Published online Dec 26, 2018. doi: 10.12998/wjcc.v6.i16.1210
Peer-review started: July 6, 2018
First decision: October 4, 2018
Revised: November 15, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: December 26, 2018
Processing time: 170 Days and 20.3 Hours
Chondromyxoid fibroma (CMF) is a rare benign bone tumour of cartilaginous origin, which usually affects the metaphysis of the long bone. Involvement of the temporal bone is extremely rare. Patients with CMF in the temporal bone can present some neurological deficits due to involvement of surrounding neural structures.
We present the first case of histopathologically proven CMF originating in the temporal bone and involving the hypoglossal canal in a 40-year-old woman. Hypoglossal nerve paralysis was identified on the cranial nerve examination. The patient underwent surgical excision and was neurologically normal except for mild left facial palsy on 5-mo follow-up examination after surgery. In the current report, the major characteristics and computed tomography/magnetic resonance imaging features of the lesion are discussed. Furthermore, previous literature regarding this pathology is reviewed.
The current study presents the first case of temporal bone CMF involving the hypoglossal canal.
Core tip: Chondromyxoid fibroma (CMF) involving the temporal bone is extremely rare. We herein present the first reported case of a histopathologically proven CMF originating in the temporal bone and involving the hypoglossal canal in a 40-year-old woman. The major characteristics and imaging features of the lesion are discussed in this report.
- Citation: Zheng YM, Wang HX, Dong C. Chondromyxoid fibroma of the temporal bone: A case report and review of the literature. World J Clin Cases 2018; 6(16): 1210-1216
- URL: https://www.wjgnet.com/2307-8960/full/v6/i16/1210.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i16.1210
Chondromyxoid fibromas (CMF), a rare benign bone tumour originating from cartilaginous tissue, more frequently arises in the metaphyseal region of long bones[1,2]. A CMF encountering in the skull base, especially in the temporal bone, is extremely rare[3]. Neurological deficits are unusual in temporal bone CMF until the tumour involves adjacent cranial nerves, such as facial nerve and vestibulocochlear nerve[4]. To the best of our knowledge, no cases of temporal bone CMF involving the hypoglossal nerve have previously been reported. Here we present a rare case of CMF originating in the temporal bone with the ipsilateral hypoglossal nerve, facial nerve and jugular foramen invaded simultaneously. Special attention is given to the major characteristics and computed tomography/magnetic resonance imaging (CT/MRI) features of temporal bone CMF. In addition, a review of the previous literature regarding this rare disease entity is presented. Written informed consent was provided by this patient.
A 40-year-old woman presented to our neurosurgery clinic with a 5-mo history of left facial nerve paralysis and a 3-mo history of dysphagia showing up as water choked coughing. She also reported a dysarthria for a period of 2 mo and a headache for 1 mo. The patient had no history of past illness, and had no family history of specific diseases.
The patient was noted to have an intact cranial nerve examination bilaterally. Hypalgesia was identified in the left half of her face. The tongue deviated to the left on attempted midline protrusion. The ipsilateral atrophy of the tongue was also identified.
Measurements of routine laboratory examinations, including routine blood tests, routine urine tests and blood biochemistry, were within normal levels. No valuable laboratory data was found.
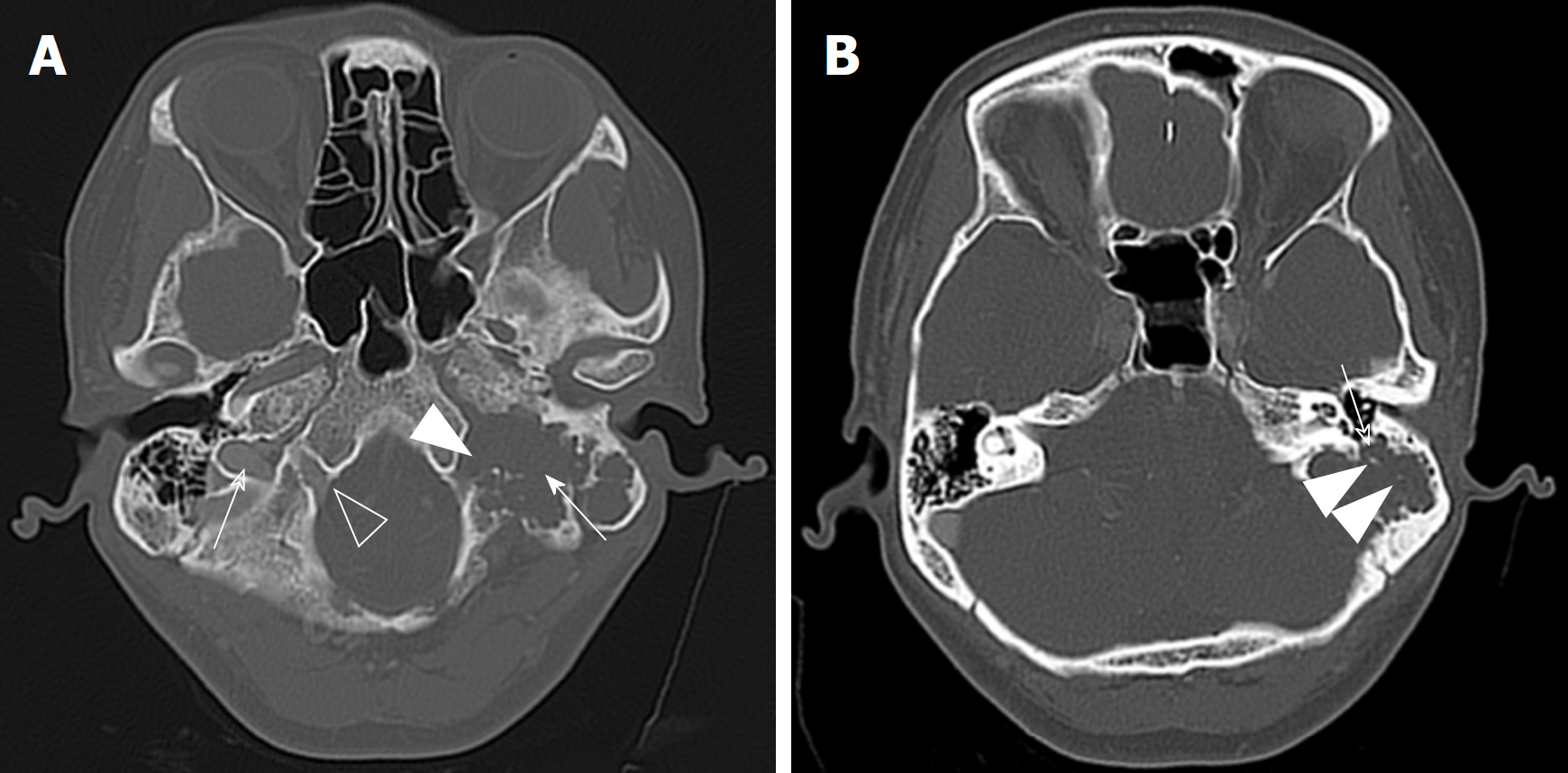
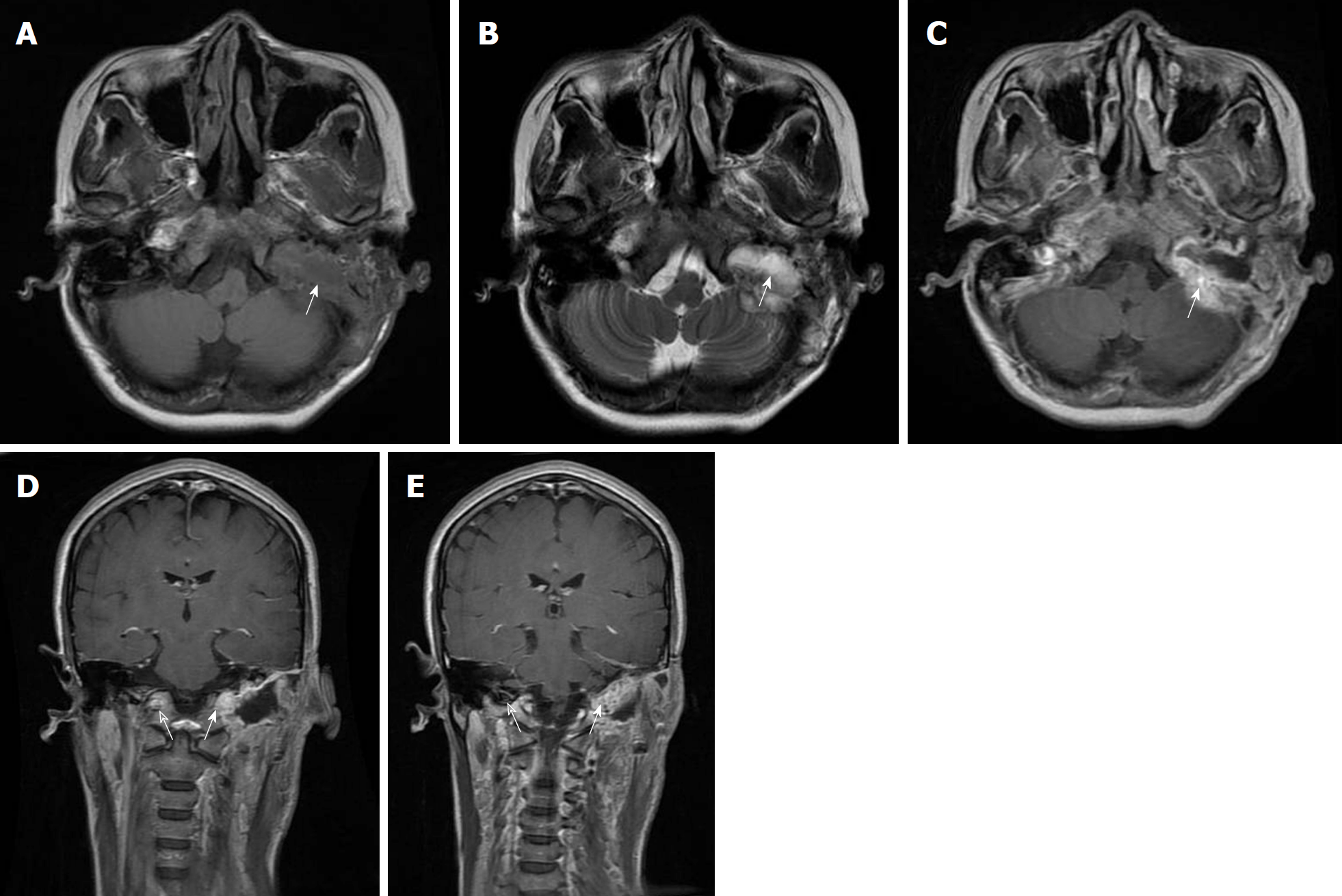
CT imaging revealed a lobulated soft tissue mass with a sclerotic rim and calcification. The tumour eroded the left mastoid air cells and the ipsilateral hypoglossal canal, as well as invaded the left mastoid portion of the facial nerve canal and jugular foramen (Figure 1). On magnetic resonance imaging without contrast enhancement, the lobulated lesion measured 4.4 cm × 2.3 cm × 4.0 cm and involved the left hypoglossal canal, exhibiting diffuse hypointensity on T1-weighted imaging and heterogeneous hyperintensity on T2-weighted imaging (Figure 2). The mastoid segment of the left facial nerve could not be demonstrated separately from the lesion. After contrast agent [gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA)] administration, the mass showed peripheral contrast enhancement, apart from the central area of hypointense that did not change in intensity.
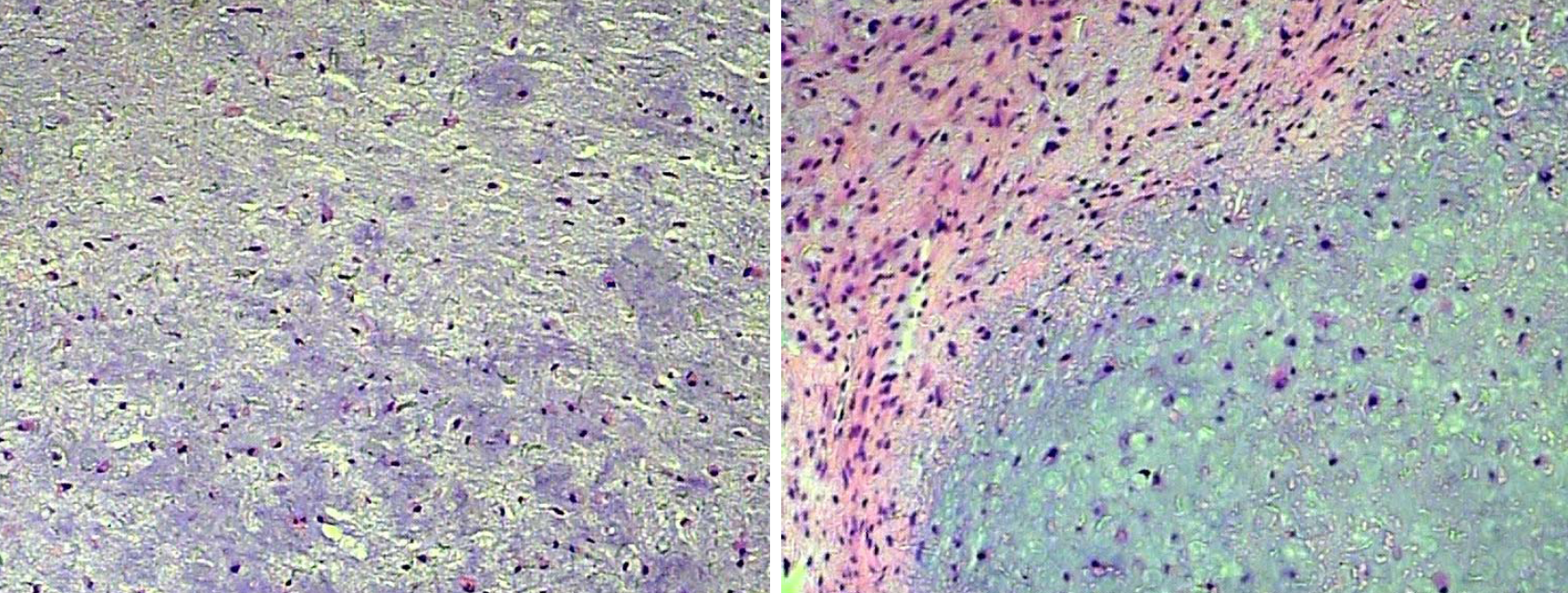
Histopathological analysis revealed a myxoid lesion consisting of cartilage material, admixed with bland stromal cells and fibroblastic tumoral cells. Immunohistochemical stains were positive for vimentin and S-100, but negative for GFAP and NF (Figure 3). These pathology results were consistent with a diagnosis of CMF.
Given uncertainty in diagnosis, the patient underwent mastoidectomy and surgical excision. At surgery, a left postauricular incision, which extended down to the level of the upper neck, was performed. Interfascial dissection was then used to expose the mastoid. The intraoperative appearance of the mass was irregular and lobulated with erosion of the mastoid air cells. Frank invasion of the ipsilateral hypoglossal canal and hypoglossal nerve was seen. The mastoid segment of the left facial nerve was compressed and invaded anteriorly by the mass. Using microscopic dissection and image guidance, excision of the tumour was performed. The portion of the tumour that had involved the hypoglossal canal and left facial nerve was also resected.
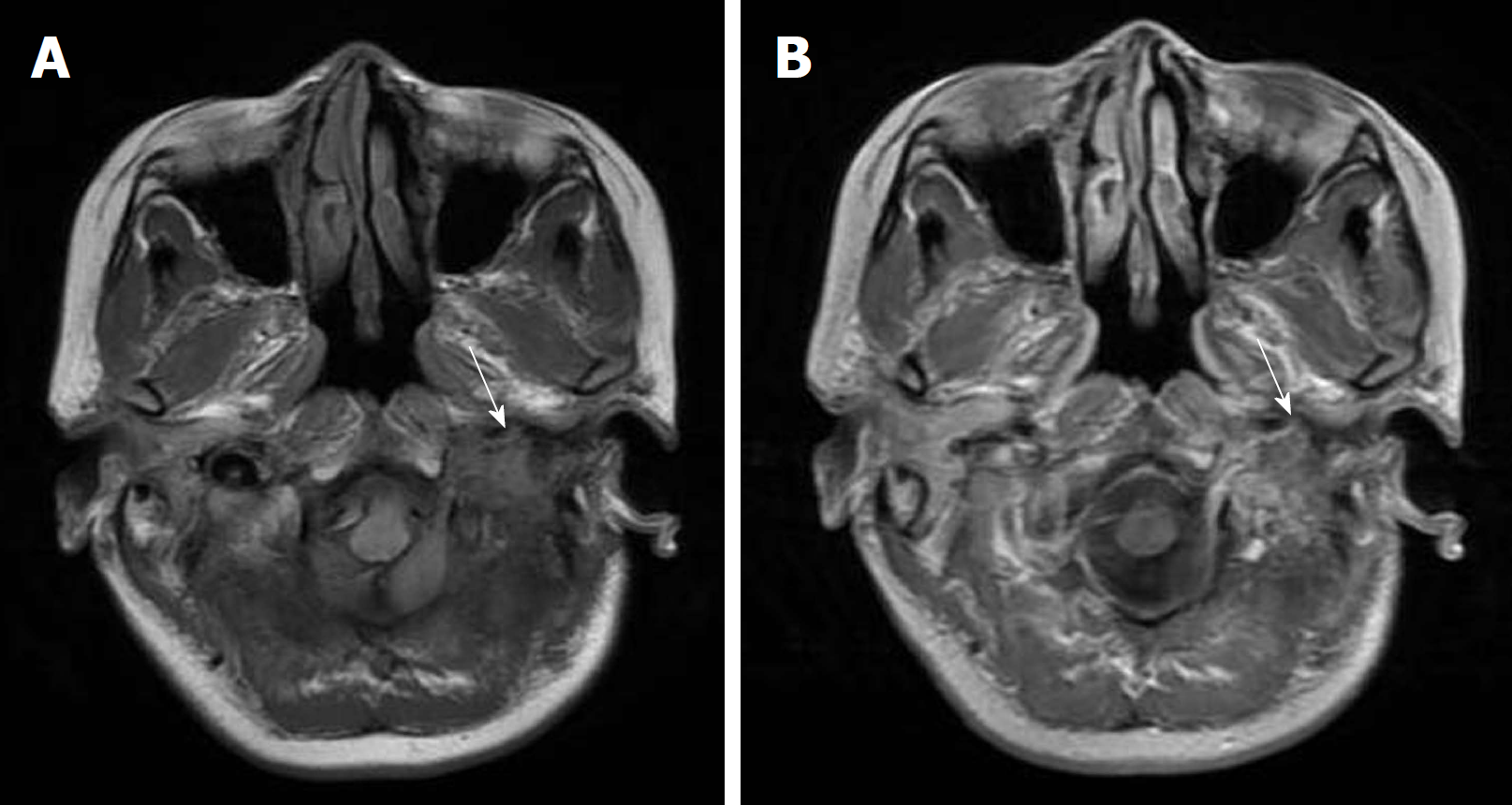
After the surgery, the patient reported improvement in dysphagia and dysarthria but still had some decreased sensation on the left side of the face and was discharged uneventfully. No other treatment was performed after the surgery. The patient was neurologically normal except for mild left facial palsy on 5-mo follow-up examination after surgery and no evidence of residual or recurrent lesion was identified on MRI scans (Figure 4).
CMF, a least common benign bone tumour characterized by chondral, myxoid and fibrous differentiation, is usually found in the metaphysis of long bones[5]. The temporal bone is an extremely rare location of CMF. Our review of the literature identifies that there have only been 13 reported CMF cases with the temporal bone involvement[1,3-14] (Table 1). CMF in the temporal bone is believed to arise from areas of synchondrosis and suture sites in the temporal bone which have embryonic cartilaginous residue[7]. Patients with skull base lesions usually present in the fourth decade of life[5]. In our case, the patient was 40 years old. The mean age of the patients in the 13 cases reported in previous studies was 36.54 years (range, 12 to 67 years). No gender predilection is found in temporal bone CMF[9]. Of these 13 patients reported, 8 patients are male.
| Ref. | Location | Age/gender | Neurological symptoms/ abnormalities on physical examination | Treatment | Follow-up outcome |
| Oh et al[5] | Left mastoid, extending into left external auditory cannal | 38/F | Hearing loss | Complete resection | Persistent conductive hearing loss |
| Sharma et al[6] | Left temporal region in the floor of the middle cranial fossa | 12/F | Headache and left-sided otalgia | Complete resection | Completely relived |
| Gupta et al[7] | Left mastoid, eroding left bony canal of the facial nerve | 42/M | Right-sided otalgia | NA | NA |
| Ozek et al[8] | Left petrous apex and left cerebellopontine angle | 17/M | Headache, diplopia, left VI and VII cranial nerve paralysis and hearing loss | Subtotally resection | Mild left facial palsy and hearing loss |
| Thompson et al[3] | Left mastoid, eroding the left mastoid portion of the facial nerve canal | 32/F | Left facial nerve paralysis | Complete resection | NA |
| Otto et al[9] | Right mastoid, eroding the posterior fossa plate | 58/F | Vertigo and syncope | Complete resection | No evidence of recurrence 6 mo after operation |
| Tarhan et al[1] | Left temporal bone, tympanic region | 44/F | Left facial pain | Complete resection | NA |
| Suzuki et al[10] | Left squamous temporal bone | 49/M | Visual disturbance with right homonymous upper quadrantanopia | Preoperative embolization and resection | NA |
| Patino-Cordoba et al[11] | Left mastoid, eroding the external auditory canal | 20/M | Hearing loss | Complete resection | NA |
| LeMay et al[4] | Left mastoid | 22/M | Headache and left-sided otalgia | Resection via left temporal craniotomy | Persistent conductive hearing loss |
| Maruyama et al[12] | Right petrous temporal bone, extending into the jugular foramen | 67/M | Right bulbar palsy, right facial palsy, complete right-sided hearing loss and trigeminal hypoesthesia | Incomplete resection due to jugular foramen involvement | Resolution of all cranial neuropathies except hearing loss and hoarseness |
| Kitamura et al[13] | Left mastoid, extending into the occipital bone and invading the foramen magnum and jugular foramen | 48/M | Left aural fullness, tinnitus and transient dizziness | Staged resections (1 yr apart) secondary to bleeding | No recurrence 2 yr after first procedure |
| Frank et al[14] | Left petrous apex, extending into the sphenoid sinus, clinoid process, sella, cavernous sinus and retrosellar area | 26/M | Diplopia and abducens nerve paresis | Complete resection | Resolution of abducens palsy |
Depending on the location within the temporal bone, the presentation of these lesions are usually insidious and various. Headache, hearing loss, facial pain, facial nerve paralysis, otalgia, tinnitus, diplopia due to compression and involvement of the neural structures are described symptoms (Table 1). Our case is unusual in that the patient presented with hypoglossal nerve paralysis due to the involvement of the hypoglossal canal. A review of the English-language literature identified no reported cases of temporal bone CMF involving the hypoglossal canal. In addition, the patient complained of dysphagia and dysarthria, which may be due to the involvement of the ipsilateral jugular foramen and are the symptoms of IX and X cranial nerve compression. Maruyama et al[12] also reported a temporal bone lesion with the jugular foramen involved and the patient in that case also presented with dysphagia and dysarthria. There is a reported case of facial nerve paralysis directly related to the involvement of the facial nerve canal[3]. Facial nerve paralysis was noted in three of the temporal bone cases reported. The patient in our case also presented facial nerve paralysis due to the involvement of the facial nerve canal and facial nerve by the mass.
The CT and MRI are useful for the preoperative diagnosis and can show the borders of the temporal bone CMF and the relationship between tumour and surrounding structures. The imaging features of CMF in the temporal bone are well described[1,3,5-7,9,15]. CT imaging can effectively demonstrate the details of bone structures surrounding the lesion. Most CMFs in temporal bones on CT images are well-defined tumours with sclerotic rims and scalloped margins, but locally invading surrounding vital structures, such as cranial nerves[1]. Intratumoral calcification has been reported in up to 75% of CMFs of the skull base[3]. In our case, the intratumoral calcification was not only appreciated on CT images but also demonstrable histologically. MRI is superior to determining the extent of the disease. CMFs typically display hypointensity on T1-weighted imaging and heterogeneous hyperintensity on T2-weighted imaging. Heterogeneous T2 signal is attributed to varying chondroid, myxoid and fibrous elements within the lesion. Furthermore, temporal bone CMFs usually display gadolinium-based contrast enhancement. Similar CT and MRI observations were displayed in our case.
There is still a challenge for differentiating a CMF from other diagnostic considerations in patients with temporal bone lesions. The differential diagnoses would include chordoma, myxoid chondrosarcoma and facial nerve schwannoma (Table 2). It is not difficult to distinguish a CMF from a chordoma which usually arises from the central areas of the skull base and has more extraosseous extension[5]. Moreover, chordoma can be differentiated easily from CMF by immunohistochemistry. Chordoma usually expresses epithelial antigens such as keratin and epithelial membrane antigen, whereas CMF does not stain with antibodies against these proteins[16]. The distinction between a CMF and myxoid chondrosarcoma is important due to their different management. However, distinguishing a CMF from a myxoid chondrosarcoma can be difficult, as they have similar MRI features and both of them stain positively for S-100 protein on immunohistochemistry[9,11]. An obviously infiltrative growth pattern in addition to bone destruction may be useful to distinguish these two pathologies. Additionally, a chondrosarcoma would be unlikely in the temporal bone[5]. The typical pathological features of CMF are the biphasic arrangement of the myxoid or cartilaginous areas and the peripheral condensation of spindle and stellate cells with eosinophilic cytoplasm imparts a lobular silhouette, which are lacking in chondrosarcoma[17]. In order to decrease the risk of diagnostic error, an accurate diagnosis must be established on the basis of careful correlation of clinical, radiographic, pathological and immunohistochemical findings. A CMF in the mastoid portion of the temporal bone can mimic facial nerve schwannoma in this location. Thompson et al[3] reported that a CMF of the mastoid facial nerve canal which was misdiagnosed as a facial nerve schwannoma. Intratumoral calcification, which is less commonly seen in a facial nerve schwannoma, but has been reported in up to 75% of CMFs of the skull base, can be used to distinguish these two pathologies[3].
| Chondromyxoid fibroma | Myxoid chondrosarcoma | Chordoma | Facial nerve schwannoma | |
| Pathological findings | Multilobular arrangement of stellate or spindle-shaped cells in an abundant myxoid background or chondroid intracellular material | Well-differentiated hyaline matrix; an absence of a fibrous component. The cells in chondrosarcoma are almost exclusively chondroblasts | Tumour cells are arranged in sheets or cords or float singly within an abundant myxoid stroma with an abundant pale vacuolated cytoplasm | Schwannoma is composed of spindle cells with wavy appearing nuclei. Areas of hypocellularity may alternate with areas of hypercellularity |
| Immunohistochemical findings | Positive staining for S-100 protein and vimentin | Positive staining for S-100 protein and vimentin | Positive staining for S-100 protein, pankeratin, low-molecular cytokeratins, and epithelial membrane antigen | Positive staining for S-100 protein |
| Radiographic findings | Well-defined tumours with sclerotic rims and scalloped margins; intratumoral calcification; low signal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighted images | Ill-defined tumours without sclerotic rims; an obviously infiltrative growth pattern and bone destruction; intratumoral calcification; low signal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighted images | Ill-defined tumours with obvious bone destruction; intratumoral calcification; low signal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighted images | Well-defined tumours without intratumoral calcification; isointensity to muscle on T1-weighted images and heterogeneous high signal intensity on T2-weighted images with well defined margins |
Complete surgical resection should be the first treatment of choice for temporal bone CMFs[5,7,9]. The patients in our case underwent complete resection for the lesion and no evidence of recurrent lesion was found at 5-mo follow-up. Of the 13 reported cases of temporal bone CMF, 12 had been treated by surgical excision and no recurrent lesion was identified at follow-up. Radiation therapy is not considered as a primary treatment, because of the potential for inducing malignancy[5,9].
In conclusion, CMF of the temporal bone is a very rare benign tumour which can cause some neurological symptoms due to involvement of vital intracranial structures. The current study presents the first case of temporal bone CMF involving the hypoglossal canal. CT and MRI are necessary for preoperative diagnosis. An accurate diagnosis of CMF must be established on the basis of careful correlation of clinical, radiographic, pathological and immunohistochemical findings. Surgical excision is the treatment of choice for CMF in the temporal bone.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chowdhury FH, Kai K, Mehdi I S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Tarhan NC, Yologlu Z, Tutar NU, Coskun M, Agildere AM, Arikan U. Chondromyxoid fibroma of the temporal bone: CT and MRI findings. Eur Radiol. 2000;10:1678-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Crocker M, Corns R, Bodi I, Zrinzo A, Gleeson M, Thomas N. Chondromyxoid fibroma of the skull base invading the occipitocervical junction: report of a unique case and discussion. Skull Base. 2010;20:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Thompson AL, Bharatha A, Aviv RI, Nedzelski J, Chen J, Bilbao JM, Wong J, Saad R, Symons SP. Chondromyxoid fibroma of the mastoid facial nerve canal mimicking a facial nerve schwannoma. Laryngoscope. 2009;119:1380-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | LeMay DR, Sun JK, Mendel E, Hinton DR, Giannotta SL. Chondromyxoid fibroma of the temporal bone. Surg Neurol. 1997;48:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Oh N, Khorsandi AS, Scherl S, Wang B, Wenig BM, Manolidis S, Jacobson A. Chondromyxoid fibroma of the mastoid portion of the temporal bone: MRI and PET/CT findings and their correlation with histology. Ear Nose Throat J. 2013;92:201-203. [PubMed] |
| 6. | Sharma M, Velho V, Binayake R, Tiwari C. Chondromyxoid fibroma of the temporal bone: A rare entity. J Pediatr Neurosci. 2012;7:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Gupta S, Heman-Ackah SE, Harris JA, Hagiwara M, Cosetti MK, Hammerschlag PE. Chondromyxoid fibroma of the temporal bone. Otol Neurotol. 2012;33:e71-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ozek E, Iplikcioglu AC. Chondromyxoid fibroma of the skull base: a case report of an unusual location. Cent Eur Neurosurg. 2011;72:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 9. | Otto BA, Jacob A, Klein MJ, Welling DB. Chondromyxoid fibroma of the temporal bone: case report and review of the literature. Ann Otol Rhinol Laryngol. 2007;116:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Suzuki S, Oka H, Tanaka R, Kawano N, Fujii K, Dobashi Y, Iwabuchi K. A chondromyxoid fibroma-like tumor of the cranial convexity: immunohistochemical and ultrastructural study. Clin Neuropathol. 1999;18:37-41. [PubMed] |
| 11. | Patino-Cordoba JI, Turner J, McCarthy SW, Fagan P. Chondromyxoid fibroma of the skull base. Otolaryngol Head Neck Surg. 1998;118:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Maruyama R, Nagaoka S, Todaka T, Nakahara T, Kishida K. Intracranial chondromyxoid fibroma extending into the jugular foramen. Pathol Int. 1994;44:857-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Kitamura K, Nibu K, Asai M, Shitara N, Niki T. Chondromyxoid fibroma of the mastoid invading the occipital bone. Arch Otolaryngol Head Neck Surg. 1989;115:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Frank E, Deruaz JP, de Tribolet N. Chondromyxoid fibroma of the petrous-sphenoid junction. Surg Neurol. 1987;27:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Cappelle S, Pans S, Sciot R. Imaging features of chondromyxoid fibroma: report of 15 cases and literature review. Br J Radiol. 2016;20160088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Feuvret L, Noël G, Calugaru V, Terrier P, Habrand JL. Chondromyxoid fibroma of the skull base: differential diagnosis and radiotherapy: two case reports and a review of the literature. Acta Oncol. 2005;44:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 17. | McClurg SW, Leon M, Teknos TN, Iwenofu OH. Chondromyxoid fibroma of the nasal septum: case report and review of literature. Head Neck. 2013;35:E1-E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |