ratio, missing lymphatic portal, PI and elasticity score were independent influencing factors of cervical lymph node metastasis of PTC (P < 0.05, Table 2).
| Factor | β | SE | Wald | P | OR | 95.0%CI | |
| Lower limit | Upper limit | ||||||
| L/S (> 2 = 0, ≤ 2 = 1) | 3.136 | 0.385 | 12.732 | 0.003 | 23.006 | 10.817 | 48.928 |
| Boundary shape (rule = 0, irregular = 1) | 0.767 | 0.681 | 3.294 | 0.134 | 2.153 | 0.567 | 8.179 |
| Lymphatic portal (with = 0, no = 1) | 2.796 | 0.582 | 13.592 | 0.002 | 16.384 | 5.236 | 51.266 |
| Blood flow distribution (central type = 0, mixed type = 1) | 0.093 | 0.209 | 1.563 | 0.329 | 1.098 | 0.729 | 1.654 |
| PI | 0.169 | 0.081 | 8.452 | 0.003 | 1.184 | 1.01 | 1.388 |
| Contrast distribution (uniform = 0, non-uniform = 1) | 0.453 | 0.301 | 3.73 | 0.065 | 1.573 | 0.872 | 2.838 |
| Elasticity score | 1.589 | 0.231 | 9.457 | 0.03 | 4.901 | 1.131 | 1.861 |
The AUC of PI in the diagnosis of lymph node metastasis was 0.698. When the elasticity score was combined with PI in the logistic regression model, the combined AUC increased to 0.890. The combination AUC of PI, elasticity score, L/S ratio and missing lymphatic portal for the diagnosis of lymph node metastasis was 0.949, which was significantly higher than the AUC of PI alone (P < 0.05, Figure 3). The fitting equation for the combined diagnosis was logit(P) = -10.230 + 1.753 × L/S ratio + 3.243 × missing lymphatic portal + 0.165 × PI + 2.248 × elasticity score.
Cervical lymph node metastasis in patients with PTC is closely related to the prognosis. Accurate diagnosis of metastatic cervical lymph nodes before surgery is essential[9-11]. Currently, ultrasound is commonly used in the diagnosis of PTC cervical lymph node metastasis[12-14]. The possible ultrasound results including L/S ratio < 2, missing lymphatic portal, liquefaction, calcification and other ultrasound signs suggest lymph node metastasis[15-17]. However, not every patient with lymph node metastasis has typical ultrasound signs. It is still necessary to combine other examination methods to improve the accuracy of diagnosis. With the development of ultrasound imaging and ultrasound elastography, its application in the diagnosis of lymph node metastasis is becoming more and more common. Contrast-enhanced ultrasound and elastography have been reported in the literature for preoperative diagnosis of PTC cervical lymph node metastasis[18-20]. However, regardless of contrast-enhanced ultrasound or elastography, the accuracy of individual diagnosis is unclear. The aim of this study was to explore the value of the combination of these two methods based on conventional ultrasound. Therefore, with the aim to investigate the diagnostic value for PTC cervical lymph node metastasis by combined contrast ultrasound and elastography on the basis of conventional ultrasound, the patient’s conventional ultrasound, contrast-enhanced ultrasound and elastography data of PTC cervical lymph node metastasis were compared and analyzed in the present study.
In this study, we found that L/S ratio ≤ 2, irregular margins, missing lymphatic portal, and non-uniform blood flow distribution were significantly higher in the metastatic group than in the reactive group, which is consistent with the report by Hong et al[21]. These indicators are typical ultrasound characteristics of PTC metastatic cervical lymph nodes. It is noteworthy that lymph node liquefaction is also a typical ultrasound characteristic but only two cases were found in the metastatic lymph nodes. Thus, it implied that although the lymph node liquefaction is typical, it is not universal enough and difficult to be used as an indicator for diagnosing lymph node metastasis. In addition, calcification, enhanced cortical echo and enhanced blood flow in metastatic lymph nodes were higher than those in reactive lymph nodes, but the difference was not statistically significant (P > 0.05). It suggested that these indexes cannot be accurately used alone for diagnosis. This may be one of the reasons why conventional ultrasound cannot accurately diagnose metastatic lymph nodes.
Contrast-enhanced ultrasound can clearly reflect microvascular information in the cervical lymph nodes[10,22,23]. Because of changes in the blood supply to lymph nodes infiltrated by cancer cells, ultrasound contrast is often displayed as non-uniform high perfusion from the periphery of the lymph nodes. The contrast-enhanced ultrasound of the reactive lymph nodes showed a uniform enhancement from the lymphatic portal. This phenomenon provides a reference for the diagnosis of cervical lymph nodes by contrast-enhanced ultrasound. In our study, PI and non-uniform contrast agent distribution in metastatic lymph nodes were significantly higher than those in reactive lymph nodes. It is suggested that the metastatic lymph nodes in our study also showed non-uniform high perfusion from the periphery of the lymph nodes. However, it must be recognized that different metastatic lymph nodes may have different blood supply status, and there is a difference in contrast-enhanced ultrasound[24-26]. Therefore, the accuracy of contrast-enhanced ultrasound in the diagnosis of PTC cervical lymph node metastasis remains controversial.
Currently, there are few studies on the relationship between elastography and PTC cervical lymph node metastasis. Duan et al[27] believed that the elasticity score of cervical lymph nodes was positively correlated with hardness, and the higher the score, the greater the possibility of metastasis. Xu et al[23] revealed the accuracy of acoustic radiation force impulse in predicting cervical lymph node metastasis. However, the value of elastography in the diagnosis of cervical lymph node metastasis was limited[28]. In the present study, the elasticity of metastatic lymph nodes was harder. The elastic score of 3 or 4 points was significantly higher than that of the reactive lymph nodes. It is also suggested that the overall hardness of the metastatic lymph nodes is higher than that of the reactive lymph nodes.
In order to further explore the role of conventional ultrasound, contrast-enhanced ultrasound and elastography in assessing PTC cervical lymph node metastasis, multivariate logistic regression analysis was performed. It was found that L/S ratio, missing lymphatic portal, PI and elasticity score were independent factors (P < 0.05). ROC curve analysis revealed that the AUC of PI in the individual diagnosis of lymph node metastases was only 0.698. Thus, it was difficult to be a diagnosis tool alone. However, when combined with elasticity score, the combined AUC increased to 0.890, which was significantly higher than that of PI alone. According to the result of logistic regression analysis in this study, missing lymphatic portal and L/S ratio ≤ 2 had a greater impact on cervical lymph node metastasis. Therefore, PI, elasticity, missing lymphatic portal and L/S ratio were combined, and it was found that the combined AUC could reach 0.949. It suggested that the combination of conventional ultrasound, contrast ultrasound and ultrasound elasticity can effectively improve the diagnostic accuracy. According to the fitting equation logit(P) = -10.230 + 1.753 × L/S ratio + 3.243 × missing lymphatic portal + 0.165 × PI + 2.248 × elasticity score, lymph node metastasis can be predicted by entering the above parameters using SPSS software.
In summary, the present study compared the differences among conventional ultrasound, contrast-enhanced ultrasound and elastography in the diagnosis of metastatic and reactive lymph nodes. A logistic regression model was used to combine the three technologies and provide a fitting equation for the diagnosis of lymph node metastasis. This combined diagnostic equation provided better diagnostic accuracy for metastatic lymph nodes than separate diagnoses. However, due to the limited sample size of the study, the fitting equation needs further verification.
Cervical lymph node metastasis in patients with papillary thyroid carcinoma (PTC) is closely related to the prognosis of patients. Therefore, the accurate diagnosis of preoperative cervical metastatic lymph nodes has an important impact on the choice of surgical plan and the prognosis of PTC patients. Ultrasound is a common imaging method for detecting cervical lymph nodes in PTC patients. Some ultrasound signs of lymph nodes can indicate the possibility of lymph node metastasis, but these ultrasound signs are not accurate in determining lymph node metastasis.
Currently, contrast-enhanced ultrasound and elastography are new techniques for ultrasound diagnosis. Studies have revealed that contrast-enhanced ultrasound and elastography can be used to diagnose metastatic lymph nodes, but the accuracy is controversial. Our study aimed to combine traditional ultrasound results and contrast-enhanced ultrasound and elastography to improve the accuracy of lymph node metastasis diagnosis.
In this study, we analyzed the conventional ultrasound, contrast-enhanced ultrasound and elastography data of lymph nodes in PTC patients. The purpose of this study was to explore the accuracy of combined use of contrast-enhanced ultrasound and elastography based on conventional ultrasound in the diagnosis of PTC cervical lymph node metastasis.
A total of 94 patients with PTC were recruited, and the patients were divided into a metastasis group and a reactive group. There were 50 nodules in the metastatic group and 63 nodules in the reactive group. Conventional ultrasound, contrast-enhanced ultrasound and elastography were performed and data were recorded. Logistic regression was used to generate predictive probability distributions for the diagnosis of lymph node metastasis with different indicators, and ROC curves were used to test the accuracy of different indicator combinations.
The long diameter/short diameter (L/S) ratio and missing lymphatic portal as revealed by traditional ultrasound, the peak intensity (PI) measured by contrast-enhanced ultrasound and the elastic score measured by elastography had an effect on the occurrence of PTC cervical lymph node metastasis (P < 0.05). The accuracy of combined PI, elastic score, missing lymphatic portal and LS ratio in diagnosing lymph node metastasis was higher than the accuracy of individual diagnosis. The fitting equation for combined diagnosis was logit(P) = -12.341 + 1.482 × L/S ratio + 3.529 × missing lymphatic portal + 0.392 × PI + 3.288 × elasticity score.
Compared with traditional ultrasound diagnosis, the combination of contrast-enhanced ultrasound and elastography based on gray-scale ultrasound is expected to accurately assess PTC lymph node metastasis.
Both contrast-enhanced ultrasound and elastography are non-invasive ultrasound diagnostic techniques. The combined diagnosis can improve the diagnostic accuracy for PTC lymph node metastasis and provide important reference for the selection of clinical surgical plans.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
STROBE Statement: The authors have read the STROBE Statement, and the manuscript was prepared and revised according to the STROBE Statement.
P- Reviewer: Knittel T, Kato S, Lin JA S- Editor: Dou Y L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Qu N, Zhang L, Ji QH, Chen JY, Zhu YX, Cao YM, Shen Q. Risk Factors for Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Meta-Analysis. World J Surg. 2015;39:2459-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Park AY, Kim JA, Son EJ, Youk JH. Shear-Wave Elastography for Papillary Thyroid Carcinoma can Improve Prediction of Cervical Lymph Node Metastasis. Ann Surg Oncol. 2016;23:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, Tae K. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Nam SY, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, Chung JH. Preoperative ultrasonographic features of papillary thyroid carcinoma predict biological behavior. J Clin Endocrinol Metab. 2013;98:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Xiang D, Hong Y, Zhang B, Huang P, Li G, Wang P, Li Z. Contrast-enhanced ultrasound (CEUS) facilitated US in detecting lateral neck lymph node metastasis of thyroid cancer patients: diagnosis value and enhancement patterns of malignant lymph nodes. Eur Radiol. 2014;24:2513-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Park YJ, Kim JA, Son EJ, Youk JH, Park CS. Quantitative shear wave elastography as a prognostic implication of papillary thyroid carcinoma (PTC): elasticity index can predict extrathyroidal extension (ETE). Ann Surg Oncol. 2013;20:2765-2771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Jung WS, Kim JA, Son EJ, Youk JH, Park CS. Shear wave elastography in evaluation of cervical lymph node metastasis of papillary thyroid carcinoma: elasticity index as a prognostic implication. Ann Surg Oncol. 2015;22:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1063] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 10. | Moon HJ, Kim EK, Yoon JH, Kwak JY. Clinical implication of elastography as a prognostic factor of papillary thyroid microcarcinoma. Ann Surg Oncol. 2012;19:2279-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Al-Hilli Z, Strajina V, McKenzie TJ, Thompson GB, Farley DR, Regina Castro M, Algeciras-Schimnich A, Richards ML. Thyroglobulin Measurement in Fine-Needle Aspiration Improves the Diagnosis of Cervical Lymph Node Metastases in Papillary Thyroid Carcinoma. Ann Surg Oncol. 2017;24:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Batawil N, Alkordy T. Ultrasonographic features associated with malignancy in cytologically indeterminate thyroid nodules. Eur J Surg Oncol. 2014;40:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Jin ZQ, Lin MY, Hu WH, Li WY, Bai SJ. Gray-scale ultrasonography combined with elastography imaging for the evaluation of papillary thyroid microcarcinoma: as a prognostic clinicopathology factor. Ultrasound Med Biol. 2014;40:1769-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Qu N, Zhang L, Ji QH, Zhu YX, Wang ZY, Shen Q, Wang Y, Li DS. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer. 2014;14:914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Gürleyik E, Gurleyik G, Karapolat B, Onsal U. Incidental Papillary Thyroid Microcarcinoma in an Endemic Goiter Area. J Thyroid Res. 2016;2016:1784397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Yuasa Y, Sueda T. Prognostic impact of para-aortic lymph node metastasis in pancreatic ductal adenocarcinoma. World J Surg. 2010;34:1900-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Shinozaki M, Hoon DS, Giuliano AE, Hansen NM, Wang HJ, Turner R, Taback B. Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clin Cancer Res. 2005;11:2156-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Hong YR, Luo ZY, Mo GQ, Wang P, Ye Q, Huang PT. Role of Contrast-Enhanced Ultrasound in the Pre-operative Diagnosis of Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Carcinoma. Ultrasound Med Biol. 2017;43:2567-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Cui Z, Gao Y, Wang W, Zhu Z, Zhang Y, Ma Z. Evaluation of Neck Lymph Node Metastasis on Contrast-Enhanced Ultrasound: An Animal Study. Clin Exp Otorhinolaryngol. 2017;10:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Azizi G, Keller JM, Mayo ML, Piper K, Puett D, Earp KM, Malchoff CD. Shear Wave Elastography and Cervical Lymph Nodes: Predicting Malignancy. Ultrasound Med Biol. 2016;42:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Hong YR, Yan CX, Mo GQ, Luo ZY, Zhang Y, Wang Y, Huang PT. Conventional US, elastography, and contrast enhanced US features of papillary thyroid microcarcinoma predict central compartment lymph node metastases. Sci Rep. 2015;5:7748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92:2917-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 23. | Xu JM, Xu XH, Xu HX, Zhang YF, Guo LH, Liu LN, Liu C, Bo XW, Qu S, Xing M, Li XL. Prediction of cervical lymph node metastasis in patients with papillary thyroid cancer using combined conventional ultrasound, strain elastography, and acoustic radiation force impulse (ARFI) elastography. Eur Radiol. 2016;26:2611-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Qiu J, Xue X, Hu C, Xu H, Kou D, Li R, Li M. Comparison of Clinicopathological Features and Prognosis in Triple-Negative and Non-Triple Negative Breast Cancer. J Cancer. 2016;7:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Yip L, Nikiforova MN, Yoo JY, McCoy KL, Stang MT, Armstrong MJ, Nicholson KJ, Ohori NP, Coyne C, Hodak SP, Ferris RL, LeBeau SO, Nikiforov YE, Carty SE. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: a study of 1510 patients. Ann Surg. 2015;262:519-25; discussion 524-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Zou M, Baitei EY, Alzahrani AS, BinHumaid FS, Alkhafaji D, Al-Rijjal RA, Meyer BF, Shi Y. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014;24:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Duan SB, Yu J, Li X, Han ZY, Zhai HY, Liang P. Diagnostic value of two-dimensional shear wave elastography in papillary thyroid microcarcinoma. Onco Targets Ther. 2016;9:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Chen BB, Li J, Guan Y, Xiao WW, Zhao C, Lu TX, Han F. The value of shear wave elastography in predicting for undiagnosed small cervical lymph node metastasis in nasopharyngeal carcinoma: A preliminary study. Eur J Radiol. 2018;103:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |