Published online Dec 26, 2018. doi: 10.12998/wjcc.v6.i16.1206
Peer-review started: September 18, 2018
First decision: October 12, 2018
Revised: November 20, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: December 26, 2018
Processing time: 97 Days and 14.2 Hours
Posaconazole is a widely used azole antifungal agent, and posaconazole-associated severe hyperbilirubinemia is usually rare in clinical practice. We herein report a 58-year-old male with acute myeloid leukemia, who developed fungal infection following chemotherapy.
After administration of posaconazole oral suspension, the patient developed severe hyperbilirubinemia and jaundice (Common Terminology Criteria for Adverse Events, CTCAE -Grade 3) with a serum total bilirubin (T-BIL) peak level of 170 μmol/L, alkaline phosphatase level of 739 U/L, alanine aminotransferase level of 99 U/L, and gamma-glutamyl transpeptidase level of 638 U/L. After posaconazole withdrawal and symptomatic treatment with liver-protective agents, the level of T-BIL and other laboratory data decreased gradually, and related symptoms disappeared. After medication analysis and literature review, we consider that the patient had a cholestatic type of posaconazole-induced liver injury, which was related to intracellular mitochondrial DNA damage. The case demonstrates that when patients with hematological malignancy develop severe infection following chemotherapy, combination of anti-infective drugs may contribute to a higher risk of severe drug-induced liver injury.
This is the first thoroughly documented case report of posaconazole-associated severe hyperbilirubinemia. Therefore, in order to avoid severe adverse events, liver and renal function should be monitored closely before and during the administration of posaconazole.
Core tip: Posaconazole is safe and tolerable in most cases and posaconazole-associated severe hyperbilirubinemia is usually very rare. For patients with hematological malignancy who develop severe infection following chemotherapy, combination of anti-infective drugs may contribute to higher risk of posaconazole-induced severe liver injury of cholestatic type. Therefore, for patients with hematological malignancy, liver and renal function should be monitored closely before and during the administration of posaconazole.
- Citation: Song ZW, Pan YC, Huang ZC, Liu WX, Zhao RS, Jing HM, Dong F. Posaconazole-associated severe hyperbilirubinemia in acute myeloid leukemia following chemotherapy: A case report. World J Clin Cases 2018; 6(16): 1206-1209
- URL: https://www.wjgnet.com/2307-8960/full/v6/i16/1206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i16.1206
Posaconazole, a novel triazole antifungal agent, is widely used for the prophylaxis and treatment of invasive Aspergillus and Candida infection. Because posaconazole is a well-tolerated antifungal agent[1], posaconazole-associated severe hyperbilirubinemia is usually rare in clinical practice. We herein report a case of a 57-year-old man with acute myeloid leukemia (AML) following chemotherapy who developed severe hyperbilirubinemia and jaundice after posaconazole administration. The causality between posaconazole and the adverse event, the mechanism of posaconazole-induced liver injury, and exacerbating risk factors were provided.
A 58-year-old man was diagnosed with AML (M2) in January 2018. Afterwards, he developed pulmonary infection, and his hepatic and renal function remained normal following a standard voriconazole dose (200 mg, ivgtt, q12h). He received idarubicin (10 mg, iv, qd, days 1-3) and cytarabine (75 mg, iv, q12h, days 1-7) for chemotherapy on January 29, and bone marrow aspiration showed morphological complete remission. At the end of chemotherapy, gingival necrosis developed in the patient on February 5 accompanied with grey tissues and clusters attached to mouth mucosa, and fungal infection following chemotherapy was considered. Fungal quest through microscopic examination showed a positive result with obvious hyphae on February 8, but the specy remained unavailable. Since aspergillosis was suspected, the patient was treated with amphotericin B for gargling, amphotericin B liposome (100 mg, ivgtt, qd) and voriconazole (200 mg, ivgtt, q12h). On February 13, pathologic examination showed mucormycosis (Rhizopus microsporus), and moderate renal damage was observed with a creatinine clearance (Ccr) level of 42.7 mL/min (normal range, > 80 mL/min) while liver function remained normal.
He had a healthy past history with no hepatic dysfunction before except for consumption of alcoholic beverages.
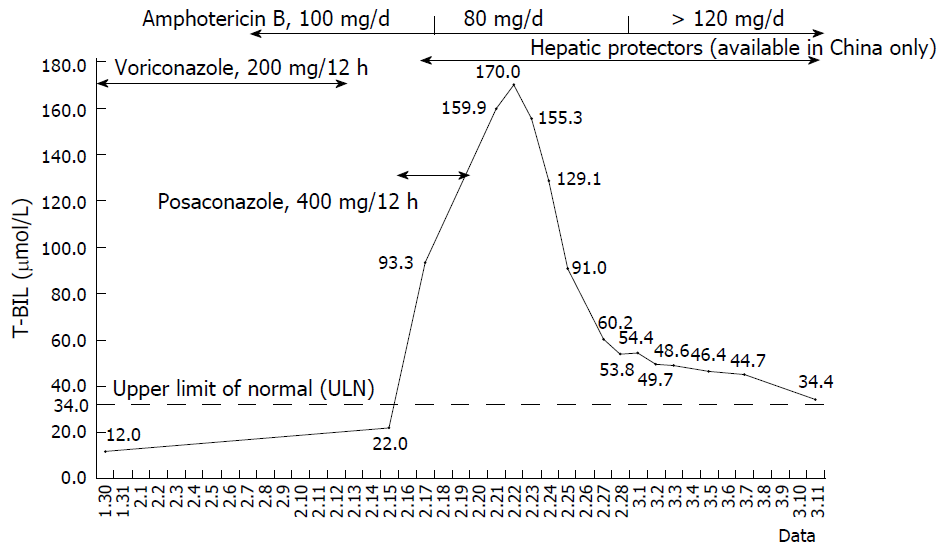
Due to the moderate renal damage and to its poor efficacy for mucormycosis, voriconazole was discontinued. The medication process was illustrated in Figure 1. On February 15, his recovery from renal damage was evidenced by a Ccr level of 79.2 mL/min, while liver function remained normal. Posaconazole (400 mg, po, q12h) was prescribed together with amphotericin B. On two days post-posaconazole, T-BIL and direct bilirubin (D-BIL) rose to 93.3 μmol/L and 92.7 μmol/L, respectively, with progressive yellowish conjunctivae and skin (jaundice). Since drug-induced liver injury (DILI) could not be excluded, amphotericin B was suspected to be the cause. The dosage of amphotericin B liposome was reduced to 80 mg/d, and single hepatic protector was prescribed. Hyperbilirubinemia and jaundice became progressively worse, and posaconazole was discontinued as an unconfirmed causative drug on four days post-posaconazole while keeping amphotericin B (80 mg). Laboratory data on February 21 revealed progressive hyperbilirubinemia with a T-BIL (D-BIL) level of 159.9 (158.3) μmol/L. T-BIL reached a peak value of 170 μmol/L on February 22; then hepatic protectors were combined to treat hepatic dysfunction. On February 23, T-BIL (D-BIL) began to decrease to 155.3 (121.5) μmol/L with an ALP level of 739 U/L, a GGT level of 638 U/L and an ALT level of 98U/L, while other laboratory data continued to decrease. On February 27, hyperbilirubinemia-related symptoms improved. In consideration that posaconazole was the causative drug and amphotericin B liposome was effective, the dosage of amphotericin B liposome was escalated gradually, and the symptoms improved gradually, while T-BIL remained decreasing consistently.
Later, jaundice disappeared and oral infection improved significantly. With a stable healthy condition and relatively normal T-BIL [34.4 μmol/L, Upper Limit Of Normal (ULN) of 34 μmol/L] on March 11, the patient was discharged from hospital.
DILI is a serious medication-induced complication with a prevalence of up to 14 out of 100000 people, accounting for 33% of acute liver failure cases[2]. Actually, posaconazole-induced severe hyperbilirubinemia is a rare type of DILI, which has not been reported in case reports. In order to diagnose DILI, medication history, withdrawal response, response to unintentional re-administration and laboratory evidence of hepatocellular or cholestatic injury will need to be combined. Liver biopsy may help identify non-drug-related (alternative) causes and define the pattern of DILI. The RUCAM (Roussel Uclaf Causality Assessment Method) scale has been established to assess the causality of DILI and suspected drug[3], and it provides a quantitative grade of causality for each suspected drug in a case report and a final score greater than 8 indicates the causal relationship that is highly probable[4]. Generally speaking, the mechanism of DILI remains incompletely understood. DILI can be classified as hepatocellular, cholestatic, or mixed type. At present, the calculation of R ratio [R = (ALT ÷ ALT’s ULN) / (ALP ÷ ALP’s ULN)] has been adopted to determine the pattern of DILI: R ≥ 5 defines hepatocellular, R ≤ 2 defines cholestatic and 2 < R < 5 defines a mixed liver injury[4].
In this case report, the patient had normal baseline liver function at initial hospitalization. His liver function remained normal prior to posaconazole administration but hyperbilirubinemia occurred on two days post-posaconazole. Afterwards, posaconazole was discontinued, and symptomatic treatment (liver-protective drugs) was prescribed. At four days after posaconazole withdrawal, T-BIL began to decrease and was normalized with jaundice improving. Analyzing the medication history, his liver function remained normal during previous combination of voriconazole and amphotericin B liposome, and hyperbilirubinemia occurred after voriconazole withdrawal, excluding voriconazole. Besides, T-BIL kept rising during amphotericin B liposome dose reduction, and T-BIL remained decreasing consistently within single therapy of amphotericin B liposome and escalading duration, excluding amphotericin B. After comprehensive analysis on his past history, medication process, causality identification, clinical manifestation and laboratory evidence of liver function, we considered that posaconazole was likely the causative drug for hyperbilirubinemia in this case, and the causality was highly probable with an RUCAM score of 9. Also, the calculated R value of 0.335 indicated that posaconazole-associated severe hyperbilirubinemia in the case was cholestatic, but the precise pattern of DILI requires liver biopsy.
Plasma protein binding ratio of posaconazole is up to 98%[5], and it is mainly metabolized through intrahepatic glucuronidation with an elimination half-life varying from 25 to 35 h. The majority of posaconazole (66%) are excreted unchanged in feces with less than 1% excreted unchanged in urine[6]. As a well-tolerated antifungal agent, posaconazole-associated severe hyperbilirubinemia is usually rare in clinical practice with unknown mechanism. One study demonstrates that mitochondrial dysfunction might account for DILI due to posaconazole[7]. When intracellular posaconazole accumulates, it decreases mitochondrial membrane potential, impairs the electron transport chain, causes mitochondrial superoxide accumulation and mitochondrial DNA decreasing, and finally induces hepatic cell apoptosis.
Interestingly, posaconazole-induced hyperbilirubinemia is not necessarily linked with an elevated plasma concentration of posaconazole[8,9]. On the contrary, hyperbilirubinemia may contribute to lowering plasma concentration of posaconazole[6]. Increased bilirubin competitively binds albumin with posaconazole, and plasma-bound posaconazole decreases. On the other hand, increased bilirubin expedites the elimination of posaconazole through up-regulation of glucuronidation.
As a well-tolerated azole antifungal agent, posaconazole is relatively safe, but its pharmacokinetics varies in different patients[10]. DILI caused by posaconazole may be aggravated by risk factors in particular groups. According to the FDA instruction, under rare situations, severe liver complication might be worsened in patients with serious primary diseases such as hematological malignancy. In this case, there were several risk factors that can contribute to worsening liver injury. First, the patient was diagnosed with severe systemic disease-AML (M2). Second, he received chemotherapy, which potentially impaired his liver and renal function. He was under myelosuppression and febrile neutropenia following chemotherapy. Additionally, he developed severe pulmonary infection with respiratory failure type I before chemotherapy, which might cause inadequate oxygen supply and carbon dioxide retention for organs. Potential early liver dysfunction could not be excluded completely despite normal laboratory values. Finally, voriconazole and amphotericin B had been prescribed earlier with moderate renal damage occurring before posaconazole administration, which might contribute to liver injury.
In summary, the case report describes a 58-year-old male patient with AML (M2) and a healthy past history who developed mucormycosis following chemotherapy. After administration of posaconazole oral suspension, he developed severe hyperbilirubinemia and jaundice (CTCAE-Grade 3). After posaconazole withdrawal and symptomatic treatment with liver-protective agents, T-BIL was normalized, and symptoms of jaundice disappeared gradually. After literature review, we considered that posaconazole was the causative drug with an RUCAM score of 9, and the severe hyperbilirubinemia was cholestatic type in this case. Additionally, DILI due to posaconazole may be linked with an intracellular concentration of posaconazole and mitochondrial DNA damage but not necessarily with elevated plasma concentration. The case demonstrates that, besides drug factors, DILI caused by posaconazole is closely related with patients’ age, past medical history, the history of adverse events, concomitant diseases, and medication combination. The condition is usually very rare, and we have found no detailed description of it in the medical literature. Therefore, to the best of our knowledge, this is the first thoroughly documented case report of posaconazole-associated severe hyperbilirubinemia. The case report provides instructive points for other healthcare professionals. When patients with hematological malignancy develop severe infection following chemotherapy, combination of anti-infective drugs may contribute to a higher risk of severe DILI. Therefore, to avoid severe adverse events, liver and renal function should be monitored closely before and during the administration of posaconazole.
The case report suggests that, to avoid severe adverse events, liver and renal function should be monitored closely before and during the administration of posaconazole, especially for patients with hematological malignancy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fukuda S, Pourshafie MR, Xavier-Elsas P S- Editor: Cui LJ L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Lenczuk D, Zinke-Cerwenka W, Greinix H, Wölfler A, Prattes J, Zollner-Schwetz I, Valentin T, Lin TC, Meinitzer A, Hoenigl M. Antifungal Prophylaxis with Posaconazole Delayed-Release Tablet and Oral Suspension in a Real-Life Setting: Plasma Levels, Efficacy, and Tolerability. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Yusuf D, Christy J, Owen D, Ho M, Li D, Fishman MJ. A case report of nifedipine-induced hepatitis with jaundice. BMC Res Notes. 2018;11:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci. 2015;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 4. | Danan G, Teschke R. Drug-Induced Liver Injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) Still Used 25 Years After Its Launch? Drug Saf. 2018;41:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Available from: www.mimsonline.com.au. |
| 6. | Maleki S, Corallo C, Coutsouvelis J, Singh J. Failure to achieve therapeutic levels with high-dose posaconazole tablets potentially due to enhanced clearance. J Oncol Pharm Pract. 2018;24:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Haegler P, Joerin L, Krähenbühl S, Bouitbir J. Hepatocellular Toxicity of Imidazole and Triazole Antimycotic Agents. Toxicol Sci. 2017;157:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Boglione-Kerrien C, Picard S, Tron C, Nimubona S, Gangneux JP, Lalanne S, Lemaitre F, Bellissant E, Verdier MC, Petitcollin A. Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol. 2018;144:127-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Tverdek FP, Heo ST, Aitken SL, Granwehr B, Kontoyiannis DP. Real-Life Assessment of the Safety and Effectiveness of the New Tablet and Intravenous Formulations of Posaconazole in the Prophylaxis of Invasive Fungal Infections via Analysis of 343 Courses. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Mattiuzzi G, Yilmaz M, Kantarjian H, Borthakur G, Konopleva M, Jabbour E, Brown Y, Pierce S, Cortes J. Pharmacokinetics of posaconazole prophylaxis of patients with acute myeloid leukemia. J Infect Chemother. 2015;21:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |