Published online Jun 16, 2018. doi: 10.12998/wjcc.v6.i6.127
Peer-review started: February 14, 2018
First decision: March 8, 2018
Revised: March 24, 2018
Accepted: April 11, 2018
Article in press: April 12, 2018
Published online: June 16, 2018
Processing time: 126 Days and 11.4 Hours
Identification of left ventricular mural thrombus (LVT) may be challenging depending on the imaging modality used. We present a case of LVT which was incidentally identified on cine cardiac magnetic resonance imaging (CMR). A sixty-four years old female presented with worsening dyspnea on exertion with troponin elevation. Transthoracic echocardiography (TTE) revealed a dilated left ventricle (LV) and ejection fraction (EF 30%) with thinning and akinesis of inferior/inferolateral wall was noted with basal and mid inferior wall aneurysm, and thrombus was not identified. CMR done to ascertain viability of myocardium revealed a mural thrombus within basal inferior aneurysm. This was not visualized on transthoracic echocardiography with and without use of contrast. She underwent coronary artery bypass grafting, bioprosthetic mitral valve replacement, resection and plication of posterior left ventricular aneurysm with removal of mural thrombus, and was started on anticoagulation with warfarin post-operatively for the apical thrombi. Cardiac magnetic resonance is a well suited imaging modality in detecting LVT due to its high resolution images and is more reproducible than TTE. In our patient, conventional TTE despite administration of echo-contrast agents failed to diagnose the presence of LVT in the basal inferior aneurysm as well as the apical thrombi. Delayed-enhancement CMR provides the greatest sensitivity for detection of left ventricular thrombus, superior to standard transthoracic and contrast-enhanced transthoracic echocardiography.
Core tip: Delayed-enhancement cardiac magnetic resonance imaging provides the greatest sensitivity for detection of left ventricular thrombus, superior to standard transthoracic and contrast-enhanced transthoracic echocardiography.
- Citation: Siddiqui I, Nguyen T, Movahed A, Kabirdas D. Elusive left ventricular thrombus: Diagnostic role of cardiac magnetic resonance imaging-A case report and review of the literature. World J Clin Cases 2018; 6(6): 127-131
- URL: https://www.wjgnet.com/2307-8960/full/v6/i6/127.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i6.127
Left ventricular mural thrombus (LVT) complicating myocardial infarction has significant morbidity and potential mortality. Studies have demonstrated high incidence of LVT following anterior myocardial infarction[1]. LVT carries both short term and long term risk of embolic events which may result in stroke and systemic complications[2,3]. Early identification and prompt treatment is crucial in management of these patients. Multiple imaging modalities may be used to diagnose LVT including transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and cardiac magnetic resonance imaging (CMR)[4]. We present a patient with worsening dyspnea on exertion and heart failure with reduced ejection fraction (HFrEF) who was later found to have a laminated LV mural thrombus which was not identified on TTE despite ultrasound contrast agent administration for LV opacification.
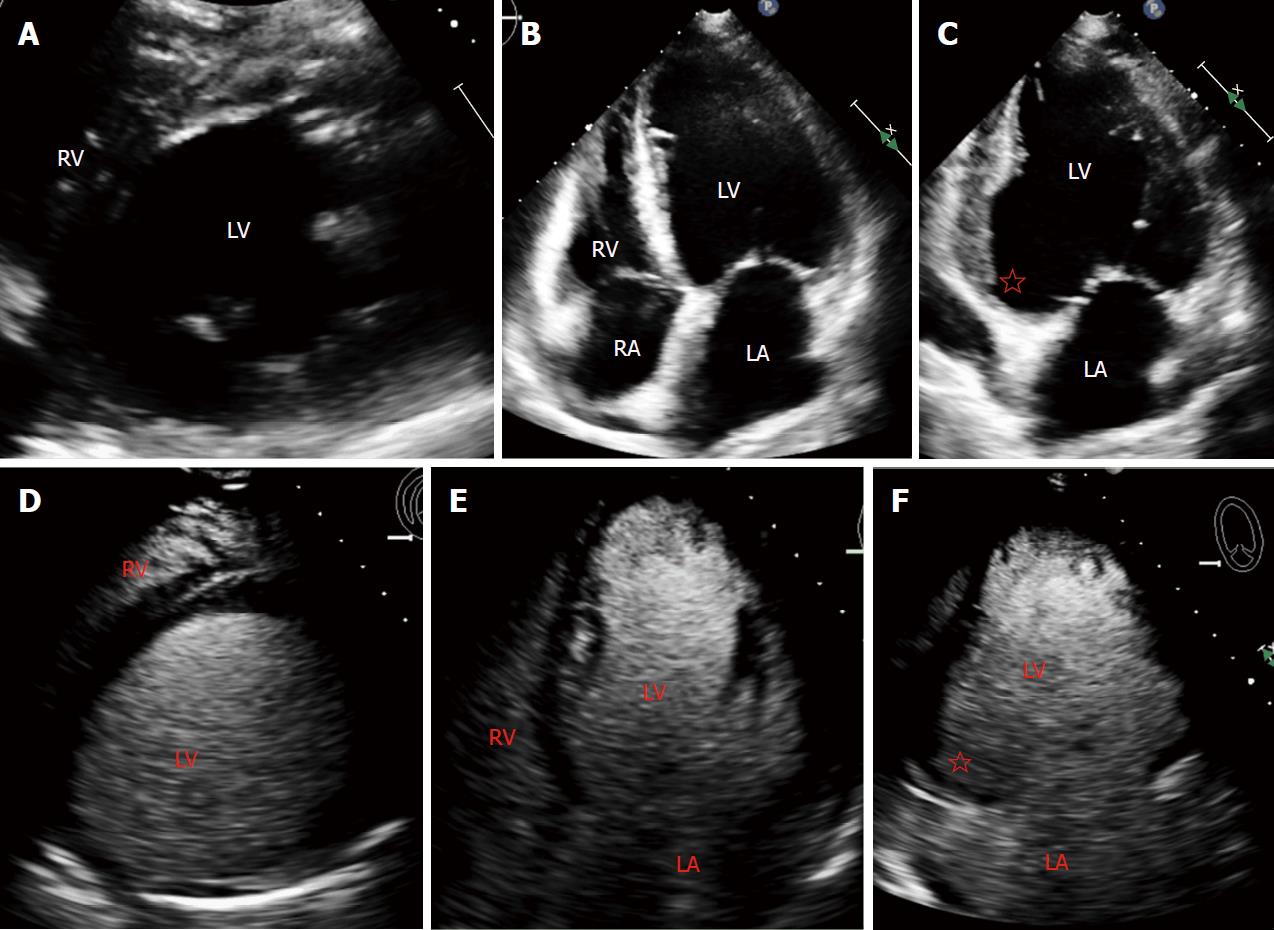
A sixty-four years old female with a past medical as well as family history of type II diabetes and hypertension presented to an outside facility with worsening dyspnea on exertion that had rapidly progressed from her baseline along with troponin elevation. Her electrocardiogram (EKG) did not show evidence for ST elevation. A transthoracic echocardiogram (TTE) was performed which revealed EF 20%-25%, severe inferior hypokinesis, mild apical hypokinesis. She underwent cardiac catheterization for presumed ischemic cardiomyopathy due to reduced EF and troponin elevation, which revealed chronic occlusion of the right coronary artery, subtotal occlusion of the proximal LAD, a severe stenosis in the first circumflex marginal and an occluded second marginal. She was transferred to our facility for coronary artery bypass grafting. TTE on presentation to our facility revealed a severely dilated left ventricle (LV) with eccentric hypertrophy and reduced ejection fraction (EF) of 30%. Thinning and akinesis of the inferior/inferolateral wall was noted with basal and mid inferior wall aneurysm which was likely supplied by an occluded right coronary artery. There was severe functional secondary mitral regurgitation arising from annular dilatation. No thrombus was identified despite administration of ultrasound contrast agent (Figure 1).
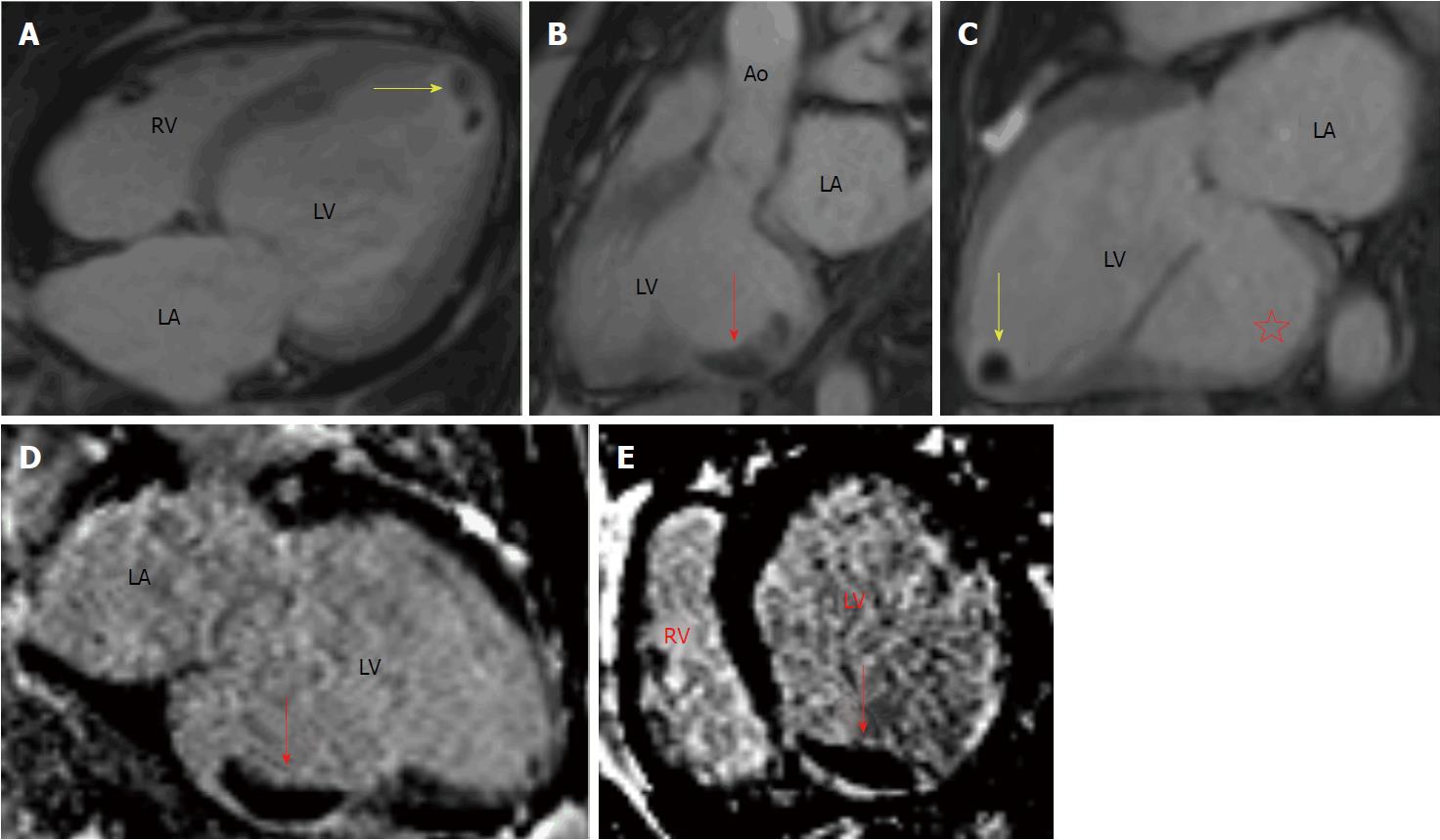
CMR was also done within 24 h of the TTE to ascertain viability of myocardium to determine candidacy for surgical revascularization. CMR revealed a severely dilated LV chamber size with large basal inferior wall aneurysm. Post Gadolinium Inversion recovery sequences with high inversion time of 600 ms revealed 3 cm x 1.3 cm mass within basal inferior aneurysm with homogenous black appearance confirming presence of mural thrombus. There were also 2 apical thrombi visualized with largest measuring 1 cm x 1 cm (Figure 2). Aneurysmal basal inferior wall was non-viable however myocardium supplied by left anterior descending (LAD) and left circumflex territory (LCX) was noted to be viable. She underwent coronary artery bypass grafting with a saphenous vein graft (SVG) to LAD and a SVG to the first obtuse marginal branch of LCX as well as bioprosthetic mitral valve replacement (MVR). She also underwent resection and plication of posterior left ventricular aneurysm with removal of mural thrombus. She was started on anticoagulation with warfarin post-operatively for the apical thrombi.
While transthoracic echocardiography is a valuable diagnostic test in assessing cardiac structure and function and remains a mainstay as initial diagnostic imaging for potential cardiac source of embolism[5], its utility in the detection and diagnosis of LVT is variable. Previous consensus documents have recommended TTE as an initial screening tool for LVT[6,7] and early studies have evaluated its sensitivity and specificity in detection of LVT[8-10]. Factors such as indication for TTE and use of echo contrast influence its sensitivity for detection of LVT as reported by Weinsaft et al[11] If the clinical indication was specifically to evaluate for LVT, the sensitivity increased from 26% to 60%, with an overall sensitivity and specificity of 33% and 91%, respectively. CMR is currently a widely available imaging modality gaining popularity for detection of cardiac thrombus. Investigators have found CMR to have a higher sensitivity and specificity of 58% and 99%, respectively for detection of LV thrombus in comparison with TTE. Additionally, they found that both cine-CMR and TTE were less likely to detect small protuberant thrombus as well as mural thrombus.
Cardiac magnetic resonance is a well suited imaging modality in detecting LVT due to its high resolution images and is more reproducible than TTE[12]. While it is the preferred method for definitive detection of LVT, differences in sensitivity exist between cine and delayed enhancement CMR (DE-CMR). DE-CMR is a technique which utilizes Inversion Recovery (IR) sequences which is typically used to identify infarcted myocardium from viable myocardium to aid in determination of candidacy for surgical revascularization in patients with severely reduced left ventricular function. Conventional DE-CMR utilizes an inversion time (also known as TI) of about 200-300 ms which makes viable myocardium appear black (null) while infarcted myocardium appears bright whereas mural thrombus appears variable, gray with an “etched” appearance. DE-CMR can be tailored to detect thrombus by adjusting the inversion time to longer intervals, i.e., long TI fixed at about 600 ms aids in nulling avascular tissue such as mural thrombus and renders a homogeneously black appearance which is characteristic and diagnostic of thrombus. Weinsaft and colleagues compared sensitivities of cine-CMR vs DE-CMR amongst a sample and found that DE-CMR identified LVT in a higher number of patients compared to cine-CMR (7% vs 4.7%) which was confirmed by pathology[13]. The limitation of cine-CMR was especially evident with mural thrombi and it was concluded by the authors that DE-CMR is a more clinically useful tool for detection of LVT. Additionally, DE-CMR with long TI has also been demonstrated to be effective in differentiating thrombus from neoplasm[14].
Our case highlights the limitations of transthoracic echocardiography in detecting laminated mural thrombi. In these instances, DE-CMR is invaluable in establishing a diagnosis. The indication for TTE in this case was not detection of LVT, and hence the literature would suggest that the sensitivity for detection in this case would be low (26%). Additional groups such as Srichai et al[4] demonstrated a 100% sensitivity of DE-CMR in detecting LV thrombi in patients with recent systemic embolism whereas TTE detected 33% and TEE detected 50% of confirmed thrombus. Diagnosis requires a high index of suspicion with careful attention to patient risk factors and predisposing anatomy on echocardiography.
In our patient, conventional TTE despite administration of echo-contrast agents failed to diagnose the presence of LVT in the basal inferior aneurysm as well as the apical thrombi. Lack of echo-contrast uptake by a laminated thrombus in an aneurysmal basal inferior wall camouflaged as the myocardium itself and hence likely concealed the thrombus. Two small apical thrombi which were well visualized in DE-CMR were also not detected in contrast-enhanced TTE which points to the decreased sensitivity of the modality to exclude LVT. Cine-CMR in our patient revealed thinned, dyskinetic basal inferior wall aneurysm and DE-CMR confirmed the presence of thrombi. CMR proved to be a comprehensive diagnostic tool in identifying myocardial viability/candidacy for surgical revascularization, confirming severity of mitral regurgitation, and detecting ventricular aneurysm and mural thrombi. Preoperative diagnosis of LVT and aneurysm was vital in the management of our patient not only to initiate anticoagulation therapy but also aided in surgical planning. Our patient successfully underwent CABG, MVR, removal of mural thrombus, and resection and plication of ventricular aneurysm.
In conclusion, Delayed-enhancement CMR provides the greatest sensitivity for detection of left ventricular thrombus, superior to standard transthoracic and contrast-enhanced transthoracic echocardiography.
A 64-year-old female with type II diabetes and hypertension presented with worsening dyspnea on exertion that had rapidly progressed from her baseline with orthopnea and paroxysmal nocturnal dyspnea.
Pulmonary rales, jugular venous distension, and a loud holosystolic murmur radiating to axilla.
New-onset heart failure due to mitral regurgitation vs cardiomyopathy.
Elevated troponin and brain natriuretic peptide.
Transthoracic echocardiogram with ultrasound enhancing agent showed severe functional secondary mitral regurgitation and reduced left ventricle ejection fraction of 30% with inferior wall aneurysm, while delayed enhancement cardiac magnetic resonance imaging (CMR) illustrated a large mural thrombus measuring 3.0 cm x 1.3 cm adherence to the basal inferior wall aneurysm as well as two apical thrombi.
Coronary artery bypass grafting, mitral valve replacement, removal of mural thrombus, and resection and plication of ventricular aneurysm.
Cardiac magnetic resonance is a well suited imaging modality in detecting left ventricular thrombus due to its high resolution images and is more reproducible than transthoracic echocardiogram.
Delayed-enhancement CMR provides the greatest sensitivity for detection of left ventricular thrombus, superior to standard transthoracic and contrast-enhanced transthoracic echocardiography.
CARE Checklist (2013): The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Satoh H S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 413] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Stratton JR, Resnick AD. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987;75:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol. 1993;22:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 293] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, Landeck BF, Maganti K, Michelena HI, Tolstrup K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J Am Soc Echocardiogr. 2016;29:1-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 6. | Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003;16:1091-1110. [PubMed] |
| 7. | Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davidson TW, Davis JL, Douglas PS, Gillam LD. ACC/AHA guidelines for the clinical application of echocardiography: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. J Am Coll Cardiol. 1997;29:862-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Visser CA, Kan G, David GK, Lie KI, Durrer D. Two dimensional echocardiography in the diagnosis of left ventricular thrombus. A prospective study of 67 patients with anatomic validation. Chest. 1983;83:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Ezekowitz MD, Wilson DA, Smith EO, Burow RD, Harrison LH Jr, Parker DE, Elkins RC, Peyton M, Taylor FB. Comparison of Indium-111 platelet scintigraphy and two-dimensional echocardiography in the diagnosis of left ventricular thrombi. N Engl J Med. 1982;306:1509-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 105] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Stratton JR, Lighty GW Jr, Pearlman AS, Ritchie JL. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation. 1982;66:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 219] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, Brosnan R, Shah DJ, Velazquez EJ, Parker M. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging. 2011;4:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1075] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 13. | Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Pazos-López P, Pozo E, Siqueira ME, García-Lunar I, Cham M, Jacobi A, Macaluso F, Fuster V, Narula J, Sanz J. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc Imaging. 2014;7:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |