Copyright
©The Author(s) 2018.
World J Clin Cases. Dec 6, 2018; 6(15): 961-984
Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.961
Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.961
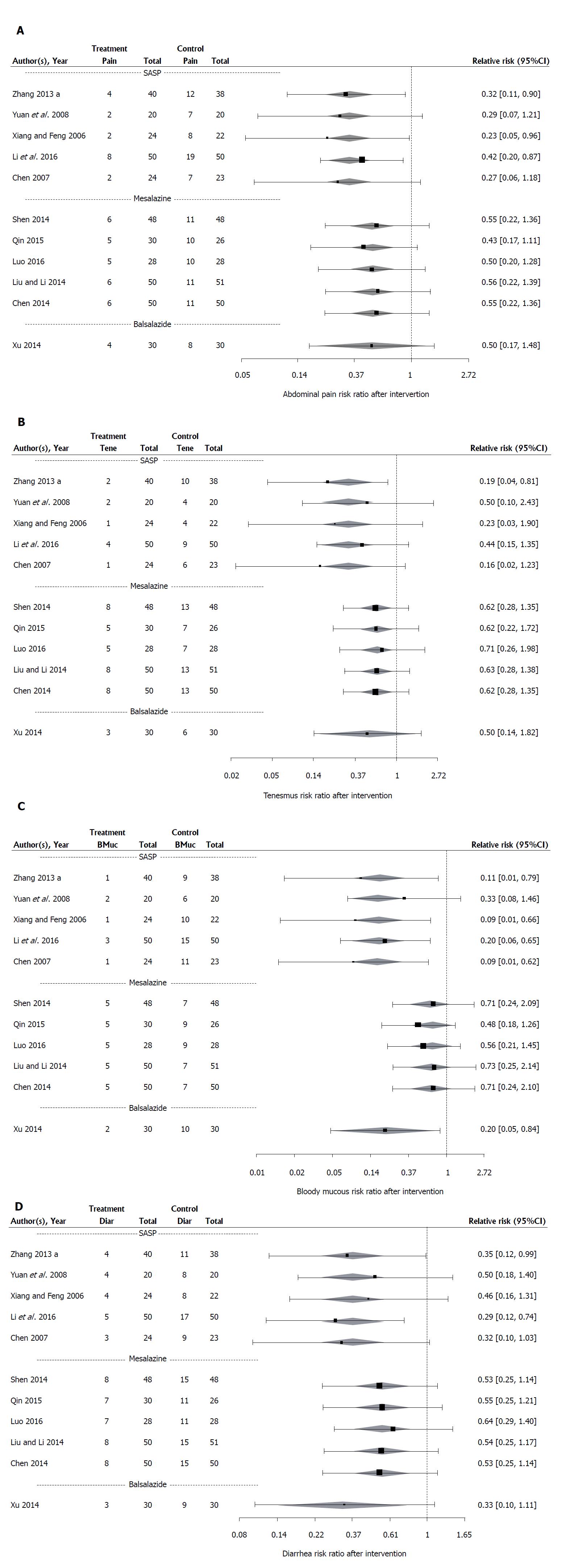
Figure 8 Forest plots of the results of fixed effects meta-analysis with a moderator for concomitant drug therapy for 11 studies evaluating the effect of Medilac-S® in combination with conventional drug therapy on number of participants reporting clinical symptoms including: (A) abdominal pain; (B) tenesmus; (C) blood and mucous in stool; and (D) diarrhea.
“Pain” is the number of participants reporting abdominal pain in each study, “Tene” is the number of participants reporting tenesmus in each study, “BMuc” is the number of participants reporting blood and mucous in stool in each study, “Diar” is the number of participants reporting diarrhea in each study, and “Total” is the total number of participants in each study. The relative risk (RR) and its 95%CI for each study is listed on the right hand side of the graph. The 95%CI for the estimated mean RR for each concomitant drug therapy category is shown as a shaded diamond with the endpoints of the diamond being the CI endpoints and the location of the maximum width of the diamond being at the estimated mean RR for that drug type. The vertical dashed line at 1 indicates a RR of 1 which occurs when there is no observed difference between the treatment and the control.
- Citation: Sohail G, Xu X, Christman MC, Tompkins TA. Probiotic Medilac-S® for the induction of clinical remission in a Chinese population with ulcerative colitis: A systematic review and meta-analysis. World J Clin Cases 2018; 6(15): 961-984
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/961.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.961