Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.694
Peer-review started: July 31, 2018
First decision: August 31, 2018
Revised: October 3, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: November 6, 2018
Processing time: 98 Days and 5.5 Hours
We report a case of natural killer (NK)/T-cell lymphoma with concomitant syndrome of inappropriate antidiuretic hormone secretion (SIADH). The patient was a 64-year-old woman with a history of nasopharyngeal carcinoma of over 30 years. She was admitted with a chief complaint of intermittent fever for 2 mo. Palpation after admission indicated a swollen lymph node below the left jaw. Multiple imaging examinations on admission indicated multiple enlarged lymph nodes throughout the body. We performed a left submandibular lymph node biopsy, and the results revealed NK/T-cell lymphoma. A biochemical examination indicated Epstein-Barr virus positivity. At the same time, the patient developed hyponatremia. Based on her laboratory examination and clinical manifestation, decreased plasma osmolality, urine osmolality greater than plasma osmolality, lack of skin swelling, normal blood pressure, normal renal function, no adrenal function detected on serology, and no abnormalities in imaging examination of the adrenal glands, the likelihood of SIADH in the patient was high. After fluid restriction and administration of sodium chloride, the patient’s blood sodium level gradually increased. Subsequently, the immune function of the patient declined, there were severe symptoms of infection, and she died of respiratory failure. NK/T-cell lymphoma associated with SIADH has not, to our knowledge, been previously reported in PubMed. This case emphasizes the importance of monitoring serum ion levels, especially serum sodium, in patients with NK/T-cell lymphoma.
Core tip: Lymphoma is known to be a cause of syndrome of inappropriate antidiuretic hormone secretion (SIADH). Moreover, Epstein-Barr virus (EBV) has a high infection rate, and in very rare cases, it can lead to extranodal natural killer (NK)/T-cell lymphoma. A very limited number of patients with lymphoma accompanied by SIADH have been reported, but NK/T-cell lymphoma with concomitant SIADH has not yet been reported in PubMed. Here, we present a case of NK/T-cell lymphoma in a 64-year-old woman with EBV infection accompanied by SIADH, suggesting the importance of monitoring serum ions, especially serum sodium, in patients with NK/T-cell lymphoma.
- Citation: Liu QB, Zheng R. Natural killer/T-cell lymphoma with concomitant syndrome of inappropriate antidiuretic hormone secretion: A case report and review of literature. World J Clin Cases 2018; 6(13): 694-702
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/694.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.694
Epstein-Barr virus (EBV) is a well-recognized carcinogen that has been implicated in the etiology of several malignancies, including nasopharyngeal carcinoma[1]. The viral tropism of EBV is toward the B lymphocyte, but in very rare cases it can infect ectopic T and/or natural killer (NK) cells, leading to extranodal NK/T-cell lymphoma. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) is a common cause of hyponatremia[2], but the symptoms of SIADH are nonspecific, which make it hard to detect and treat promptly. Untreated acute hyponatremia can cause substantial morbidity and mortality[3]. Many cancers can lead to SIADH[4], including lymphoma. According to the 2016 revision of the World Health Organization classification of lymphoid neoplasms[5], lymphomas are classified as either Hodgkin or non-Hodgkin lymphomas, and NK-cell lymphomas are in the latter class. Although non-Hodgkin lymphoma with concomitant SIADH has been reported internationally, NK/T-cell lymphoma with concomitant SIADH has not been reported in PubMed. Herein, we present a case of NK/T-cell lymphoma in a 64-year-old woman with EBV infection accompanied by SIADH, and suggest that monitoring serum ions, especially serum sodium, is vital in patients with NK/T-cell lymphoma.
A 64-year-old woman was admitted with a chief complaint of intermittent fever for 2 mo. Two months previously, the patient had a sinus infection resulting from a cold, accompanied by intermittent fevers in the afternoon, with body temperature reaching as high as 38.9 °C. The patient had a history of nasopharyngeal carcinoma of over 30 years, which was controlled with chemotherapy; there was no recurrence to this point. One year prior, she had traveled to Europe, where she ate local food including sausage and fish.
Physical examination after admission indicated a body temperature of 37.1 °C, heart rate of 80 bpm, respiratory rate of 18 breaths per minute, and blood pressure of 14.7/9.3 kPa. A 2.5 cm × 1 cm swollen, hard, unfixed, and painless lymph node was palpated below the left jaw. No blood congestion was found in the throat area. The left abdomen was soft with tender. No other obvious abnormalities were observed.
Laboratory tests indicated the following biochemical results: serum sodium, 137.6 mmol/L; white blood cell (WBC) count, 2.4 × 109/L; neutrophil count, 4.9 × 109/L; C-reactive protein, 11.7 mg/L; EBV quantitative DNA test, 2.19E × 104 copies/mL; EBV NA-IgG antibodies, positive (+) (> 600); EBV VCA-IgG antibodies, positive (+) (> 750); and EBV-IgM antibodies, negative. No abnormalities were found in the remaining tests.
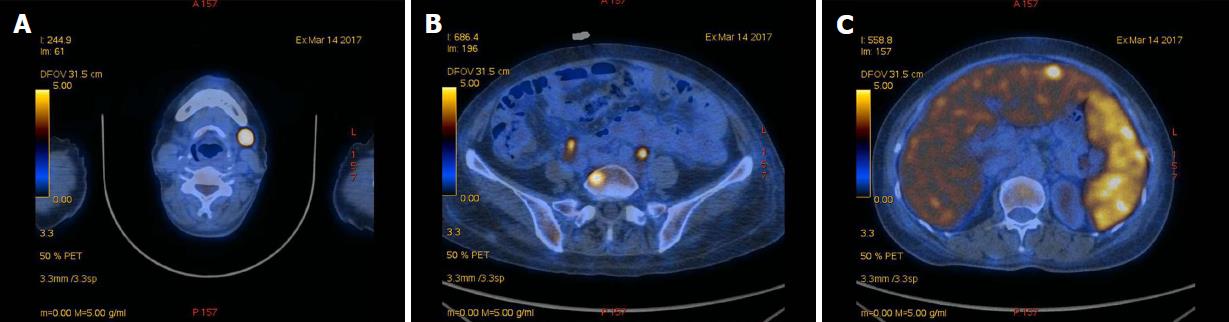
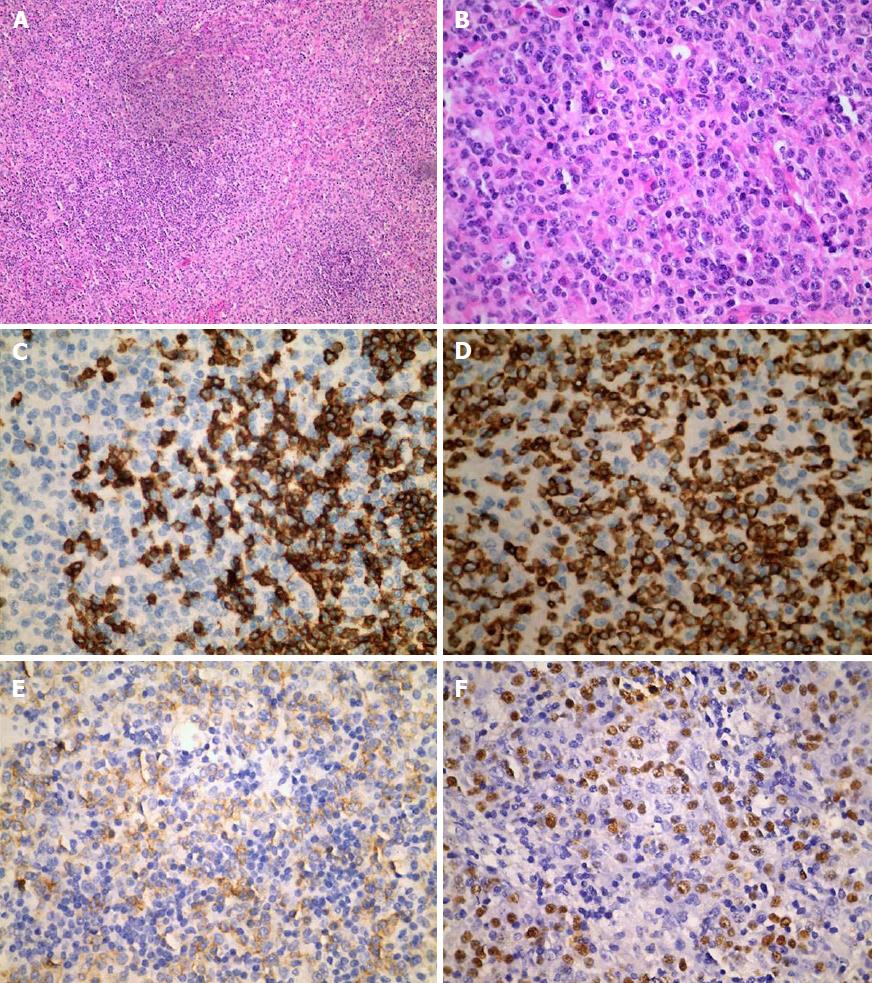
An imaging examination was performed on admission. Computed tomography of the lungs indicated that the bilateral axillary and mediastinal lymph nodes were slightly enlarged; enhanced computed tomography of the whole abdomen indicated swollen lymph nodes in the hepatic portal region and retroperitoneum, which did not rule out lymphoma; and bilateral ultrasound of the submandibular glands indicated several visible bilateral swollen lymph nodes. To determine the characteristics of the lymph nodes, whether lymphoma was present, and the margins of the lesion, we performed positron emission tomography-computed tomography, bone marrow biopsy, and left submandibular lymph node biopsy. Positron emission tomography-computed tomography indicated multiple swollen lymph nodes throughout the body accompanied by increased fluorodeoxyglucose metabolism, consistent with lymphoma (Figure 1). Bone marrow biopsy and immunotyping showed a myelogram with hyperplastic activity and a small number of abnormal lymphocytes, accounting for 4.80% of the visible cells other than NK lymphocytes. Biopsy of the left submandibular lymph nodes confirmed NK/T-cell lymphoma, and in situ hybridization showed multiple cells with strongly positive signal for EBV-encoded small RNA (Figure 2).
At day 10 after admission, the patient developed lethargy, and had a serum Na+ level of 116.9 mmol/L. Once daily concentrated sodium chloride solution of 20 mL + 250 mL 0.9% normal saline was administered intravenously. One day later, the serum Na+ level decreased rapidly to 109.3 mmol/L. The serum K+ level was 3.36 mmol/L, urea level was 2.99 mmol/L, serum creatinine was 47.8 μmol/L, blood glucose level was 7.08 mmol/L, plasma osmolality was 235.39 mOsm/kg, and urine osmolality was 494 mOsm/kg. The patient’s blood pressure was normal during that time. The fluid intake of the patient was immediately restricted, and three times daily, two salt capsules and 10 mL of concentrated sodium chloride were administered orally. After 2 d, the patient’s blood Na+ level gradually increased to 126.5 mmol/L (8.6 mmol/L per day). Her mental state returned to normal, and the endocrinology department was consulted. Based on the patient‘s decreased plasma osmolality, urine osmolality greater than plasma osmolality, lack of skin swelling, normal blood pressure, normal renal function, no adrenal function detected on serology, and no abnormalities in imaging examination of the adrenal glands, as well as the effect of treatment, the likelihood of SIADH in the patient was high. The supplemental infusion of intravenous concentrated sodium chloride solution was discontinued, and renal sodium secretion was assayed. The hematology department was also consulted, and the patient was administered with epirubicin, vinorelbine sulfate, flumethasone, cyclophosphamide, and asparaginase chemotherapy, along with supportive treatment. We believed that the patient might be involved in stage 4B NK/T-cell lymphoma with concomitant SIADH, although with slightly lower renal sodium levels (20 mmol/L).
On day 30 after admission, the patient’s white blood cell (WBC) count gradually decreased to 0.1 × 109/L, and the neutrophil count rapidly decreased to 0/L. The immune function of the patient declined, there were severe symptoms of infection, and respiratory function further deteriorated. On day 31 after admission, the WBC count gradually decreased to 0, various vital signs declined, and the patient died after failed resuscitation.
SIADH has a hidden onset and is a syndrome caused by excessive antidiuretic hormone (ADH) secretion by the posterior pituitary[6]. It has a high mortality rate[3]. SIADH has many causes, and many cancers can lead to SIADH. It has been reported that lymphoma cells secrete ADH, and this prolonged ADH production results in SIADH[7-9]. SIADH secondary to lymphoma is relatively rare, and SIADH secondary to NK/T-cell lymphoma has not yet been reported.
The symptoms of SIADH are nonspecific, and are primarily based on diagnostic standards published by Bartter et al[10] in 1967: (1) decreased plasma osmolality; (2) urine osmolality greater than plasma osmolality; (3) increased renal sodium secretion; (4) no skin swelling and normal blood pressure; and (5) normal renal and adrenal function. Recent studies showed that a fractional excretion of uric acid > 12% has a high sensitivity and specificity for diagnosing SIADH[11] and can serve as a new basis for confirming an SIADH diagnosis. The first-line treatment for SIADH is restriction of fluid intake. If urine osmolality is higher than 500 mOsm/kg H2O and fluid restriction is ineffective, demethylchlortetracycline, urea, tolvaptan, and other drugs are instead used for treatment[12-14]. Intravenous infusion of 20-40 mg furosemide is used to manage volume overload, and 3% hypertonic saline solution by mouth or continuous intravenous infusion can be used as necessary for correcting hyponatremia[10,15]. During sodium solution infusion, the focus should be on changes in blood sodium rather than the rate of sodium solution infusion. In the first 24-48 h, changes in blood sodium should be closely monitored, and treatment should be adjusted accordingly to rapidly correct blood sodium to within the safe range; otherwise, correction to normal ranges will not be achieved.
Studies have shown that drugs and malignant tumors are the most common causes of SIADH[16], and the prognosis for SIADH secondary to malignant tumors is poorer than that caused by drugs[4]. To reduce the development of SIADH, regular examinations of blood sodium should be performed when malignant tumors are discovered as well as when beginning to administer drugs that are known to cause SIADH; this can help facilitate the immediate discovery of decreased blood sodium and thus immediately manage symptoms and improve prognosis.
According to the 2016 revision of the World Health Organization classification of lymphoid neoplasms[5], lymphomas are classified as either Hodgkin or non-Hodgkin lymphomas. Non-Hodgkin lymphomas are classified as either mature B cell lymphomas or mature T-cell lymphomas, and NK-cell lymphomas are the latter. Currently, 34 cases of non-Hodgkin lymphoma with concomitant SIADH have been reported internationally[7,8,17-34] (Table 1).
| Classification | Ref. | Country | Gender | Age | EBV status | Lymphoma classification | Symptoms | Treatment | Outcome |
| T-cell lymphoma | Chubachi et al[18] 1995 | Japan | M/ F | 53/78 yr | Infected | Nasal T-cell lymphoma | Fever following adjuvant chemotherapy | Fluid restriction | Died |
| Demirkan et al[19] 2001 | Turkey | M | 23 yr | Unknown | Anaplastic large cell lymphoma | Weight loss | Fluid restriction | Died | |
| Night sweats | |||||||||
| Fever | |||||||||
| Hirata et al[8] 2012 | Japan | F | 40 yr | Unknown | Primary cutaneous anaplastic large cell lymphoma | Erythema | Fluid restriction | Died | |
| Right shoulder ulceration | Isotonic saline | ||||||||
| Nishiwaki et al[30] 2014 | Japan | F | 70 yr | Unknown | Acute adult T-cell leukemia/lymphoma | Rash | Fluid restriction | Cured | |
| Isotonic saline | |||||||||
| Sun et al[34] 2018 | China | M | 71 yr | Uninfected | Extranodal nasal type natural killer (NK) /T cell lymphoma | Testicular enlargement | Fluid restriction | Died | |
| Multiple rashes with eschar | Sodium chloride for oral | ||||||||
| Obstinate hyponatremia | Hypertonic saline infusion | ||||||||
| Hydrocortisone infusion | |||||||||
| Phan et al[25] 1998 | Indonesia | M | 53 yr | Unknown | Burkitt’s lymphoma | Left maxillary swelling | Plasma exchanges | Died | |
| Methotrexate | |||||||||
| Cytarabine | |||||||||
| Hydrocortisone | |||||||||
| B-cell lymphoma | Sica et al[22] 1999 | Italy | F | 41 yr | Unknown | Primary central nervous system lymphoma | None | Fluid restriction | Cured |
| Normal saline | |||||||||
| Diuretics | |||||||||
| Watabe et al[23] 2000 | Japan | M | 70 yr | Infected | Angiotropic B-cell lymphoma | Anemia | Fluid restriction | Died | |
| High LDH | |||||||||
| Ohara et al[27] 2007 | Japan | F | 65 yr | Unknown | Diffuse large B-cell lymphoma | Abdominal pain | Fluid restriction | Cured | |
| Acyclovir infusion | |||||||||
| Morimoto et al[20] 2007 | Japan | M | 75 yr | Unknown | Intravascular large B-cell lymphoma | Nothing | Fluid restriction | Died | |
| Hypertonic saline infusion | |||||||||
| Furosemide | |||||||||
| Fludrocortisone acetate | |||||||||
| Brodmann et al[26] 2007 | Switzerland | F | 76 yr | Unknown | Mantle cell lymphoma | Unknown | Fluid restriction | Cured | |
| Sodium chloride | |||||||||
| Potassium chloride | |||||||||
| Kobayashi et al[7] 2008 | Japan | F | 84 yr | Unknown | Diffuse large B-cell lymphoma | Left cervical tumor increased | Fluid restriction | Cured | |
| Isotonic saline | |||||||||
| Polprasert et al[21] 2011 | Thailand | F | 64 yr | Unknown | Follicular lymphoma | Watery diarrhea | Ganciclovir | Cured | |
| Rituximab | |||||||||
| Cyclophosphamide | |||||||||
| Vincristine | |||||||||
| Prednisolone | |||||||||
| Onishi et al[28] 2011 | Japan | F/ M/ M | 73/80/83 yr | Unknown | Asian variant of intravascular large B cell lymphoma | Unknown | Unknown | Unknown | |
| M | 69/83 yr | Died | |||||||
| Onishi et al[28] 2011 | Japan | M/M/F | 75/60/83 yr | Unknown | Diffuse large B-cell lymphoma | Unknown | Unknown | Unknown | |
| F/M | 84/64 yr | Died | |||||||
| Onishi et al[28] 2011 | Japan | M/M/F/M | 74/69/75/75 yr | Unknown | Plasmablastic lymphoma/ Primary central nervous system lymphoma/ Mantle cell lymphoma/ Lymphoplasmacytic lymphoma | Unknown | Unknown | Died/Died/Unknown/ Died | |
| Bockorny et al[29] 2012 | America | M | 70 yr | Unknown | Marginal zone lymphoma | Fatigue | Fluid restriction | Cured | |
| Confusion | Demeclocycline | ||||||||
| Skin lesions | Rituximab | ||||||||
| Zhu et al[24] 2013 | China | F | 70 yr | Unknown | Diffuse large B-cell lymphoma | Nausea | Fluid restriction | Cured | |
| Vomiting | CHOP | ||||||||
| Left leg radiating pain | |||||||||
| Akhtar et al[17] 2013 | United Kingdom | M | 75 yr | Unknown | Intravascular large B-cell lymphoma | Weight loss | Fluid restriction | Died | |
| Clammy | Demeclocycline Fludrocortisone | ||||||||
| Incontinent hypoxic | |||||||||
| Sumiyoshi et al[31] 2014 | Japan | F | 61 yr | Unknown | Follicular lymphoma | Epigastralgia | Aciclovir | Cured | |
| Peritoneal irritation | |||||||||
| Intestinal pseudo-obstruction | |||||||||
| Itaya et al[32] 2015 | Japan | M | 81 yr | Unknown | Diffuse large B-cell lymphoma | Fever | Hypertonic saline | Died | |
| Fatigue | Hydrocortisone sodium succinate | ||||||||
| Anorexia | |||||||||
| Shimizu et al[33] 2017 | Japan | F | 73 yr | Unknown | Mucosa-associated lymphoid tissue lymphoma | Unknown | Fluid restriction | Cured | |
| Hypertonic saline |
The male/female ratio of these 34 cases was 19:15, mean age was 69 years (range 23-84 years), and patients aged between 60 and 90 years accounted for 85% (29/34) of the patients. The clinical manifestations of SIADH in these cases were nonspecific, and large B-cell lymphoma made up 47% (16/34) of all the pathological types. Based on the known treatment data, the main treatment was fluid restriction, which was administered to 80% (16/20). The ratio of outcomes died/cured/unknown was 17:10:7, and the mortality rate of known outcomes was 63% (17/27). The clinical characteristics of known cases may be useful for clinicians to remain aware for these characteristics in patients who may be at risk for SIADH.
The pathogenesis of SIADH secondary to lymphoma is complex. One potential cause is abnormal secretion of ADH by lymphocytes[7,10,30]. In our case, because of the death of the patient, whether hyponatremia was recurrent could not be determined, and abnormal secretion of ADH by lymphocytes could not be ruled out. Central nervous system injury secondary to non-Hodgkin lymphomas may also play an important role in the pathogenesis of SIADH[10]. Hyponatremia can also lead to nervous system symptoms[10], but the likelihood of central nervous system injury in our patient was low. The use of chemotherapy drugs such as vinca alkaloids and cyclophosphamide can also induce SIADH[35]. However, before SIADH was discovered in our patient, no chemotherapy drugs had been administered, so this did not apply in our case.
Hypercytokinemia is another important factor in the development of SIADH secondary to lymphoma. Studies have shown that the levels of epidermal growth factor (EGF), granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-5, IL-6, IL-12, IP-10, sIL-2Rα, membrane immunoglobulin (MIG), IL-1RA, and other cytokines are significantly elevated in T-cell lymphomas[36,37]. In extranodal nasal type NK/T-cell lymphomas, sIL-2Rα, IL-6, and IL-10 are significantly elevated[38]. Increased IL-2, sIL-2R, IL-6, IL-1β, and tumor necrosis factor (TNF)-α can lead to abnormal secretion of ADH[39]. Watabe et al[23] found that patients with high levels of the cytokine IL-6 are more likely to develop SIADH. We did not examine the levels of cytokines in our patient; however, it cannot be ruled out that SIADH in our patient was associated with abnormal secretion of ADH by lymphocytes or that the lymphoma regulated ADH secretion through cytokines.
EBV is a member of the human herpesvirus family and its genome consists of a single double-stranded DNA molecule. It has a high infection rate, with potentially 90% or more of all individuals infected worldwide[40,41]. Humans are the only host for EBV and they become lifelong carriers of the virus after infection. Most hosts can live with EBV for a long period without any serious effects, but in some individuals, EBV is closely associated with the development of malignant tumors[42], including nasopharyngeal carcinoma and lymphomas. The viral tropism of EBV is toward B lymphocytes, but in very rare cases, it can infect ectopic T and/or NK cells, leading to chronic active EBV infection, extranodal NK/T-cell lymphoma, or invasive NK cell leukemia[43].
EBV can be divided into three latency programs. Latency II shows expression of EBV nuclear antigen 1 (EBNA1) and latent membrane proteins (LMPs), and is closely associated with the development of peripheral NK/T-cell lymphoma and nasopharyngeal carcinoma in elderly patients[42,44-46]. Our patient had been diagnosed with both nasopharyngeal carcinoma and NK/T-cell lymphoma, and the possibility of long-term latent EBV infection cannot be ruled out.
According to the 2016 revision of the World Health Organization classification of lymphoid neoplasms, NK/T-cell lymphoma is categorized into mature T-cell lymphoma and extranodal nasal type NK/T-cell lymphoma. The latter type is a relatively rare lymphoma characterized by high invasiveness, poor prognosis, and high likelihood of recurrence. According to a 2010 report, extranodal nasal type NK/T-cell lymphoma accounts for 6.9% of non-Hodgkin lymphomas and 28.2% of T-cell and NK cell lymphomas in China[47,48]. Eighty to ninety percent of patients with nasal type NK/T-cell lymphoma report symptoms of nasal congestion, sinus infection, ulceration, and epistaxis[48]. Traditional treatments include chemotherapy, radiotherapy, and multimodal therapy, but even in patients with stage I or II disease, the 3-year survival rate is only 40%-50%[49-51]. Most published studies related to the efficacy of chemotherapy in extranodal NK/T-cell lymphoma have shown that the recurrence rate is as high as 50%[52]. Our patient was diagnosed with nasopharyngeal carcinoma 30 years earlier without recurrence following control with chemotherapy, but specific pathological examination results were not obtained. In addition, paraffin sections from over 30 years ago cannot be re-stained. Given the limitations of previous pathological examinations, the possibility that the patient developed extranodal nasal type NK/T-cell lymphoma cannot be ruled out. However, based on its biological characteristics, namely, a high recurrence rate, high invasiveness, and poor prognosis, the likelihood that our patient developed extranodal nasal type NK/T-cell lymphoma 30 years ago is small.
In summary, NK/T-cell lymphoma with concomitant SIADH is relatively rare. Blood sodium must be closely monitored in lymphoma patients as an indicator of the development of SIADH, and immediate treatment by restricting fluid intake and replenishing sodium should be administered based on blood sodium levels. At the same time, actively searching for the cause of the disease and planning adjunctive therapy with drugs as needed will prevent critical patients from developing hyponatremia and hypo-osmolality, thereby improving their prognosis.
A 64-year-old woman was admitted with intermittent fever for 2 mo.
Stage 4B natural killer (NK)/T-cell lymphoma with concomitant syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Infection, typhia, brucellosis, etc.
As determined by blood and urine sampling examination, serum Na+ was 109.3 mmol/L, urea was 2.99 mmol/L, serum creatinine was 47.8 μmol/L, plasma osmolality was 235.39 mOsm/kg, and urine osmolality was 494 mOsm/kg.
Positron emission tomography-computed tomography indicated multiple swollen lymph nodes throughout the body accompanied by increased fluorodeoxyglucose metabolism, consistent with lymphoma.
Biopsy of the left submandibular lymph nodes confirmed NK/T-cell lymphoma.
Chemotherapy, fluid restriction, and administration of sodium chloride.
This is the first known report of NK/T-cell lymphoma with concomitant SIADH in PubMed.
Lymphoma is one of the causes of SIADH; however, NK/T-cell lymphoma with concomitant SIADH has not been reported.
This case report emphasizes the importance of monitoring serum ions and etiological treatment in patients with NK/T-cell lymphoma.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): E
P- Reviewer: Akbulut S, Chowdhury FH, Dasgupta S, Ohashi N, Vaudo G, Vidal EIO S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Song H
| 1. | Khan G, Hashim MJ. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010. Infect Agent Cancer. 2014;9:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Adrogué H, Madias N. Hyponatremia. N Engl J Med. 2000;342:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1106] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 3. | Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126:S1-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 656] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 4. | Goldvaser H, Rozen-Zvi B, Yerushalmi R, Gafter-Gvili A, Lahav M, Shepshelovich D. Malignancy associated SIADH: Characterization and clinical implications. Acta Oncol. 2016;55:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5427] [Article Influence: 603.0] [Reference Citation Analysis (0)] |
| 6. | Crowley RK, Thompson CJ. Syndrome of inappropriate antidiuresis. Expet Rev Endocrinol Metabol. 2006;1:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kobayashi K, Yokote T, Akioka T, Takubo T, Tsuji M, Hanafusa T. Inappropriate antidiuretic hormone production in diffuse large B-cell lymphoma. Br J Haematol. 2008;143:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hirata Y, Yokote T, Nishiwaki U, Tsuji M, Hanafusa T. Syndrome of inappropriate antidiuretic hormone secretion associated with primary cutaneous anaplastic large cell lymphoma. Br J Haematol. 2012;157:412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Yue L, Li YL. Clinical Significance of Peripheral blood EB Virus Detection in NK/T Cell Lymphoma Patients. Zhongguo Shiyan Xueyexue Zazhi. 2017;25:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42:790-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 751] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, Blum CA, Nickel CH, Bingisser R, Bock A. Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: 'The Co-MED Study'. Clin Endocrinol (Oxf). 2017;86:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Dousa TP, Wilson DM. Effects of demethylchlortetracycline on cellular action of antidiuretic hormone in vitro. Kidney Int. 1974;5:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Verbalis JG, Adler S, Schrier RW, Berl T, Zhao Q, Czerwiec FS; SALT Investigators. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Verbalis JG, Baldwin EF, Neish PN, Robinson AG. Effect of protein intake and urea on sodium excretion during inappropriate antidiuresis in rats. Metabolism. 1988;37:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Janicic N, Verbalis JG. Evaluation and management of hypo-osmolality in hospitalized patients. Endocrinol Metab Clin North Am. 2003;32:459-481, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Shepshelovich D, Leibovitch C, Klein A, Zoldan S, Milo G, Shochat T, Rozen-zvi B, Gafter-Gvili A, Lahav M. The syndrome of inappropriate antidiuretic hormone secretion: Distribution and characterization according to etiologies. Eur J Intern Med. 2015;26:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Akhtar S, Cheesman E, Jude EB. SIADH and partial hypopituitarism in a patient with intravascular large B-cell lymphoma: a rare cause of a common presentation. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Chubachi A, Miura I, Hatano Y, Ohshima A, Nishinari T, Miura AB. Syndrome of inappropriate secretion of antidiuretic hormone in patients with lymphoma-associated hemophagocytic syndrome. Ann Hematol. 1995;70:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Demirkan F, Vural F, Ozsan GH, Ozcan MA, Ozkal S, Undar B. Hemophagocytic syndrome associated with inappropiate secretion of antidiuretic hormone in lymphoma and acute myeloblastic leukemia: report of two cases. Leuk Lymphoma. 2001;42:1401-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Morimoto K, Ogihara T, Shiomi T, Awaya N. Intravascular large B-cell lymphoma with preceding syndrome of inappropriate secretion of antidiuretic hormone. Intern Med. 2007;46:1569-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Polprasert C, Wongjitrat C, Wisedopas N. Case report: severe CMV colitis in a patient with follicular lymphoma after chemotherapy. J Med Assoc Thai. 2011;94:498-500. [PubMed] |
| 22. | Sica S, Cicconi S, Sorà F, Chiusolo P, Piccirillo N, Laurenti L, La Barbera EO, Giordano G, Leone G. Inappropriate antidiuretic hormone secretion after high-dose thiotepa. Bone Marrow Transplant. 1999;24:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Watabe R, Shibata K, Hirase N, Kodera T, Muta K, Nishimura J, Nawata H. Angiotropic B-cell lymphoma with hemophagocytic syndrome associated with syndrome of inappropriate secretion of antidiuretic hormone. Ann Hematol. 2000;79:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Zhu CG, Zhang QZ, Zhu M, Zhai QL, Liang XY, Shao ZH, Ver Hoeve EC, Qu HQ. A case report of syndrome of inappropriate antidiuretic hormone secretion with Castleman's disease and lymphoma. BMC Endocr Disord. 2013;13:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Phan TG, Manoharan A, Pryor D. Relapse of central nervous system Burkitt's lymphoma presenting as Guillain-Barre syndrome and syndrome of inappropriate ADH secretion. Aust N Z J Med. 1998;28:223-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Brodmann S, Gyr Klaas E, Cathomas R, Girardi V, von Moos R. Severe hyponatremia in a patient with mantle cell lymphoma treated with bortezomib: a case report and review of the literature. Onkologie. 2007;30:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Ohara F, Kobayashi Y, Akabane D, Maruyama D, Tanimoto K, Kim SW, Watanabe T, Tobinai K. Abdominal pain and syndrome of inappropriate antidiuretic hormone secretion as a manifestation of visceral varicella zoster virus infection in a patient with non-Hodgkin's lymphoma. Am J Hematol. 2007;82:416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Onishi C, Ikejiri F, Kawakami K, Miyake T, Kumanomido S, Inoue M, Takahashi T, Tanaka J, Yamamoto M, Sugimoto T. Asian variant of intravascular large B cell lymphoma causes patients to frequently develop the syndrome of inappropriate antidiuretic hormone secretion. Ann Hematol. 2011;90:1293-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Bockorny B, Dasanu CA. Syndrome of inappropriate antidiuretic hormone secretion due to marginal zone lymphoma: responding to rituximab. Clin Lymphoma Myeloma Leuk. 2012;12:220-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Nishiwaki U, Hirata Y, Yokote T, Iwaki K, Tsuji M, Hanafusa T. Syndrome of inappropriate antidiuretic hormone secretion associated with adult T-cell leukaemia/lymphoma. Br J Haematol. 2014;166:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Sumiyoshi H, Kobayashi Y, Makita S, Tatsuno M, Kitahara H, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Tobinai K. Viscerally disseminated herpes zoster presenting abdominal pain, siadh, and skin rash in a follicular lymphoma patient. Ann Oncol. 2014;25 Suppl 5:v97-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Itaya M, Nagata S, Ogino S, Ohura M, Kuriki K, Fukaya T, Ohta N, Sugiyama K, Oki Y, Ikegaya N. A case of primary adrenal diffuse large B cell lymphoma presenting with severe hyponatremia. CEN Case Rep. 2016;5:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Shimizu N, Tanaka S, Watanabe Y, Tokuyama W, Hiruta N, Ohwada C, Sakaida E, Nakaseko C, Tatsuno I. Syndrome of Inappropriate Antidiuretic Hormone Secretion in a Patient with Mucosa-associated Lymphoid Tissue Lymphoma. Intern Med. 2017;56:3225-3229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Sun Y, Lin S, Lin KY, Li WM, Xu W, Zeng LY. Extranodal nasal type natural killer/T-cell lymphoma with refractory hyponatremia: A case report and literature review. Tumor. 2018;38:140-144. |
| 35. | Liamis G, Filippatos TD, Elisaf MS. Electrolyte disorders associated with the use of anticancer drugs. Eur J Pharmacol. 2016;777:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Gupta M, Stenson M, O'Byrne M, Maurer MJ, Habermann T, Cerhan JR, Weiner GW, Witzig TE. Comprehensive serum cytokine analysis identifies IL-1RA and soluble IL-2Rα as predictors of event-free survival in T-cell lymphoma. Ann Oncol. 2016;27:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Nakayama S, Yokote T, Kobayashi K, Takubo T, Tsuji M, Hanafusa T. Multiple cytokine-producing peripheral T-cell lymphoma. Br J Haematol. 2009;146:125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Ohno T, Ueda Y, Nagai K, Takahashi T, Konaka Y, Takamatsu T, Suzuki T, Sasada M, Uchiyama T; Kyoto University Hematology/Oncology Study Group. The serum cytokine profiles of lymphoma-associated hemophagocytic syndrome: a comparative analysis of B-cell and T-cell/natural killer cell lymphomas. Int J Hematol. 2003;77:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Mastorakos G, Weber JS, Magiakou MA, Gunn H, Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 1994;79:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 41. | Dunleavy K, Roschewski M, Wilson WH. Lymphomatoid granulomatosis and other Epstein-Barr virus associated lymphoproliferative processes. Curr Hematol Malig Rep. 2012;7:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10:803-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 519] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 43. | Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B Biol Sci. 2017;372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 44. | Cesarman E, Mesri EA. Virus-associated lymphomas. Curr Opin Oncol. 1999;11:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol. 2003;121:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Li X, Li G, Gao Z, Zhou X, Zhu X. The relative frequencies of lymphoma subtypes in china: a nationwide study of 10002 cases by the chinese lymphoma study group. Ann Oncol. 2011;22:141. |
| 48. | Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, Konoplev S, Bueso-Ramos CE, Vega F, Medeiros LJ. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013;37:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 49. | Kim GE, Cho JH, Yang WI, Chung EJ, Suh CO, Park KR, Hong WP, Park IY, Hahn JS, Roh JK. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000;18:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 236] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Kim WS, Song SY, Ahn YC, Ko YH, Baek CH, Kim DY, Yoon SS, Lee HG, Kang WK, Lee HJ. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 51. | Cheung MM, Chan JK, Lau WH, Ngan RK, Foo WW. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 2002;54:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | Lee J, Kim CY, Park YJ, Lee NK. Sequential chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in patients with stage I/II extranodal natural killer/T-cell lymphoma, nasal type. Blood Res. 2013;48:274-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |