Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.116
Peer-review started: August 14, 2018
First decision: October 8, 2018
Revised: October 16, 2018
Accepted: October 22, 2018
Article in press: October 22, 2018
Published online: January 6, 2019
Processing time: 143 Days and 15.4 Hours
The most common organ where follicular dendritic cell sarcoma (FDCS) occurs is in cervical lymph nodes, while few cases are found in extranodal organs such as liver, spleen, and soft tissue. This is a case report that FDCS occurs in the hepatogastric ligament. To our knowledge, there is no such case that has been reported previously. A 47-year-old male patient was found to have an intraabdominal mass during an annual physical examination. Computed tomography showed a 4.2 cm × 4.1 cm mass located at the lesser curvature of the stomach, above the pancreas. During operation, a tumor mass was found in the hepatogastric ligament and a radical resection was performed. The tumor was diagnosed as FDCS by pathology and immunohistochemical testing. The patient had a favorable recovery, and no obvious abnormality was found 3 months post-operation.
Core tip: Follicular dendritic cell sarcoma (FDCS) is a rare malignant tumor that is derived from hyperplasia dendritic cells. There have been no cases reported of FDCS located in the hepatogastric ligament. This is a very rare localization for FDCS and necessitates attention from clinicians regarding the possibility of an abdominal mass in FDCS patients.
- Citation: Yan WX, Yu YX, Zhang P, Liu XK, Li Y. Follicular dendritic cell sarcoma detected in hepatogastric ligament: A case report and review of the literature. World J Clin Cases 2019; 7(1): 116-121
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/116.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.116
Follicular dendritic cell sarcoma (FDCS) is a rare malignant tumor derived from hyperplasia dendritic cells. FDCS cases were first reported and discussed by Monda et al[1] in 1986. Unlike normal dendritic cells, follicular dendritic cells have no ability to present antigens[2]. FDCS, derived from hyperplastic dendritic cells, usually occurs in lymph nodes, especially in the cervical lymph node. Few cases occur in extranodal organs such as liver, spleen and soft tissue[3]. Most FDCS patients are young adults, with no notable gender differences[4]. Surgery is presently the main therapeutic strategy. Although more than 200 cases were found by searching PubMed databases from 1986 to present using “follicular dendritic cell sarcoma” as the key word, no cases were reported of FDCS in the hepatogastric ligament. To our knowledge, this is a very rare localization for FDCS and necessitates attention from clinicians regarding the possibility of an abdominal mass in FDCS patients.
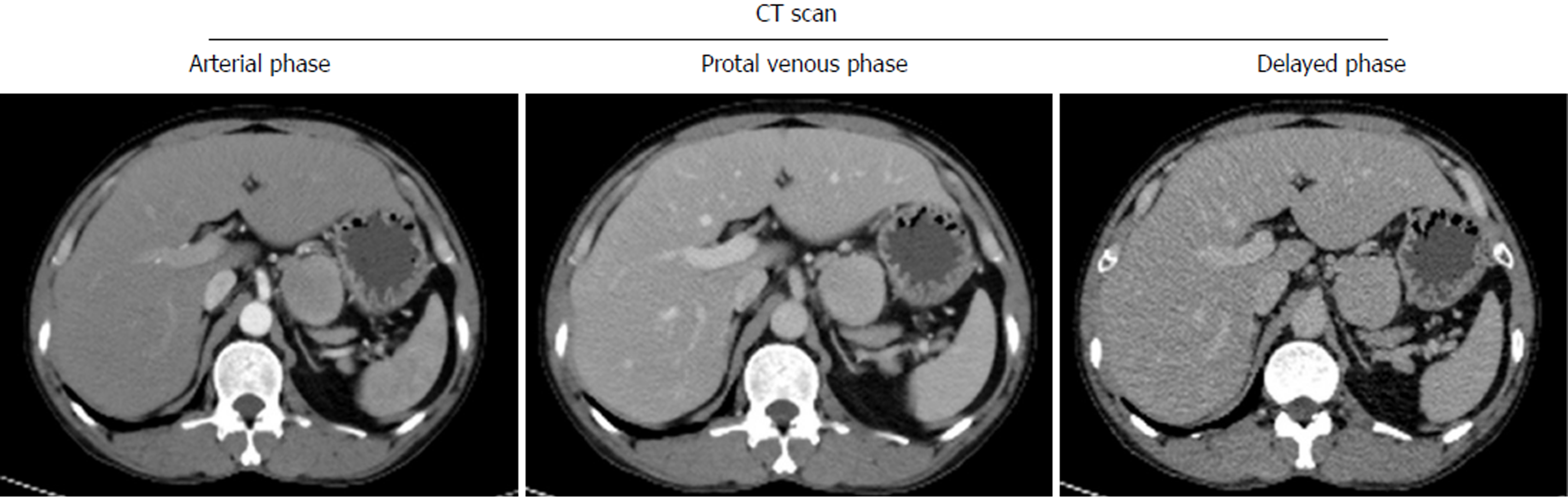
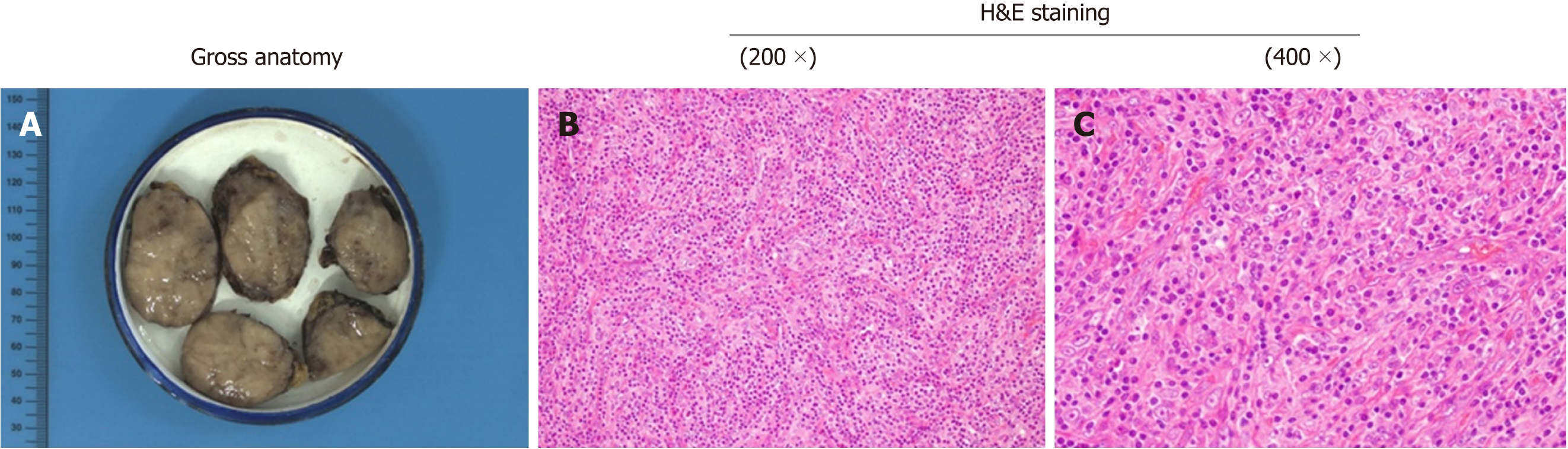
A 47-year-old male patient was found to have an enterocoelic mass during an annual physical exam. There were no any gastrointestinal symptoms when the patient was admitted to our hospital. Relevant past medical history included a long period of outdoor work, irregular eating times, smoking and alcohol abuse over about thirty years. Computed tomography (CT) scans showed a 4.2 cm × 4.1 cm mass located at the lesser curvature of the stomach, above the pancreas (Figure 1). The clinical diagnosis was an abdominal occupying mass. During operation, the tumor mass was found to be located in the hepatogastric ligament next to the lesser curvature of the stomach and cardia. A radical resection was performed and tumor size was measured to be 4.5 cm × 5.5 cm × 3 cm, with the appearance of a smooth surface, brown coloration, abundant blood supply and firm texture (Figure 2).
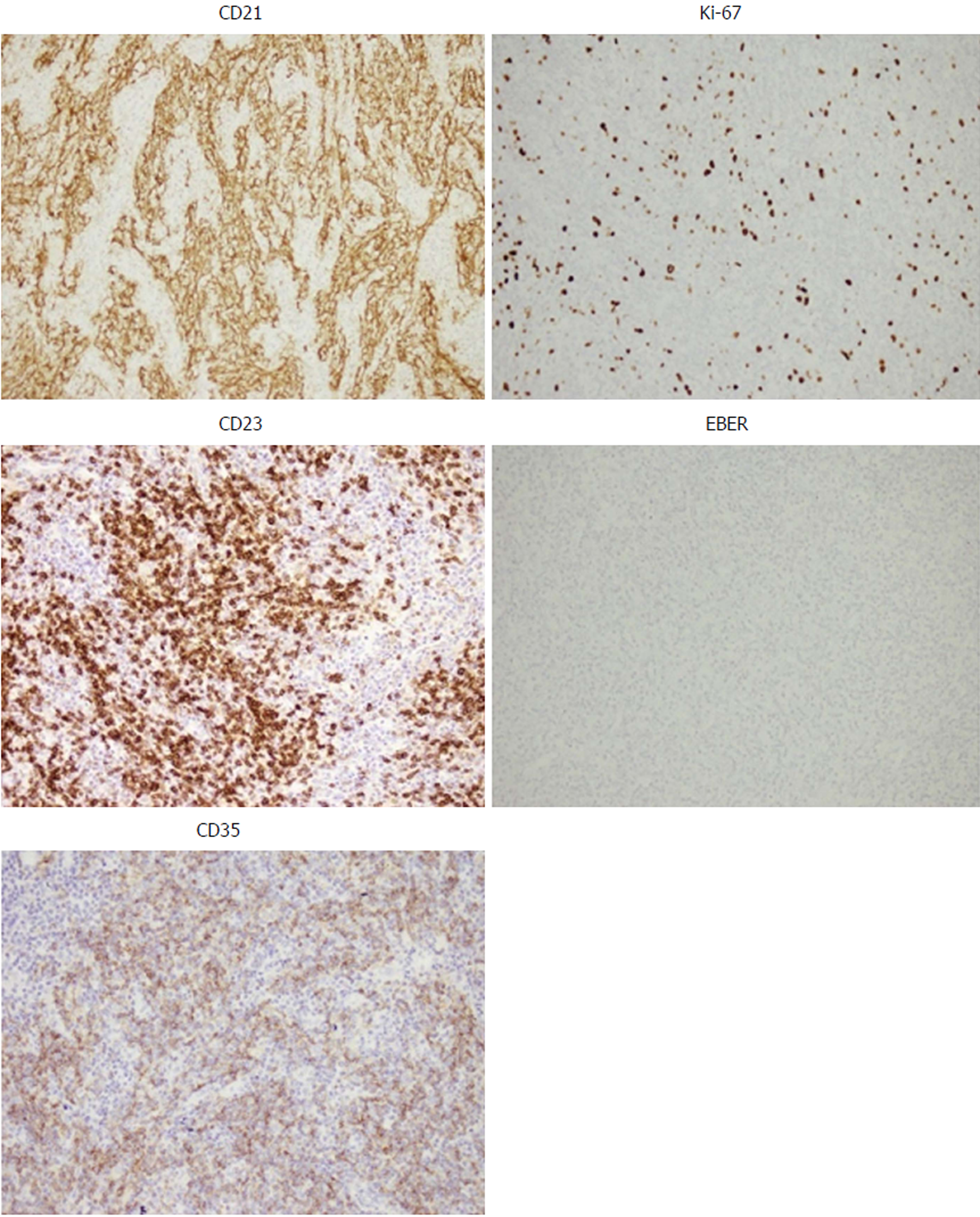
The pathological reports included the following: (1) Marginal sinus, non-expanded vessels, vestigial lymphoid follicles and vestigial reticular cells, tumor tissues are surrounded by adipose tissue and incrassated fiber capsule; (2) The spindle or oval tumor cells are arranged in a whirlpool or woven pattern; (3) Cells show abundant cytoplasm but no clear boundary between cells; and (4) Other pathological features include nearly-circular cell nucleus, slender chromatin, and few mitoses (Figure 2). The immunohistochemical test results included the following: CD21(+), CD23(+), CD35(+), S-100(partial+), BC12(+), BC16(+), CDε(T+), CD5(+), CD20(B+), CD38(little+), CD43(T+), Cyclind1(+), Ki67(20%+), Mum(partial+), CD10(-), Pax5(-), EBER(-). The representative immunohistochemical tests with positive staining and negative staining are shown in Figure 3. In summary, the pathological diagnosis was FDCS. Post-operation, the patient had a favorable recovery without complications. After the operation, the patient had no obvious abnormalities at the follow-up consultations in the first month and third months.
FDCS is a low-moderate malignant tumor but with a higher rate of relapse and metastasis[5]. The carcinogenic mechanisms of FDCS initiation and progression are largely unknown. About 10-20% of patients usually suffer from hyaline-vascular Castleman disease (HVCD)[6]. Since FDCS with HVCD has increased expression of vascular endothelial growth factor (VEGF), it is speculated that FDCS may be derived from hyperplastic dendritic cells stimulated by VEGF[7,8]. As a malignant tumor of the lymphatic system, no evidence shows that FDCS has any relationship with Epsteim-Barr virus and Epstein-Barr-encoded-RNA, which is typically used as a negative control for diagnosis[9].
There are about 200 cases of FDCS reported after Monda[1]’s first report in 1986. There is an incidence of morbidity in young adults with a median age of 43-year-old, however there are no gender differences. FDCS is usually detected in lymph nodes, especially the cervical lymph node. FDCS is also found in other lymph nodes such as the mediastinum, retroperitoneum, mesentery, and tonsil[10]. FDCS may occur in extronodal glands, including the liver, stomach, spleen, pancreas, intestine, muscles, and skin. Since the clinical manifestation of FDCS has no specificity, it is difficult to find or make an accurate diagnosis based solely on a general clinical examination. FDCS occurring in the lymph gland is typically in chronic processing, with painless swollen lymph nodes. FDCS that occurs intraabdominally may easily metastasize to other organs, such as the liver and lung.
The diagnosis of FDCS is mainly based on microscopy-based observations of cytological features and immunohistochemical tests. In low-power fields, the cytological features are characterized by spindle to ovoid or fusiform cell forming fascicles, whorle, diffuse sheet or nodular shapes with lymphocyte infiltration[11,12]. In high-power fields, cytoplasm appears as thin eosinophilic. Tumor cells have symplasma with non-clear boundaries. Cell nuclei are circular or ovular with clear nuclear envelopes. The chromatin usually exhibits vacuolization or a stipping shape. Mitochondria are occasionally seen in the nucleus. A large area of coagulative necrosis is usually found in patients with poor prognoses[13].
FDCS in electron microscopy shows long villar cells linked together by desmosome junctions[12]. Immunohistochemical characteristics of FDCS are positive staining for CD21, CD23 and CD35, which are the main diagnostic markers to differentiate from other diseases, including dendritic sarcoma, soft tissue sarcoma, lymphoma, and especially interdigitating dendritic cell sarcoma (IDCS). There are other non-specific positive immunohistochemistry biomarkers including CD68, S-100, CD1a, D2-40 and Ki-67. Positive Ki-67 expression is also an important index of prognosis[11].
The potential misdiagnosis of FDCS includes histiocytic sarcoma (HS), Langerhans cell sarcoma (LCS), and IDCS. The immunohistochemical biomarkers being used most often for these 4 diseases (FDCS, HS, LCS and IDCS) are CD68, CD1a, CD21, CD23, LYS and S-100. According to previous reports[13], the expression profile is the following: HS: CD68 (100%), CD1a (0%), CD21/CD35 (0%), LYS (94%), S100 (33%); LCS: CD68 (96%), CD1a (100%), CD21/CD35 (0%), LYS (42%), S100 (100%); IDCS: CD68 (50%), CD1a (0%), CD21/CD35 (0%), LYS (25%), S100 (100%); FDCS: CD68 (54%), CD1a (0%), CD21/CD35 (100%), LYS (8%), S-100 (16%). In addition, the diagnosis of FDCS also needs to be distinguished from ectopic thymoma, malignant melanoma, lymphoepithelioma-like carcinoma and malignant peripheral nerve sheath tumors. Immunohistochemical tests of CD21, CD23 and CD35 are the most accurate indicators for distinguishing FDCS from non-FDCS diseases.
Currently, there is no valid therapeutic strategy for FDCS. The Cytoxan, Hydroxyrubicin, Oncovin and Prednisone (CHOP) program is generally used for malignant lymphoma or soft tissue sarcoma. Reports indicate that the CHOP program has no satisfactory effect, however doxorubicin (DXR), etoposide, methylprednisolone, cisplatin, and cytarabine (ESHAP) are recommended[2]. Due to lack of sufficient data for statistical analysis and effective follow-up work, there is no affirmative answer to the curative effects. Radiotherapy and chemotherapy may be appropriated to those patients who have no chance of receiving an operation. However, we cannot treat patients with standard radiotherapy or chemotherapy regimens because FDCS is not diagnosed before surgery. The first choice is therefore radical resection. For prognosis, it is known that age (< 40-years-old), large tumor size (> 6 cm), mitotic counts (> 5/10 high-power field), positive Ki-67, and large areas of coagulative necrosis are indicators of a poor prognosis[2]. In addition, intra-abdominal FDCS, metastasis and recurrence more commonly lead to a poor prognosis.
Without any obvious or special clinical symptoms, an enterocoelic mass is found by computed tomography (CT) scanning during an annual physical examination.
An enterocoelic mass is found by CT.
Using different methods (location, imageology, histopathology) to distinguish it from other tumours.
Diagnosis of follicular dendritic cell sarcoma (FDCS) is mainly based on microscopical analysis of cytological features and immunohistochemistry.
CT can reveal occupying masses.
Immunohistochemical detection of CD21, CD23 and CD35 are the most accurate indicators that distinguish FDCS from non-FDCS diseases.
Radical resection to remove the tumor.
FDCS: Follicular dendritic cell sarcoma.
FDCS is still an uncommon disease and it is therefore necessary to find more effective diagnostic indicators and better treatment strategies.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): O
Grade B (Very good): O
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aghakhani A, Aykan NF, De Silva AP S- Editor: Ji FF L- Editor: Filipodia E- Editor: Bian YN
| 1. | Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562-572. [PubMed] |
| 2. | Sasaki M, Izumi H, Yokoyama T, Kojima M, Hosono A. Follicular dendritic cell sarcoma treated with a variety of chemotherapy. Hematol Oncol. 2017;35:905-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Sahay A, Bal M, Patil A, Kane S, Pai P. Follicular Dendritic Cell Sarcoma of the Larynx: Apropos a Rare Case with Review of the Literature. Turk Patoloji Derg. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5412] [Article Influence: 601.3] [Reference Citation Analysis (0)] |
| 5. | Lu H, Wang J. Extranodal follicular dendritic cell sarcoma in small intestinal mesentery. J Clin Exp Pathol. 2003;19:22-26. |
| 6. | Ruco LP, Gearing AJ, Pigott R, Pomponi D, Burgio VL, Cafolla A, Baiocchini A, Baroni CD. Expression of ICAM-1, VCAM-1 and ELAM-1 in angiofollicular lymph node hyperplasia (Castleman’s disease): evidence for dysplasia of follicular dendritic reticulum cells. Histopathology. 1991;19:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Chang YC, Chau IY, Yeh YC, Chau GY. Small intestine follicular dendritic cell sarcoma with liver metastasis: A case report. Medicine (Baltimore). 2017;96:e7261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol. 2006;36:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Shen SC, Wu CC, Ng KF, Wu RC, Chen HM, Chen TC. Follicular dendritic cell sarcoma mimicking giant cell carcinoma of the pancreas. Pathol Int. 2006;56:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Li L, Shi YH, Guo ZJ, Qiu T, Guo L, Yang HY, Zhang X, Zhao XM, Su Q. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16:2504-2519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Wang Q, An L, Cui N, Sha J, Zhu D. [Follicular dendritic cell sarcoma: a case report and review of literature]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25:100-102. [PubMed] |
| 13. | Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, Favera RD, Delsol G, De Wolf-Peeters C, Falini B, Gascoyne RD, Gaulard P, Gatter KC, Isaacson PG, Jaffe ES, Kluin P, Knowles DM, Mason DY, Mori S, Múller-Hermelink HK, Piris MA, Ralfkiaer E, Stein H, Su JJ, Warnke RA, Weiss LM. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |