Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.995
Peer-review started: September 14, 2018
First decision: November 1, 2018
Revised: November 8, 2018
Accepted: November 14, 2018
Article in press: November 15, 2018
Published online: December 6, 2018
Processing time: 84 Days and 10.6 Hours
To compare the accuracy of the scoring systems Child-Turcotte-Pugh (CTP), Model for End-stage Liver Disease score (MELD), MELD-Na, and MELD to Serum Sodium ratio (MESO) to predict the mortality in decompensated liver cirrhosis.
The PubMed, Web of Science, Cochrane Library, EMBASE, and Ovid databases were systematically searched from inception to September 2018 for relevant articles, and we evaluated the quality of the included studies. The accuracy of scoring systems was analyzed with Stata 12 and MetaDiSc 1.4.
Sixteen studies involving 2337 patients were included. The pooled areas under the summary receiver operating characteristic curves (AUROCs) of CTP, MELD, MELD-Na, and MESO to predict mortality were 0.81, 0.78, 0.85, and 0.86, respectively. Within 3 mo, the AUROCs of CTP, MELD, and MELD-Na in predicting mortality were 0.78, 0.76, and 0.89, respectively. The AUROCs of CTP, MELD, and MELD-Na at 3 mo were 0.86, 0.78, and 0.86, respectively. The AUROCs of CTP, MELD, and MELD-Na at 6 mo were 0.91, 0.83, and 0.90, respectively. The AUROCs of CTP, MELD, and MELD-Na at 12 mo were 0.72, 0.75 and 0.84, respectively. In cirrhotic patients with bleeding, the AUROCs of CTP and MELD were 0.76 and 0.88, respectively.
MESO has the highest AUROC in all assessed scoring systems. Considering the different time points, MELD-Na has good accuracy in predicting the mortality of decompensated liver cirrhosis. Compared to CTP, MELD is better in predicting variceal bleeding.
Core tip: Liver cirrhosis, especially decompensated liver cirrhosis, is a common chronic disease that is also the leading cause of death among nonmalignant diseases worldwide. The poor survival of decompensated cirrhosis has pushed doctors to find more accurate prognostic scoring systems to recognize and manage patients. No meta-analysis has focused on comparison of the prediction accuracy for those patients in the past. This study aimed to compare the test accuracy of four systems [Child-Turcotte-Pugh, Model for End-Stage Liver Disease score (MELD), MELD-Na, and MELD to Serum Sodium ratio] quantitatively and to pinpoint the more reliable scoring system for forecasting the mortality of decompensated liver cirrhosis patients clinically.
- Citation: Wu SL, Zheng YX, Tian ZW, Chen MS, Tan HZ. Scoring systems for prediction of mortality in decompensated liver cirrhosis: A meta-analysis of test accuracy. World J Clin Cases 2018; 6(15): 995-1006
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/995.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.995
Liver cirrhosis is a common chronic disease that is also the leading cause of deaths among nonmalignant diseases worldwide. Its development includes an asymptomatic phase called “compensated” cirrhosis, followed by a progressive phase characterized by hypertension and/or liver dysfunction, including ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, variceal hemorrhage, hepatorenal syndrome, and hepatocellular carcinoma, which is called “decompensated” cirrhosis[1]. Decompensated cirrhosis is associated with a risk of death that is 9.7 times higher than the risk in the general population[2]. The poor survival of patients with decompensated cirrhosis has pushed doctors to explore more efficient treatment methods and to find more accurate prognostic scoring systems to recognize and manage the patients[3-5]. On one hand, accurate prognostic scoring systems could help clinicians make better diagnoses and select effective therapies with less time, thus improving the prognosis of patients. In addition, the mathematical model could be used as a tool to better allocate donated organs to recipients in need among the liver transplantation community[6,7].
Until now, various scoring systems have been used to predict the mortality of liver decompensated cirrhosis, including the Child–Turcotte–Pugh (CTP), Model for End-stage Liver Disease score (MELD), MELD-Na, MELD to Serum Sodium ratio (MESO) and so on. The CTP[8] is a “modified Child score” that was first proposed in 1964, and it has been widely used for several decades for the prognostication of patients with cirrhosis[9]. Due to the lack of statistical weighting and factors resulting from complications, such as renal and pulmonary dysfunction, CTP is limited in predicting the mortality of decompensated cirrhosis patients[10]. Thus, in 2001, MELD was established by UNOS for allocation. This scoring system added serum creatinine, total serum bilirubin, international normalized ratio for prothrombin time evaluation, and the etiology of cirrhosis as predicting factors. Compared to CTP, MELD is more objective and clinically useful for defining disease severity[11,12].
In the following years, research emerged claiming that serum Na was a predictor of mortality in patients with cirrhosis and might improve the accuracy of MELD[13-15]. Then, based on MELD, refined models were established for prediction of mortality in liver disease. Biggins et al[16] developed an evidence-based model, “MELD-Na,” which was calculated with the formula “MELD-NA = MELD + 1.59 (135-Na)”. Huo et al[17], based on the MELD, devised serum sodium ratio index (MESO) to enhance the precision of MELD’s predictions in compensated and decompensated patients. It is expressed as “MESO index = (MELD Score/SNa mEq/L)*10”. The performances of all scoring systems are diverse in their application, and it remains unknown which scoring system is better. Previous meta-analyses, which used simple pooling to evaluate prediction accuracy for the assessment of the prognostic value in liver cirrhosis, have only compared CTP and MELD, which made the conclusions less convincing[18].
To our knowledge, no meta-analysis has focused on the comparison of the prediction accuracy of mortality in all four scoring systems in decompensated liver cirrhosis patients. This study aimed to quantitatively compare the test accuracy of all four systems (CTP, MELD, MELD-Na, and MESO) and to pinpoint the more reliable scoring system to forecast the mortality of decompensated liver cirrhosis patients clinically.
The PubMed, Web of Science, Cochrane Library, EMBASE, and Ovid databases were systematically searched from inception to September 2018. For the literature search, the following keywords or corresponding terms were used: (“Child-Turcotte-Pugh score” OR “CTP” OR “Child score” OR “Child-Pugh score” OR “Model for End-stage liver disease score” OR “MELD” OR “MELD-Na” OR “MELD to Serum sodium ratio” OR MESO) AND “Decompensated liver cirrhosis” AND (“outcome” OR “prediction” OR “sensitivity” OR “specificity” OR “diagnostic accuracy”). In addition, relevant original articles were retrieved through literature review. Studies that met the following requirements were included in our research: (1) The study population included decompensated liver cirrhosis patients with various causes; (2) Scoring systems were used to predict the mortality of decompensated liver cirrhosis patients; and (3) It provided sufficient data with evaluation outcomes such as true negativity (TN), true positivity (TP), false negativity (FN), and false positivity (FP), or data that could be used to calculate these results. Language, publication year, sample size, and study design were not strictly limited in the inclusion criteria.
When the same patient population was studied in more than one publication, only the one with most relevant data was included in this review. Two investigators, Wu and Tian, independently reviewed the potentially eligible studies and then cross-checked their results. Disagreements between them were resolved by discussion. Unsettled disagreements were referred to a third researcher, Zheng, for a final decision.
Apart from evaluation outcomes such as TN, TP, FN, and FP, we also extracted the following data from the selected studies: first author, country, sample size, etiology, endpoint, cut-off values of the scoring system, and sex and age of the population.
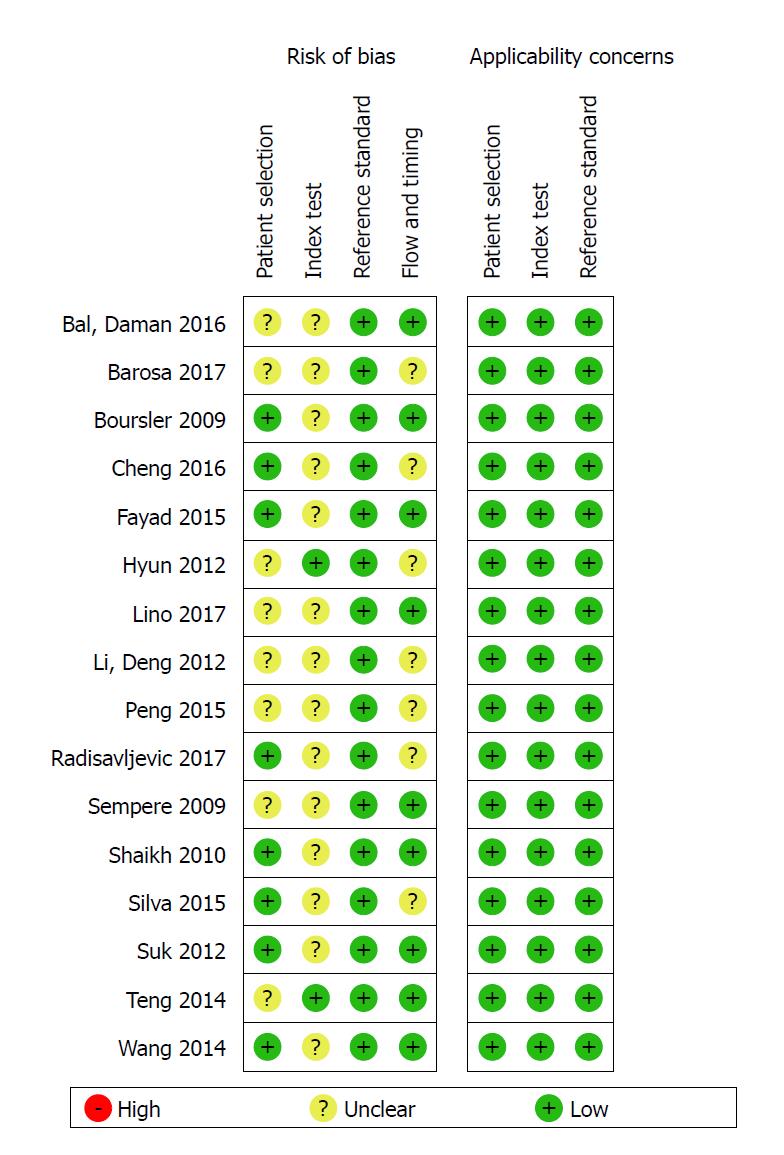
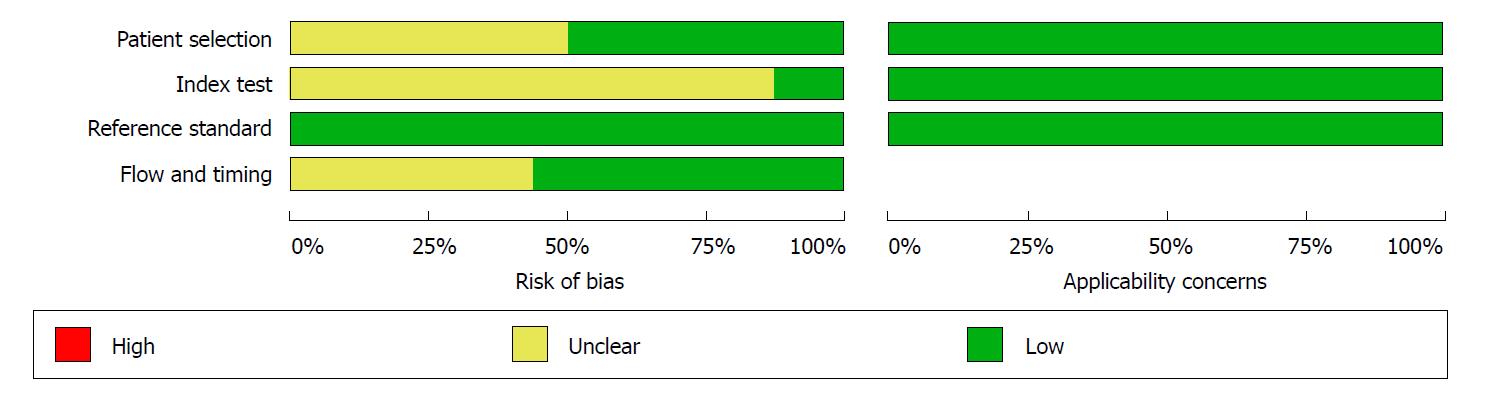
The Quality Assessment of Diagnostic Accuracy Studies 2 scale[19] was used to evaluate the quality of the included studies. Four domains were related to bias (patient selection, index text, reference standard, and flow and timing) and three domains were related to applicability (patient selection, index text, and reference standard). For quality assessment, 14 of 16 articles were graded as having low bias in more than four domains, which indicated that the quality was good. The detailed results of the quality assessment of all included studies is shown in Figures 1 and 2.
Due to the diversity of the cut-off values in different scoring systems, a bivariate model (a random model) was used to estimate the summary diagnostic odd ratios (DORs), summary sensitivities, and summary specificities. The summary receiver operating characteristic curve (SROC) and the area under the SROC (AUROC) were used to measure the predictive value of each scoring system. When the number of studies was ≤ 3, we considered simple pooling to evaluate the above results.
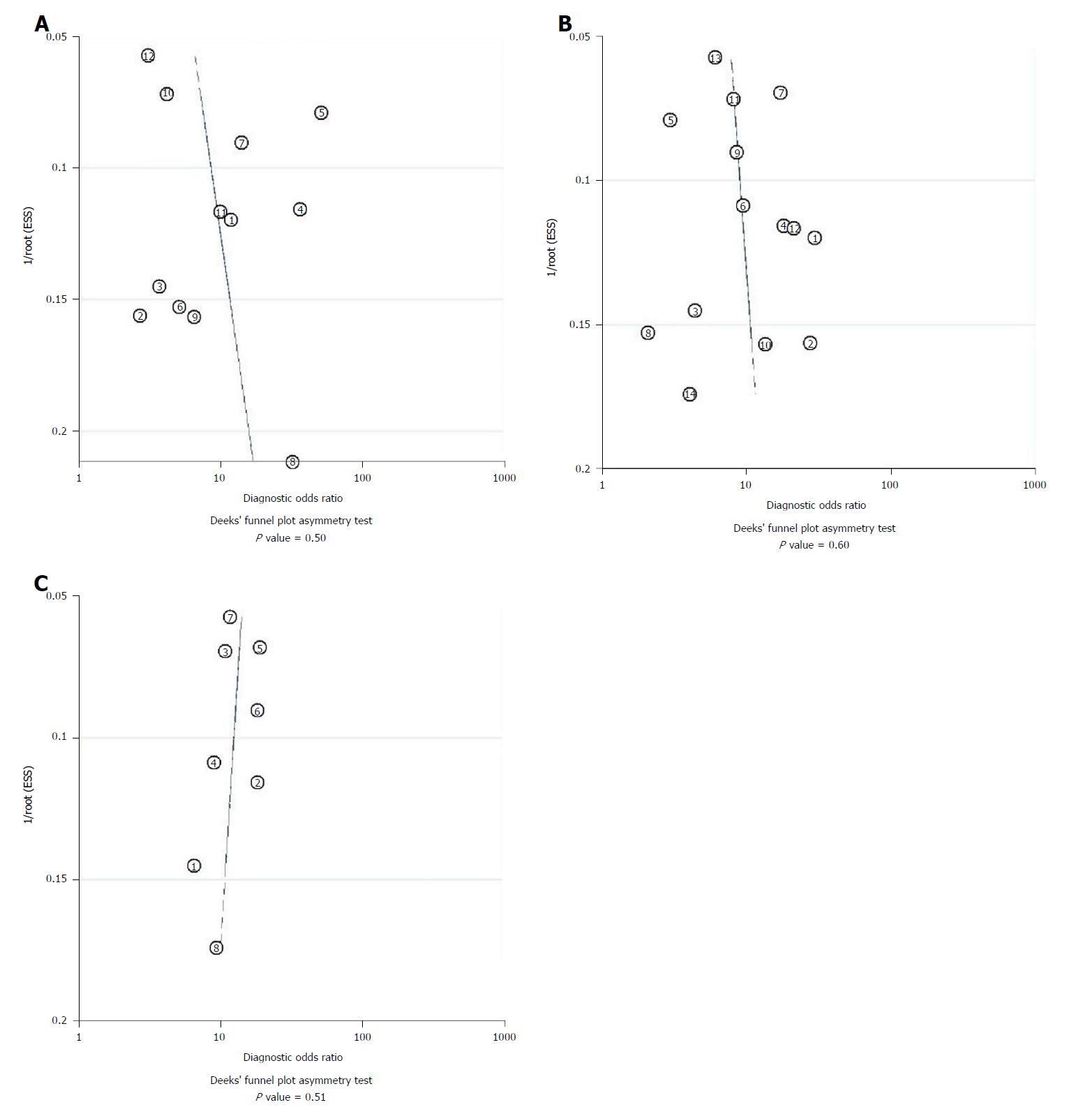
Statistical heterogeneity among studies included the threshold effect and the non-threshold effect. Spearman correlation analysis of the sensitivity logarithm and (1-specificity) logarithm [logit (true positive rate) vs logit (false positive rate)] was used to assess the effect of the threshold. The merge sensitivity, specificity, and DOR would be used to evaluate the diagnostic efficiency when the effect of threshold was removed (P > 0.1); otherwise, fitting SROC and computing AUROC were alternatives. Cochrane’s Q-test and the I2 statistic were used to assess the non-threshold effect. I2 > 50% indicates substantial heterogeneity between studies; if so, a random effects model was used in simple pooling. Subgroup analyses were conducted according to the end-points to identify the potential source of heterogeneity. Further, we used Deek’s funnel plot asymmetry to assess potential publication bias.
Stata 12.0, Meta-DiSc 1.4, and Review Manager 5.3 were used to analyze the data and calculate all the parameters. All comparisons were considered statistically significant if P ≤ 0.05 (two-sided test). To our knowledge, no protocol of the present review has been published or registered.
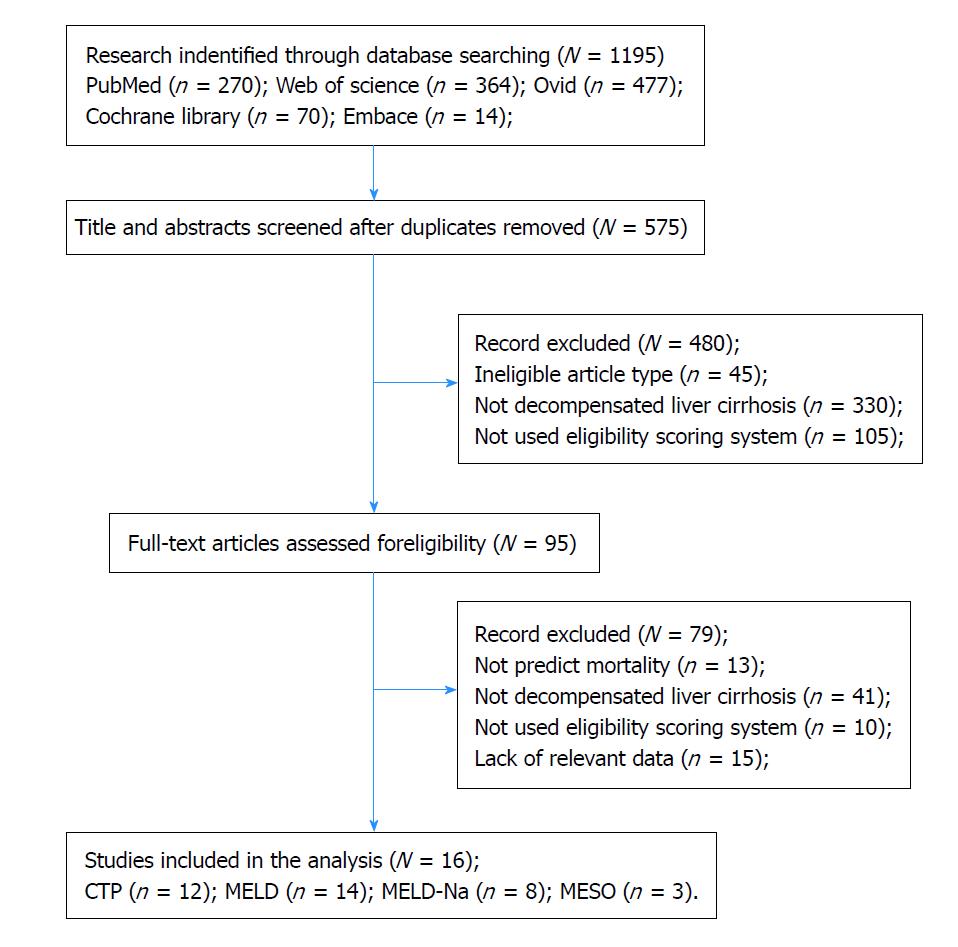
Sixteen eligible[20-35] studies involving 2337 decompensated liver cirrhosis patients were included in this meta-analysis, and the flow diagram for the selection of the articles is shown in Figure 3.
Overall, there were 12 studies involving 1722 decompensated liver cirrhosis patients for the analysis of CTP; 14 studies involving 2034 patients for MELD; 8 studies involving 1340 patients for MELD-Na; and 3 studies involving 422 patients for MESO. The characteristics of the included studies are listed in Table 1.
| Study | Country | Study design | Sex (M/F) | Age (yr) | Number of patients | End-point of observation | Scoring system | Etiology |
| Radisavljevic et al[20], 2017 | Peru | p | 79/8 | 54 | 87 | 29 mo | CTP, MESO, MELD | Alcohol |
| Iino et al[21], 2017 | Japan | r | 39/8 | 60 | 47 | 1.5 mo | CTP, MELD | HBV, HCV, Alcohol, Others |
| Barosa et al[22], 2017 | Portugal | r | Unknown | 62 | 49 | 1, 3 mo | CTP, MELD, MELD-Na | Alcohol, HCV |
| Cheng et al[23], 2016 | China | p | 87/12 | 48 | 98 | 1, 3, 6, 12 mo | CTP, MELD, MELD-Na | HBV |
| Bal et al[24], 2016 | India | r | 177/41 | 50 | 218 | 1.6 mo | MELD-Na | Ethanol, Crypto/NAFLD,HCV |
| Silva et al[25], 2015 | Brazil | p | 140/52 | 54 | 192 | 1 mo | MELD, CTP | Alcohol, HBV, HCV, Cryptogenic, Others |
| 189 | 3 mo | MELD, CTP | ||||||
| Fayad et al[26], 2015 | Brazil | p | 94/29 | 54 | 123 | In hospital | MELD, MELD-Na, MESO | HBV, HCV, Alcohol, Cryptogenic, Others |
| Suk et al[27], 2012 | Korea | p | 46/11 | 48 | 57 | 36 mo | MELD, MELD-Na | Alcohol, Viral, Alcohol + Viral |
| Li et al[28], 2012 | China | r | 133/79 | 56 | 212 | 3, 6, 12 mo | MELD, MELD-Na, MESO | HBV, HCV, Alcohol, Primary biliary cirrhosis, Other |
| Shaikh et al[29], 2010 | India | p | 72/38 | 47 | 110 | In hospital | MELD, CTP | HBV, HCV |
| Boursier et al[30], 2009 | France | p | 93/61 | 59 | 154 | 6 mo | CTP, MELD, MELD-Na | Alcohol, Viral, Others |
| Hyun et al[31], 2012 | Korea | r | 63/23 | 54 | 83 | 6 mo | CTP | HBV |
| Peng et al[32], 2015 | China | r | 94/51 | 57 | 145 | In hospital | MELD, CTP | HBV, HCV, Alcohol, Unknown, Others |
| Sempere et al[33], 2009 | Spain | r | 142/59 | 59 | 201 | 1.5, 3, 12, 36 mo | MELD, CTP | Alcohol, Viral, Others |
| Teng et al[34], 2014 | China | r | 110/22 | 51 | 132 | 1.5 mo | MELD, CTP | Alcohol, HBV, HCV, |
| Wang et al[35], 2014 | China | p | 340/89 | 49 | 429 | 3, 12 mo | CTP, MELD-, MELA-Na | HCV, HBV, Alcohol, Biliary, Others |
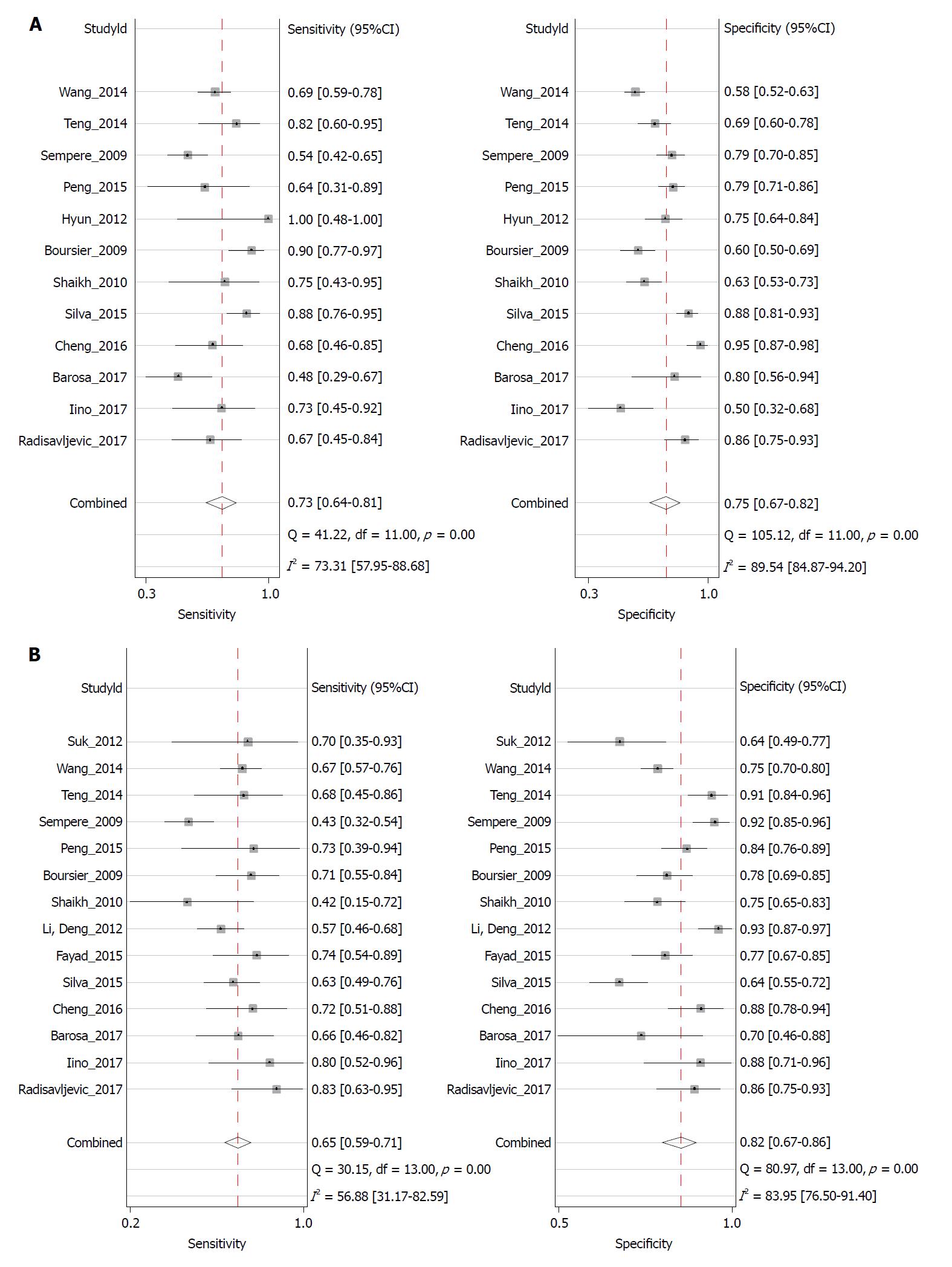
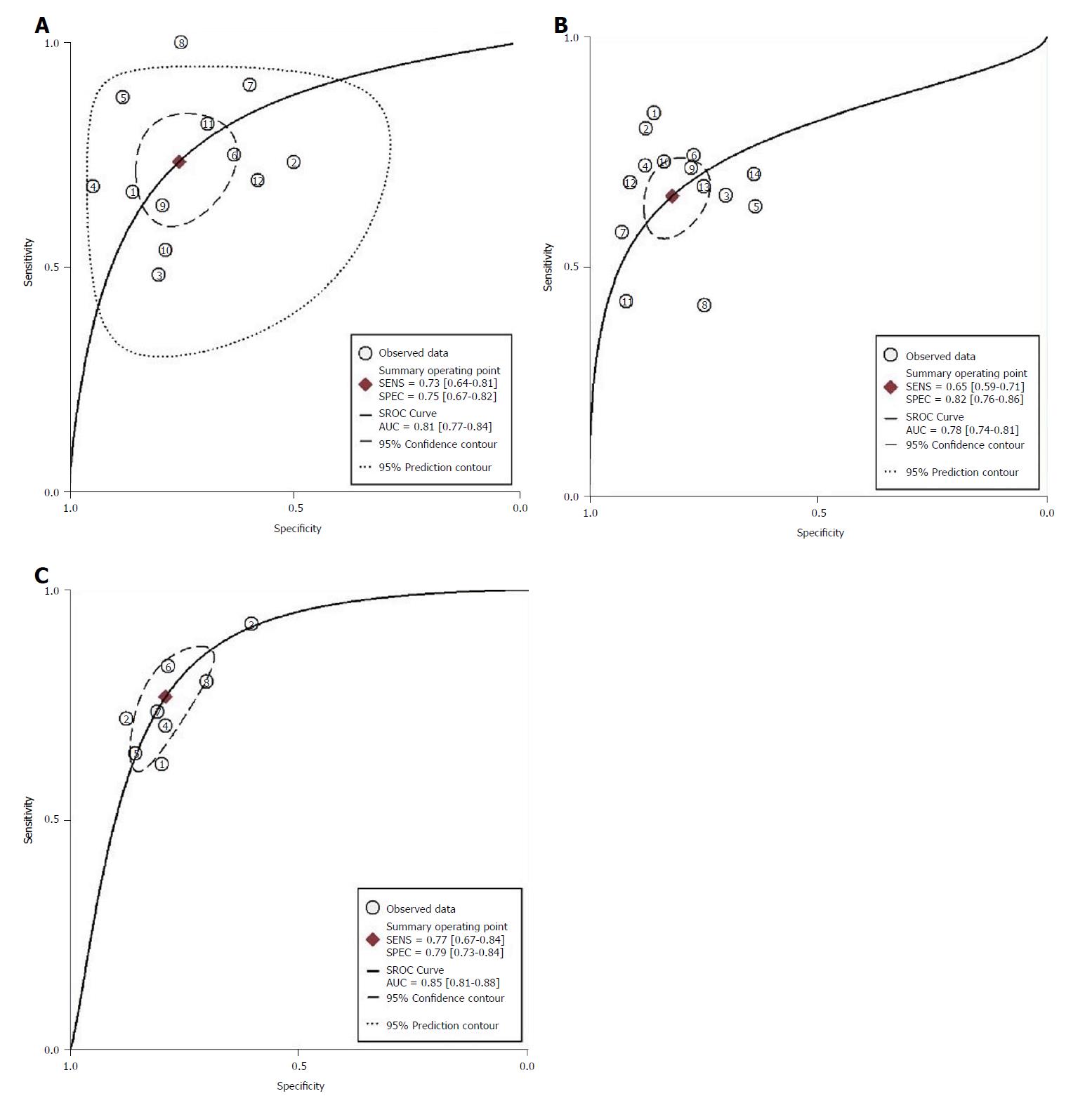
Twelve studies assessed the performance of CTP in predicting the mortality of patients. The spearman correlation coefficient for logit (true positive rate) vs logit (false positive rate) for CTP was 0.34 (P = 0.28), indicating no effect of threshold. The summary sensitivity was 0.73 (95%CI: 0.64-0.81) and the summary specificity was 0.75 (95%CI: 0.67-0.82). The forest plot is shown in Figure 4A. The AUROC of CTP was 0.81 (95%CI: 0.77-0.84) (Figure 5A). The linear regression of funnel plot asymmetry found no publication bias for the performance of CTP (P = 0.50) (Figure 6A).
Fourteen studies assessed the performance of the MELD score in predicting the mortality of patients. The spearman correlation coefficient for MELD was -0.10 (P = 0.74), indicating no effect of threshold. The summary sensitivity was 0.65 (95%CI: 0.59-0.71) and the summary specificity was 0.82 (95%CI: 0.76-0.86). The forest plot is shown in Figure 4B. The AUROC of the MELD score was 0.78 (95%CI: 0.74-0.81) (Figure 5B). The linear regression of funnel plot asymmetry found no publication bias for the performance of MELD (P = 0.60) (Figure 6B).
Eight studies assessed the performance of MELD-Na in predicting the mortality of patients. The Spearman correlation coefficient for MELD-Na was 0.67 (P = 0.07), indicating the threshold effect; thus, the sensitivity and specificity were not calculated. The AUROC of the MELD-Na score was 0.85 (95%CI: 0.81-0.88) (Figure 5C). The linear regression of funnel plot asymmetry found no publication bias for the performance of MELD-Na (P = 0.51) (Figure 6C).
There were only three studies available to assess the prediction value of MESO. We used simple pooling to evaluate the ability for prediction. The Spearman correlation coefficient was 0.50 (P = 0.67), indicating no threshold effect. The I2 was 66.0%, indicating high heterogeneity, so we used a random model. The summary sensitivity was 0.67 (95%CI: 0.58-0.74) and summary specificity was 0.84 (95%CI: 0.79-0.88). The AUROC of MESO was 0.86 (95%CI: 0.79-0.93).
Overall, MESO was the best model in predicting the mortality of the decompensated cirrhosis patients according to the AUROC.
We analyzed the prediction ability at different time points for CTP, MELD, and MELD-Na. The time points were divided as within 3 mo, 3 mo, 6 mo, and 12 mo. The results of analysis at different time points are shown in Table 2. In all models (CTP, MELD, and MELD-Na), AUROC was the highest at 6 mo and the lowest at 12 mo. For the prediction ability at different time points, MELD-Na had the highest AUROC in the within 3 mo group (0.89) and the 12-mo group (0.84). Both MELD-Na and CTP had the highest AUROC in the 3-mo group (0.86) among the four models. The AUROC of CTP was the highest in the 6-mo group (0.91).
| End-point (mortality) | Number of studies | Sensitivity (95%CI) | Specificity (95%CI) | Positive (95%CI) | Negative (95%CI) | DOR (95%CI) | I2 | AUROC |
| CTP | ||||||||
| < 3 mo | 8 | 0.72 (0.61, 0.81) | 0.73 (0.65, 0.79) | 2.6 (1.9, 3.7) | 0.39 (0.26, 0.58) | 7 (3, 14) | 0 | 0.78 |
| 3 mo | 5 | 0.79 (0.60, 0.91) | 0.79 (0.59, 0.90) | 3.7 (1.8, 7.6) | 0.26 (0.13, 0.55) | 14 (4, 46) | 96 | 0.86 |
| 6 mo | 3 | 0.90 (0.80, 0.96) | 0.69 (0.64, 0.75) | 3.0 (2.1, 4.5) | 0.16 (0.08, 0.33) | 18 (8, 40) | 0 | 0.91 |
| 12 mo | 3 | 0.68 (0.58, 0.72) | 0.68 (0.64, 0.72) | 3.3 (1.5, 7.3) | 0.49 (0.40, 0.60) | 7 (3, 20) | 84 | 0.72 |
| MELD | ||||||||
| < 3 mo | 9 | 0.66 (0.59, 0.73) | 0.87 (0.81, 0.91) | 5.1 (3.4, 7.7) | 0.39 (0.31, 0.48) | 13 (7, 24) | 6 | 0.76 |
| 3 mo | 6 | 0.70 (0.60, 0.78) | 0.78 (0.65, 0.87) | 3.1 (2.0, 4.9) | 0.39 (0.29, 0.52) | 8 (4, 15) | 95 | 0.78 |
| 6 mo | 3 | 0.74 (0.65, 0.82) | 0.83 (0.79, 0.87) | 4.4 (2.4, 8.2) | 0.30 (0.23, 0.43) | 15 (7, 34) | 56 | 0.83 |
| 12 mo | 4 | 0.60 (0.51, 0.68) | 0.88 (0.79, 0.93) | 4.8 (3.0, 7.7) | 0.46 (0.39, 0.55) | 10 (6, 17) | 92 | 0.75 |
| MELD-Na | ||||||||
| < 3 mo | 4 | 0.82 (0.69, 0.91) | 0.82 (0.64, 0.92) | 4.6 (2.2, 9.8) | 0.21 (0.12, 0.37) | 22 (9, 53) | 89 | 0.89 |
| 3 mo | 4 | 0.75 (0.63, 0.84) | 0.84 (0.80, 0.87) | 4.6 (3.5, 6.0) | 0.30 (0.20, 0.46) | 15 (8, 30) | 0 | 0.86 |
| 6 mo | 3 | 0.82 (0.73, 0.88) | 0.85 (0.80, 0.88) | 5.3 (3.7, 7.7) | 0.22 (0.15, 0.32) | 25 (14, 43) | 0 | 0.90 |
| 12 mo | 3 | 0.72 (0.63, 0.76) | 0.83 (0.80, 0.86) | 4.2 (3.4, 5.1) | 0.37 (0.30, 0.45) | 12 (8, 18) | 0 | 0.84 |
Furthermore, the overall mortality rates of variceal hemorrhage patients have been reported to be up to 30%-50% and 1-year mortality as high as 70% historically[36]. Thus, we conducted subgroup analysis to assess the performance of CTP and MELD score in predicting short-term mortality between variceal hemorrhage and others. The results showed that the AUROC of MELD was higher than that of CTP score (0.88 vs 0.76) in variceal hemorrhage patients. The detailed results are shown in Table 3.
| Subgroup | Number of study | Sensitivity (95%CI) | Specificity (95%CI) | Positive (95%CI) | Negative (95%CI) | DOR (95%CI) | I2 | AUROC |
| CTP | ||||||||
| Variceal hemorrhage | 4 | 0.70 (0.58, 0.80) | 0.71 (0.62, 0.78) | 2.4 (1.8, 3.1) | 0.42 (0.30, 0.60) | 6 (3, 10) | 19 | 0.76 |
| Others | 4 | 0.78 (0.46, 0.94) | 0.74 (0.61, 0.84) | 3.1 (1.5, 6.3) | 0.30 (0.09, 1.02) | 10 (1, 72) | 0 | 0.81 |
| MELD | ||||||||
| Variceal hemorrhage | 4 | 0.66 (0.53, 0.77) | 0.87 (0.84, 0.90) | 5.2 (3.9, 7.1) | 0.39 (0.27, 0.56) | 13 (7, 24) | 0 | 0.88 |
| Others | 5 | 0.68 (0.57, 0.77) | 0.87 (0.75, 0.93) | 5.1 (2.4, 10.7) | 0.37 (0.25, 0.54) | 14 (5, 40) | 0 | 0.79 |
This systematic review of the prognostic accuracy of CTP, MELD, MELD-Na, and MESO index included 16 studies involving 2337 patients. All their AUROC values were greater than 0.7, which demonstrated that the CTP, MELD, MELD-Na, and MESO index have certain prognostic value. MESO has the highest AUROC in all the assessed scoring systems.
CTP, as a reference for cirrhosis prognosis, has been used for more than 30 years. The drawback was that its indexes were subjective and unstable. Compared with the CTP score, MELD has some advantages. On one hand, three in four parameters were from a laboratory, which was more objective, stable, and easy, and only the index “etiology of cirrhosis” was affected by subjective factors explained by a clinician[37]. Additionally, the MELD score value was constant, and there was no “top value” or “bottom value” to predict the state of an illness. As a tool for allocation[12,38], MELD was based on verifiable measures of disease severity with minimal emphasis on waiting time[39]. Based on this meta-analysis with decompensated liver cirrhosis patients, MELD cannot replace CTP completely, and CTP still has good value for clinical application, particularly in 6-mo and 12-mo mortality prediction. CTP remains a reliable model to predict, and it might be suitable for the individual assessment of decompensated liver disease in daily clinical practice due to its simplicity and practicality[40]. Although CTP seemed subjective to some extent, clinical experience may be used to estimate the real state of illness rapidly and exhaustively, while some objective laboratory measurement parameters cannot. After all, there is significant clinical uncertainty.
Our meta-analysis results demonstrated that the prognostic accuracy of MELD-Na and MESO was higher than that of MELD. At different end-points, all the AUROC of MELD-Na was greater than or equal to 0.84. MELD-Na was superior to CTP and MELD for predicting mortality after 12 mo, indicating that MELD-Na was a good model to predict long-term mortality in decompensated liver cirrhosis patients than CTP and MELD. As hyponatremia was associated with increased morbidity and mortality in cirrhosis[41], MELD-Na and MESO were developed by incorporating the important parameter “serum Na” and using the novel algorithm in the MELD score, considering the deficiency of MELD. Some academics also confirmed that MELD-Na was a valid model to predict mortality in short- or long-term liver disease[42-44], and so did MESO[17,45].
When comparing CTP with MELD in variceal hemorrhage patients, the better choice has been inconsistent[46,47]. In our results, we found that the MELD was seemingly superior to CTP. The AUROC of MELD was 0.88 while that of CTP was 0.76 in predicting short-term mortality in variceal hemorrhage patients. The specific application of MELD would help the clinical management of variceal hemorrhage patients and suggest the prognostic evaluation.
The AUROC was the largest for 6-mo mortality among all end-points for all models (including CTP, MELD, and MELD-Na), which indicated that different end-points may affect the prediction accuracy of the scoring systems in decompensated patients. This result implied that the scoring systems would have the best effect for predicting the 6-mo mortality of decompensated cirrhosis patients.
According to our result and clinical practice, the indicators included in CTP is subjective, however, CTP is easy for doctors to get in daily practice and has high prognosis accuracy in medium term (6 mo). As for MELD, the score is presented in a continuous manner and the indicators are objective, but it does not consider the complication which doctors think might put threat on patients directly. There is no denying that MELD has prognosis value in variceal hemorrhage patients in this study. MELD-Na and MESO both consider the effect of serum Na on mortality of patients, and their prognosis accuracy is high. To be noticed, the number of studies about the MESO for prognosis in decompensated cirrhosis patients is not enough, and further study is needed to validate our finding with more original studies taken into consideration.
There were some limitations in our meta-analysis. First, the number of the included studies was relatively small, which restricted the detailed analysis for heterogeneity. Second, several closely related studies were not included in the analysis because of the lack of necessary data.
Overall, MESO shows promising value with the highest AUROC in all assessed scoring systems. MELD-Na has the best performance for predicting mortality at various time points. In particular, MELD has a unique advantage for patients with variceal hemorrhage.
In the future, more sensitive indicators could be added into the model for optimizing the original scoring system, or a more accurate model should be proposed for prognosis. Multicenter and long-term studies with larger samples would be helpful for the scoring systems to predict the mortality more precisely in decompensated liver cirrhosis patients.
Liver cirrhosis is a common chronic disease worldwide and decompensated cirrhosis is associated with a high risk of death. To find accurate prognostic scoring system not only could help clinicians to make better decisions but also has a wide significance in the context of organ allocation for decompensated cirrhosis patients.
There are so many scoring systems to predict the mortality of decompensated cirrhosis patients, while it is uncertain which scoring system is better. We performed a meta-analysis to compare the accuracy of four scoring systems: Child–Turcotte–Pugh (CTP), Model for End-stage Liver Disease score (MELD), MELD-Na, and MELD to Serum Sodium ratio (MESO) for predicting the mortality in decompensated liver cirrhosis. It is beneficial for confirming a high accuracy scoring system to use in clinical practice.
The main objective is to quantitatively compare the test accuracy of scoring systems and to pinpoint the more reliable scoring systems to forecast the mortality of decompensated cirrhosis patients. It will help us to assess the state of an illness and make better decision.
We searched PubMed, Web of science, Cochrane Library, EMBASE, and Ovid databases from inception to September 2018 for relevant articles and evaluated the quality of original articles by the Quality Assessment of Diagnostic Accuracy Studies 2 scale. As for statistical heterogeneity, threshold effect and non-threshold effect were assessed by Spearman correlation and Cochrane’s Q test, respectively. And optimum model was chosen to estimate the accuracy like diagnostic odd ratios, area under the summary receiver operating characteristic curve (AUROC). We used Deek’s funnel plot asymmetry to assess potential publication bias. Stata 12.0, Meta-DiSc 1.4, and Review Manager 5.3 were tools to be used.
Sixteen eligible studies involving 2337 decompensated liver cirrhosis patients were included in this meta-analysis. The overall analysis showed MESO had promising value with highest AUROC in all assessed scoring systems. MELD-Na had the best performance for predicting mortality at various time points. MELD had a unique advantage for patients with variceal hemorrhage.
The study confirmed the best model in predicting the mortality of the decompensated cirrhosis patients at different time points, and MELD or CTP is better for predicting short-term mortality in variceal hemorrhage patients. Additionally, the number of the included studies was relatively small, which restricted the detailed analysis for heterogeneity.
Further research would focus on more sensitive indicators that could be added into the model for optimizing the original scoring system, and a new model should be proposed for prognosis prediction more accurately. In addition, multicenter and long-term studies with larger samples could answer the question more convincingly.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koksal AS, Kreisel W, Ruiz-Margáin A S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Song H
| 1. | Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2132] [Article Influence: 112.2] [Reference Citation Analysis (3)] |
| 4. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 5. | Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573-84.e1-2; quiz e88-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Papatheodoridis GV, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V, Archimandritis AJ. MELD vs Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol. 2005;11:3099-3104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Lee M, Lee JH, Oh S, Jang Y, Lee W, Lee HJ, Yoo JJ, Choi WM, Cho YY, Cho Y. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int. 2015;35:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5733] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 9. | Forman LM, Lucey MR. Predicting the prognosis of chronic liver disease: an evolution from child to MELD. Mayo End-stage Liver Disease. Hepatology. 2001;33:473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Everson GT. MELD: the answer or just more questions? Gastroenterology. 2003;124:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 13. | Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 347] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 16. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 547] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 17. | Huo TI, Wang YW, Yang YY, Lin HC, Lee PC, Hou MC, Lee FY, Lee SD. Model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor and its correlation with portal pressure in patients with liver cirrhosis. Liver Int. 2007;27:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore). 2016;95:e2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 19. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9555] [Article Influence: 682.5] [Reference Citation Analysis (0)] |
| 20. | Radisavljevic MM, Bjelakovic GB, Nagorni AV, Stojanovic MP, Radojkovicn MD, Jovic JZ, Ignjatovic AM, Radisavljevic MM, Simonovic MM. Predictors of Mortality in Long-Term Follow-Up of Patients with Terminal Alcoholic Cirrhosis: Is It Time to Accept Remodeled Scores? Med Princ Pract. 2017;26:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Iino C, Shimoyama T, Igarashi T, Aihara T, Ishii K, Sakamoto J, Tono H, Fukuda S. Usefulness of the Glasgow-Blatchford score to predict 1-week mortality in patients with esophageal variceal bleeding. Eur J Gastroenterol Hepatol. 2017;29:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Barosa R, Roque Ramos L, Patita M, Nunes G, Fonseca J. CLIF-C ACLF score is a better mortality predictor than MELD, MELD-Na and CTP in patients with Acute on chronic liver failure admitted to the ward. Rev Esp Enferm Dig. 2017;109:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Cheng XP, Zhao J, Chen Y, Meng FK, Xu B, Yu HW, Meng QH, Liu YM, Zhang SB, Meng S. Comparison of the ability of the PDD-ICG clearance test, CTP, MELD, and MELD-Na to predict short-term and medium-term mortality in patients with decompensated hepatitis B cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:444-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Bal CK, Daman R, Bhatia V. Predictors of fifty days in-hospital mortality in decompensated cirrhosis patients with spontaneous bacterial peritonitis. World J Hepatol. 2016;8:566-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Fayad L, Narciso-Schiavon JL, Lazzarotto C, Ronsoni MF, Wildner LM, Bazzo ML, Schiavon Lde L, Dantas-Corrêa EB. The performance of prognostic models as predictors of mortality in patients with acute decompensation of cirrhosis. Ann Hepatol. 2015;14:83-92. [PubMed] |
| 27. | Suk KT, Kim CH, Park SH, Sung HT, Choi JY, Han KH, Hong SH, Kim DY, Yoon JH, Kim YS. Comparison of hepatic venous pressure gradient and two models of end-stage liver disease for predicting the survival in patients with decompensated liver cirrhosis. J Clin Gastroenterol. 2012;46:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Li JY, Deng Q, Wang Y, Xu MY, Lu LG. Prognostic value of the model for end-stage liver disease combined with serum sodium levels in patients with decompensated cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2012;20:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Shaikh S, Ghani H, Memon S, Baloch GH, Jaffery M, Shaikh K. MELD era: is this time to replace the original Child-Pugh score in patients with decompensated cirrhosis of liver. J Coll Physicians Surg Pak. 2010;20:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Boursier J, Cesbron E, Tropet AL, Pilette C. Comparison and improvement of MELD and Child-Pugh score accuracies for the prediction of 6-month mortality in cirrhotic patients. J Clin Gastroenterol. 2009;43:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Hyun JJ, Seo YS, Yoon E, Kim TH, Kim DJ, Kang HS, Jung ES, Kim JH, An H, Kim JH. Comparison of the efficacies of lamivudine versus entecavir in patients with hepatitis B virus-related decompensated cirrhosis. Liver Int. 2012;32:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Peng Y, Qi X, Dai J, Li H, Guo X. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8:751-757. [PubMed] |
| 33. | Sempere L, Palazón JM, Sánchez-Payá J, Pascual S, de Madaria E, Poveda MJ, Carnicer F, Zapater P, Pérez-Mateo M. Assessing the short- and long-term prognosis of patients with cirrhosis and acute variceal bleeding. Rev Esp Enferm Dig. 2009;101:236-248. [PubMed] |
| 34. | Teng W, Chen WT, Ho YP, Jeng WJ, Huang CH, Chen YC, Lin SM, Chiu CT, Lin CY, Sheen IS. Predictors of mortality within 6 weeks after treatment of gastric variceal bleeding in cirrhotic patients. Medicine (Baltimore). 2014;93:e321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Wang J, Wang AJ, Li BM, Liu ZJ, Chen L, Wang H, Shi F, Zhu X. MELD-Na: effective in predicting rebleeding in cirrhosis after cessation of esophageal variceal hemorrhage by endoscopic therapy. J Clin Gastroenterol. 2014;48:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Rajoriya N, Tripathi D. Historical overview and review of current day treatment in the management of acute variceal haemorrhage. World J Gastroenterol. 2014;20:6481-6494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Pagliaro L. MELD: the end of Child-Pugh classification? J Hepatol. 2002;36:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Lee J, Kim DG, Lee JY, Lee JG, Joo DJ, Kim SI, Kim MS. Impact of the Model for End-Stage Liver Disease Score Based Allocation System in Korea. Transplantation. 2017;101:S82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L; UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 534] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 40. | Zheng YX, Zhong X, Li YJ, Fan XG. Performance of scoring systems to predict mortality of patients with acute-on-chronic liver failure: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:1668-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Zhang JY, Qin CY, Jia JD, Wang BE. Serum sodium concentration profile for cirrhotic patients and its effect on the prognostic value of the MELD score. Zhonghua Gan Zang Bing Za Zhi. 2012;20:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Wong VW, Chim AM, Wong GL, Sung JJ, Chan HL. Performance of the new MELD-Na score in predicting 3-month and 1-year mortality in Chinese patients with chronic hepatitis B. Liver Transpl. 2007;13:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Ahmed R, Santhanam P, Rayyan Y. MELD-Na as a prognostic indicator of 30- and 90-day mortality in patients with end-stage liver disease after creation of transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2015;27:1226-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Lv XH, Liu HB, Wang Y, Wang BY, Song M, Sun MJ. Validation of model for end-stage liver disease score to serum sodium ratio index as a prognostic predictor in patients with cirrhosis. J Gastroenterol Hepatol. 2009;24:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Benedeto-Stojanov D, Nagorni A, Bjelaković G, Stojanov D, Mladenović B, Djenić N. The model for the end-stage liver disease and Child-Pugh score in predicting prognosis in patients with liver cirrhosis and esophageal variceal bleeding. Vojnosanit Pregl. 2009;66:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412-419.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |