Published online Mar 16, 2018. doi: 10.12998/wjcc.v6.i3.20
Peer-review started: November 1, 2017
First decision: November 20, 2017
Revised: December 31, 2017
Accepted: February 28, 2018
Article in press: February 28, 2018
Published online: March 16, 2018
Processing time: 134 Days and 10.9 Hours
To evaluate whether there was any correlation between the clinical parameters and final pathological results among patients who underwent thyroid surgery.
We retrospectively analyzed parameters, including age, sex, complete blood cell count parameters, nodule diameter, nodule localization, thyroid function testing, and pathology reports, in patients who underwent thyroid surgery. The patients were divided into malignant (n = 92) and benign (n = 413) groups depending on the final pathological results. Both groups were compared for demographic and clinical parameters. The Kolmogorov-Smirnov normality test was used to determine if the quantitative variables had a normal distribution. The nonparametric Mann-Whitney U test was used to compare quantitative data that were not normally distributed, and Pearson’s chi-squared test was used to compare the qualitative data. The correlation between the final pathological results and fine-needle aspiration biopsy findings was calculated using the cross-tabulation method.
This study included 406 women and 99 men aged between 15 and 85 years. No significant differences were found between the groups with respect to age, sex, white blood cell count, neutrophil count, lymphocyte count, thrombocyte count, red cell distribution width, platelet distribution width, mean platelet volume, platecrit, nodule localization, and thyroid function testing. On the other hand, there were significant differences between the groups with respect to nodule size (P = 0.001), cervical lymphadenopathy (P = 0.0001) and nodular calcification (P = 0.0001). Compared with the malignant group, the benign group had a significantly greater nodule size (35.4 mm vs 27.6 mm). The best cut-off point (≤ 28 mm) for nodule size, as determined by the receiver operating characteristic curve, had a sensitivity and specificity of 67.7% and 64.4%, respectively. The correlation between fine-needle aspiration biopsy and the final pathological results was assessed using the cross-table method. The sensitivity and specificity of fine-needle aspiration biopsy were 60% and 98%, respectively.
This study showed that significant differences existed between the malignant and benign groups with regard to nodule size, cervical lymphadenopathy, and nodular calcification.
Core tip: This retrospective study aimed to evaluate whether there was any correlation between the clinical parameters and the final pathological results among patients who underwent thyroid surgery. This study found no significant differences between the malignant and benign groups with respect to age, sex, white blood cell count, neutrophil count, lymphocyte count, thrombocyte count, red cell distribution width, platelet distribution width, mean platelet volume, platecrit, nodule localization, and thyroid function testing. On the other hand, there were significant differences between the groups with respect to nodule size, cervical lymphadenopathy, nodular calcification, and ultrasonographic examination findings. Compared with the malignant group, the benign group had a significantly larger nodule size. The sensitivity and specificity of fine-needle aspiration biopsy were 60% and 98%, respectively.
- Citation: Emre A, Akbulut S, Sertkaya M, Bitiren M, Kale IT, Bulbuloglu E, Colak C. Assessment of clinical and pathological features of patients who underwent thyroid surgery: A retrospective clinical study. World J Clin Cases 2018; 6(3): 20-26
- URL: https://www.wjgnet.com/2307-8960/full/v6/i3/20.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i3.20
Thyroid diseases occur at varying prevalences in various parts of the world, probably because of geographical and socioeconomic differences, and are one of the most common endocrine disorders. Surgical interventions are used for many benign and malignant thyroid disorders. Today, thyroid nodules have an ever-increasing prevalence and are diagnosed with substantial accuracy thanks to the improved diagnostic ability provided by technological advances. Despite this, a mismatch may occur between preoperative diagnosis and definitive pathological diagnosis of thyroid disorders. Excluding the childhood era, thyroid cancers constitute 1%-1.5% of all adulthood cancers[1]. Although previous studies have reported varying prevalences for thyroid nodules, their prevalence is 2%-6% when physical examination is used for diagnosis and 19%-35% based on imaging studies[2]. The prevalence of both malignant and benign thyroid disorders is increased in women and in the elderly.
Many studies to date have investigated whether any correlation exists between thyroid cancers and various clinical parameters for which a consensus has largely been reached in the literature. Among these are age, sex, nodule diameter, presence of cervical lymphadenopathy, some ultrasonographic nodule properties, and fine-needle aspiration biopsy (FNAB) findings. Recently, some laboratory parameters have been used as markers for the differentiation and prognosis of thyroid cancers. The examples include the use of the correlation between neutrophil/lymphocyte ratio and age in patients with papillary cancer; the use of the correlation between neutrophil/lymphocyte ratio and tumor diameter; the use of neutrophil/lymphocyte ratio as a marker in papillary microcarcinoma screening; and the use of mean platelet volume as a biomarker in thyroid cancers[3-9]. This study evaluated whether there was any correlation between the demographic and clinical parameters and the final pathological results of thyroidectomy specimens among patients who underwent thyroid surgery.
Between January 2010 and November 2015, 525 patients who underwent thyroidectomy for any reason in the Department of Surgery at Sutcu Imam University Faculty of Medicine in Kahramanmaras, Turkey were included in the study. The demographic, clinical and histopathological features of 505 of these patients were analyzed for this retrospective clinical trial. All the data were recorded in an Excel file by two authors (Emre A, Sertkaya M). The remaining twenty patients were excluded from the study because more than one piece of data, such as clinical or radiological data, were missing. This study was approved by the local ethics committee (Session No: 2016/03, Decree No: 09). Based on the final pathological results, the patients were divided into 2 groups: the malignant and benign groups. Both groups were compared with each other for age, sex, white blood cell count (WBC), lymphocyte count, neutrophil count, red cell distribution width (RDW), platelet distribution width (PDW), mean platelet volume (MPV), platecrit (PCT), thyroid function testing (euthyroidism, hyperthyroidism, hypothyroidism), ultrasonographic (US) findings (number of nodules, diameter of the largest nodule, nodule localization, nodule calcification, and lymphadenopathy in neck), fine-needle aspiration biopsy (FNAB), and pathological results. The results were recorded in an Excel file. Among the patients included in the present study, 379 underwent bilateral near total thyroidectomy, 85 underwent unilateral total lobectomy + isthmectomy, and 29 underwent bilateral subtotal thyroidectomy. Eight patients underwent bilateral total thyroidectomy + bilateral neck dissection, and 4 patients underwent bilateral total thyroidectomy + unilateral neck dissection.
The patients were referred to our clinic with benign thyroid disorders or suspected thyroid malignancies from other centers where they were examined with thyroid function testing and thyroid ultrasonography after a routine physical examination. Thyroid scintigraphy was carried out for patients with signs and symptoms of hyperthyroidism or suspected malignancy. Among the 505 patients included in this study, 406 underwent FNAB guided by palpation and/or US.
The statistical analyses of the present study were performed using IBM SPSS Statistics v23.0 and Medcalc 9.0 software packages. The study data were expressed as the mean ± SD, median, min-max and number of cases. The Kolmogorov-Smirnov normality test was used to determine if the quantitative variables had a normal distribution. The nonparametric Mann-Whitney U test was used to compare the quantitative data that were not normally distributed, and Pearson's chi-squared test was used to compare the qualitative data. The correlation between the final pathological results and the FNAB findings was calculated using the cross-table method. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FNAB were presented as percentages. Exact receiver operating characteristic (ROC) curves were drawn to calculate the cut-off points for nodule size. The best cut-off point for nodule size was calculated using Youden’s J Statistic. The confidence intervals were calculated using the bootstrap technique with 1000 samples and the random number seed approach. A P value of less than 0.05 was considered statistically significant for all the statistical analyses.
This study included a total of 505 patients aged 15 to 85 years, of whom 406 (80.4%) were women and 99 (19.6%) were men; thus, the female/male ratio was 4.1:1. According to the final pathological results, 261 patients had nodular hyperplasia; 53 had follicular adenoma; 52 had adenomatous hyperplasia; 49 had papillary carcinoma; 29 had papillary microcarcinoma; 20 had Hashimoto’s thyroiditis; 17 had focal lymphocytic thyroiditis; 8 had follicular carcinoma; 7 had Graves’ disease; 5 had medullary thyroid carcinoma; 3 had subacute granulomatous thyroiditis; and 1 had anaplastic cancer. The patients were grouped into two groups, namely, the malignant group (n = 92; 18.2%) and the benign group (n = 413; 81.8%). The patients in the benign group were 15 to 85 years old (mean ± SD: 49.8 ± 13.5 years), while the patients in the malignant group were 18 to 79 years old (mean ± SD: 47.2 ± 13.4 years). The two groups were not significantly different with respect to age (P = 0.09).
The groups were compared for preoperative complete blood count parameters. The two groups did not differ significantly in terms of WBC count (P = 0.703), thrombocyte count (P = 0.066), neutrophil count (P = 0.298), lymphocyte count (P = 0.295), RDW (P = 0.446), PDW (P = 0.883), MPV (P = 0.092), and PCT (P = 0.359) (Table 1). In other words, these parameters had no effect on the development of benign or malignant thyroid disorders.
| Clinical parameters | Pathology | Mean | SD | Median | Min | Max | Kolmogorov-Smirnov | P value |
| Age (yr) | Benign | 49.8 | 13.5 | 51 | 15 | 85 | 0.0001 | 0.09 |
| Malign | 47.2 | 13.4 | 45 | 18 | 79 | |||
| Total | 49.3 | 13.5 | 50 | 15 | 85 | |||
| Preoperative WBC | Benign | 7762 | 2200 | 7500 | 1005 | 16090 | 0.0001 | 0.703 |
| Malign | 7801 | 1998 | 7395 | 4020 | 13090 | |||
| Total | 7769 | 2163 | 7490 | 1005 | 16090 | |||
| Preoperative neutrophil count (%) | Benign | 0.61 | 0.09 | 0.61 | 0.35 | 0.95 | 0.0001 | 0.298 |
| Malign | 0.62 | 0.08 | 0.62 | 0.31 | 0.93 | |||
| Total | 0.6 | 0.09 | 0.61 | 0.31 | 0.95 | |||
| Preoperative Lymphocyte count (%) | Benign | 0.29 | 0.07 | 0.3 | 0.03 | 0.57 | 0.007 | 0.295 |
| Malign | 0.28 | 0.06 | 0.29 | 0.05 | 0.44 | |||
| Total | 0.29 | 0.07 | 0.29 | 0.03 | 0.57 | |||
| Preoperative Thrombocyte count | Benign | 289633 | 78647 | 285000 | 95000 | 621000 | 0.003 | 0.066 |
| Malign | 304148 | 77386 | 297000 | 155000 | 560000 | |||
| Total | 292257 | 78542 | 287000 | 95000 | 621000 | |||
| Preoperative RDW (%) | Benign | 0.14 | 0.02 | 0.14 | 0.12 | 0.3 | 0.0001 | 0.446 |
| Malign | 0.15 | 0.02 | 0.14 | 0.12 | 0.23 | |||
| Total | 0.15 | 0.02 | 0.14 | 0.12 | 0.3 | |||
| Preoperative PDW | Benign | 47.7 | 8.4 | 47 | 17 | 71 | 0.003 | 0.883 |
| Malign | 47.8 | 7.6 | 46.5 | 33 | 65 | |||
| Total | 47.7 | 8.2 | 47 | 17 | 71 | |||
| Preoperative MPV | Benign | 8.91 | 1.26 | 8.81 | 5.1 | 13.1 | 0.0001 | 0.092 |
| Malign | 8.72 | 1.25 | 8.65 | 6.5 | 12.7 | |||
| Total | 8.87 | 1.25 | 8.8 | 5.1 | 13.1 | |||
| Preoperative PCT | Benign | 0.25 | 0.07 | 0.25 | 0.09 | 0.56 | 0.0001 | 0.359 |
| Malign | 0.26 | 0.06 | 0.26 | 0.13 | 0.47 | |||
| Total | 0.25 | 0.07 | 0.25 | 0.09 | 0.56 | |||
| Nodule size (mm) | Benign | 35.4 | 16.9 | 35 | 5 | 115 | 0.0001 | 0.001 |
| Malign | 27.6 | 18.2 | 20.5 | 7 | 110 | |||
| Total | 34.0 | 17.4 | 32 | 5 | 115 |
Among the 99 males included in the study population, 86 (86.9%) had benign thyroid disease, whereas 13 (13.1%) had malignant thyroid disease. Among the female subjects, 327 (80.5%) had benign thyroid disease, whereas 79 (19.5%) had malignant thyroid disease. There was no statistical correlation between sex and final thyroid pathological results (P = 0.143) (Table 2). Preoperative blood tests revealed euthyroidism in 243 (48.1%) patients; hyperthyroidism in 214 (42.4%) patients; and hypothyroidism in 48 (9.5%) patients. In the benign group, 47.9% of the patients had euthyroidism; 43.6% had hyperthyroidism; and 8.5% had hypothyroidism. The figures for the malignant group were 48.9%, 37%, and 14.1%, respectively. The two groups did not show significant differences with regard to thyroid function testing (P = 0.190). In other words, there was no significant correlation between preoperative thyroid function testing and the final pathological results. Fifty-seven patients (14.0%) were found to have cervical lymphadenopathy on physical and ultrasonographic examinations. Among the patients in the benign group, 8.7% had cervical lymphadenopathy, whereas 22.5% of the patients in the malignant group had cervical lymphadenopathy (P = 0.0001).
| Demographic and clinical parameters | Pathological findings | P value | |||
| Benign | Malignant | Total | |||
| Sex | Male | 86 | 13 | 99 | 0.143 |
| Female | 327 | 79 | 406 | ||
| FNAB | Benign | 205 | 14 | 219 | 0.0001 |
| Malign | 4 | 21 | 25 | ||
| Suspicious | 97 | 42 | 139 | ||
| Non-diagnostic | 22 | 1 | 23 | ||
| Cervical LAP | Present | 36 | 21 | 57 | 0.0001 |
| Absent | 377 | 71 | 448 | ||
| Nodular Calcification (USG) | Present | 105 | 46 | 151 | 0.0001 |
| Absent | 308 | 46 | 354 | ||
| Large Nodule Side | Right Lobe | 222 | 48 | 270 | 0.863 |
| Left Lobe | 165 | 39 | 204 | ||
| Isthmus | 26 | 5 | 31 | ||
| Thyroid USG | MNG | 326 | 77 | 403 | 0.422 |
| Diffuse goiter | 13 | 1 | 14 | ||
| Solitary nodule | 74 | 14 | 88 | ||
| Preop TFT | Euthyroid | 198 | 45 | 243 | 0.190 |
| Hyperthyroid | 180 | 34 | 214 | ||
| Hypothyroid | 35 | 13 | 48 | ||
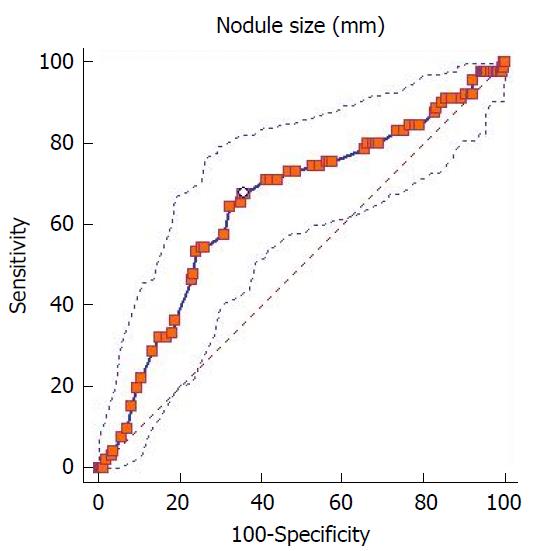
The diameter of the largest thyroid nodule ranged between 5 mm and 115 mm (mean ± SD: 35.4 ± 16.9 mm) in the benign group, whereas it ranged between 7 mm and 110 mm (mean ± SD: 27.6 ± 18.2 mm) in the malignant group. Both groups were significantly different with regard to nodule size [(P = 0.001), std error: 2.00, 95%CI: 3.59-11.45]. ROC curve analysis was used to determine the cut-off points for the correlation between nodule size and final pathological results, while Youden’s index was used to determine the best cut-off points. The area under the ROC curve was calculated to be 0.650 (95%CI: 0.60-0.69) (P = 0.0001). The best cut-off point calculated by Youden’s index was ≤ 28 mm [Youden’s index: 0.322 (95%CI: 0.18-0.41), associated criterion: ≤ 28 (95%CI: 26-33)]. This cut-off point had a sensitivity of 67.7% (95%CI: 57.1-77.2), a specificity of 64.4% (95%CI: 59.5-69.1), a positive likelihood ratio of 1.91 (95%CI: 1.6-2.3), and a negative likelihood ratio of 0.50 (95%CI: 0.4-0.7) (Figure 1).
Percutaneous or ultrasonography-guided FNAB sampling was performed in a total of 406 patients. Cytological examinations revealed benign histology in 219 patients; suspected malignancy in 139 patients; non-diagnostic histology in 23 patients; and malignant histology in the remaining patients. The final pathological examinations in the same patient group were consistent with benign histology in 328 patients and malignant histology in 78 patients (Table 2). The cross-tabulation technique was used to determine whether any correlation existed between the FNAB and final pathological results. Accordingly, FNAB had a sensitivity of 60% (95%CI: 42.1%-76.1%), a specificity of 98% (95%CI: 95.2%-99.5%), an area under the curve of 0.79 (95%CI: 0.73-0.84), a disease prevalence of 14.3% (95%CI: 10.2%-19.4%), a positive likelihood ratio of 31.35 (95%CI: 11.45-85.87), a negative likelihood ratio of 0.41 (95%CI: 0.27-0.61), a positive predictive value of 84% (95%CI: 63.92%-95.46%), and a negative predictive value of 93.6% (95%CI: 89.5%-96.46%).
The prevalence of thyroid disorders varies among different regions of the world because of geographical, socioeconomical, and nutritional differences. Thyroid cancers constitute approximately 92% of all endocrine tumors[10]. Recent developments in diagnostic imaging methods, as a result of technological advances, have resulted in an ever-increasing prevalence of newly diagnosed cases of this cancer in both genders. Today, thyroid cancer accounts for 1.0%-1.5% of all newly diagnosed cancers[1].
The prevalence of thyroid nodules, which ranges between 8% and 65%, has been determined most realistically by autopsy series in the literature[2]. The prevalence based on ultrasonography is 19%-35% because ultrasound can also detect nodules that are not palpable[2]. The prevalence of palpable nodules is lower, ranging between 2% and 7%[2]. The prevalence of thyroid nodules is higher among women, the elderly, and those who have been exposed to radiation or live in regions where iodine deficiency is endemic.
Benign disorders, such as diffuse multinodular goiter, diffuse toxic goiter, nodular goiter, toxic multinodular goiter, toxic adenomas, and thyroiditis, constitute the majority of thyroid disorders. The pathology specimens of benign disorders usually display nodular hyperplasia, follicular adenoma, adenomatous hyperplasia, and thyroiditis. In our study, the most common benign pathological finding was nodular hyperplasia, which was diagnosed in more than half of the patients. Since an association between Hashimoto’s thyroiditis and thyroid papillary cancer was reported by Dailey et al[13] in 1955, this topic has been a subject of debate in many publications[11-14]. The present study identified no cases of malignancy among patients who had Hashimoto’s thyroiditis during their 20s.
The prevalence of thyroid cancer ranges between 7% and 17% among patients who undergo surgery for nodular goiter[15-20]. These cancers are ranked by decreasing order of prevalence as papillary (73%), follicular (17.3%), medullary (3.2%), anaplastic (2.3%) and other types (4.2%)[10]. Although the cancer prevalence was higher in our study (18.2%) than has been previously reported, the order of the prevalence of cancer subtypes was similar. From a gender-based perspective, the data in the literature suggest that the prevalence of thyroid cancers is 4 times greater in women than men. Among 92 cancer patients included in our study, 85.9% were women. From a different perspective, however, we calculated a cancer prevalence of 19.4% in women and 13.1% in men who underwent thyroidectomy. This inter-gender difference in cancer prevalence was statistically insignificant (P = 0.143). These results suggest that women undergo a greater number of thyroid surgeries and therefore have a relatively greater rate of thyroid cancer.
Many studies have shown a correlation between age and thyroid cancer prevalence and have suggested that age is a risk factor for cancer development[20,21]. Therefore, age has been included as a parameter in many classifications that are used to determine the prognosis of differentiated thyroid cancers. In this study, we failed to show any significant correlation between age and thyroid cancer; that is, there was no age difference between patients with and without cancer. However, the retrospective design of our study limited our ability to reach a strong conclusion about the correlation between age and thyroid cancer development.
Recently, some laboratory parameters have been used as markers for evaluating the differentiation and prognosis of thyroid cancers. Examples include the use of the correlation between neutrophil/lymphocyte ratio and age in patients with papillary cancer; the use of the correlation between neutrophil/lymphocyte ratio and tumor diameter; the use of neutrophil/lymphocyte ratio as a marker in papillary microcarcinoma screening; and the use of mean platelet volume as a biomarker in thyroid cancers[3-9]. A comparison of our groups with respect to preoperative complete blood count parameters revealed that the two groups did not differ significantly in leukocyte, thrombocyte, neutrophil, or lymphocyte counts or RDW, PDW, MPV, and PCT values (Table 1). In other words, these parameters did not play a role in the development of benign or malignant thyroid disorders.
Even today, the relationship between thyroid function testing and thyroid malignancies is a subject of debate. Although some studies have reported that a low serum TSH level is associated with a reduced papillary carcinoma risk, we failed to show such an association in euthyroid, hyperthyroid, and hypothyroid cases[22]. We did not detect any significant differences in the thyroid function testing of the two groups. In other words, we failed to establish any significant link between preoperative thyroid function testing and final pathological results. Some researchers have argued that the prevalence of thyroid cancer is greater in patients with solitary nodules than in patients with multiple nodules. On the other hand, some studies have rejected an association between the number of nodules and cancer. According to a widely accepted view, the prevalence of thyroid cancer and nodule size are proportionally correlated. Nevertheless, a few researchers have shown that nodule size and cancer nodule size were not predictive of thyroid cancer[23]. McHenry et al[23] studied 676 thyroidectomy specimens and showed that malignant nodules had a significantly smaller nodule diameter compared to benign ones (P < 0.05); our results were parallel with these findings[23]. Furthermore, our results showed significantly better statistical significance (P = 0.001).
Many algorithms and classification schemes have been proposed and published in textbooks regarding the use of FNAB for the differentiation of malignant thyroid lesions from benign lesions. Moreover, FNAB may also yield false negative or false positive results despite its high accuracy for differentiating benign and malignant lesions. This is highly correlated with nodule size and number. It should be noted that FNAB may be associated with diagnostic errors, particularly for MNG and nodules smaller than 10 mm in diameter[22,24-26]. Four hundred and six of our patients underwent FNAB for the differential diagnosis of nodular lesions. Four patients with presumed false negative FNAB results were diagnosed as having malignancy after histopathological examination of the surgical specimens. Among the patients with equivocal cytopathology results, 30.2% received a diagnosis of malignancy after histopathological examination of the surgical specimens. Our results also demonstrated that FNAB may yield misleading results despite its high predictive value.
One of the main limitations of the present study was that there was no standardized algorithm to determine which patients should undergo surgery using a particular surgical procedure. The major cause of this limitation was the rapid physician circulation in our clinic. The second limitation of our study was its retrospective design. The third and most important limitation was the difficulty in accessing the medical records regarding whether laryngeal nerve and parathyroid gland injury, bleeding, or wound infection occurred, or whether a secondary exploration was performed. Therefore, we cannot discuss any findings about this subject.
The main objectives of this retrospective trial study were to evaluate whether there was any correlation between the clinical parameters and final pathological results among patients who underwent thyroid surgery.
We retrospectively analyzed the demographic, clinical and histopathological parameters of 505 patients who underwent thyroid surgery. The patients were divided into malignant (n = 92) and benign (n = 413) groups depending on the final pathological results. We analyzed whether there was any difference between the groups in terms of demographic and clinical parameters.
No significant differences were found between the groups with respect to age, sex, WBC count, neutrophil count, lymphocyte count, thrombocyte count, RDW, PDW, MPV, PCT, nodule localization, and thyroid function testing. On the other hand, there were significant differences between the groups with respect to nodule size, cervical lymphadenopathy and nodular calcification. The correlation between FNAB and final pathological results was assessed using the cross-table method, and the sensitivity and specificity for FNAB were 60% and 98%, respectively.
This study showed that significant differences existed between the malignant and benign groups with regard to nodule size, cervical lymphadenopathy, and nodular calcification.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Indrasena B, Zhang ZY S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 834] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 2. | Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 3. | Liu J, Du J, Fan J, Liu K, Zhang B, Wang S, Wang W, Wang Z, Cai Y, Li C. The Neutrophil-to-Lymphocyte Ratio Correlates with Age in Patients with Papillary Thyroid Carcinoma. ORL J Otorhinolaryngol Relat Spec. 2015;77:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Kocer D, Karakukcu C, Karaman H, Gokay F, Bayram F. May the neutrophil/lymphocyte ratio be a predictor in the differentiation of different thyroid disorders? Asian Pac J Cancer Prev. 2015;16:3875-3879. [PubMed] |
| 5. | Kim SM, Kim EH, Kim BH, Kim JH, Park SB, Nam YJ, Ahn KH, Oh MY, Kim WJ, Jeon YK. Association of the Preoperative Neutrophil-to-ymphocyte Count Ratio and Platelet-to-Lymphocyte Count Ratio with Clinicopathological Characteristics in Patients with Papillary Thyroid Cancer. Endocrinol Metab (Seoul). 2015;30:494-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Kim JY, Park T, Jeong SH, Jeong CY, Ju YT, Lee YJ, Hong SC, Ha WS, Choi SK, Jung EJ. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014;46:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Seretis C, Gourgiotis S, Gemenetzis G, Seretis F, Lagoudianakis E, Dimitrakopoulos G. The significance of neutrophil/lymphocyte ratio as a possible marker of underlying papillary microcarcinomas in thyroidal goiters: a pilot study. Am J Surg. 2013;205:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol. 2013;107:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Bayhan Z, Zeren S, Ozbay I, Kahraman C, Yaylak F, Tiryaki C, Ekici M. Mean Platelet Volume as a Biomarker for Thyroid Carcinoma. Int Surg. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Correa P, Chen VW. Endocrine gland cancer. Cancer. 1995;75:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Zeng RC, Jin LP, Chen ED, Dong SY, Cai YF, Huang GL, Li Q, Jin C, Zhang XH, Wang OC. Potential relationship between Hashimoto’s thyroiditis and BRAF(V600E) mutation status in papillary thyroid cancer. Head Neck. 2016;38 Suppl 1:E1019-E1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 259] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204:764-773; discussion 773-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Bukhari U, Sadiq S. Histopathological audit of goiter: A study of 998 thyroid lesions. Pakistan J Med Sci. 2008;24:442-446. |
| 16. | Wang C, Crapo LM. The epidemiology of thyroid disease and implications for screening. Endocrinol Metab Clin North Am. 1997;26:189-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Al-Maghrabi JA, Al-Enazi MH. Histopathological pattern of thyroid lesions in western region of Saudi Arabia. New Egypt J Med. 2009;40:580-585. |
| 18. | Nilubol N, Zhang L, Kebebew E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid. 2013;23:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Leeman-Neill RJ, Brenner AV, Little MP, Bogdanova TI, Hatch M, Zurnadzy LY, Mabuchi K, Tronko MD, Nikiforov YE. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119:1792-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6445] [Article Influence: 429.7] [Reference Citation Analysis (0)] |
| 21. | Qu N, Shi RL, Luo TX, Wang YL, Li DS, Wang Y, Huang CP, Ji QH. Prognostic significance and optimal cutoff of age in medullary thyroid cancer. Oncotarget. 2016;7:15937-15947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zhong LC, Lu F, Ma F, Xu HX, Li DD, Guo LH, Sun LP. Ultrasound-guided fine-needle aspiration of thyroid nodules: does the size limit its efficiency? Int J Clin Exp Pathol. 2015;8:3155-3159. [PubMed] |
| 23. | McHenry CR, Huh ES, Machekano RN. Is nodule size an independent predictor of thyroid malignancy? Surgery. 2008;144:1062-1068; discussion 1068-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Paja M, del Cura JL, Zabala R, Corta I, Lizarraga A, Oleaga A, Expósito A, Gutiérrez MT, Ugalde A, López JI. Ultrasound-guided core-needle biopsy in thyroid nodules. A study of 676 consecutive cases with surgical correlation. Eur Radiol. 2016;26:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Ucler R, Usluogulları CA, Tam AA, Ozdemir D, Balkan F, Yalcın S, Kıyak G, Ersoy PE, Guler G, Ersoy R. The diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy for thyroid nodules three centimeters or larger in size. Diagn Cytopathol. 2015;43:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Sharma C. Diagnostic accuracy of fine needle aspiration cytology of thyroid and evaluation of discordant cases. J Egypt Natl Canc Inst. 2015;27:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |