Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.671
Peer-review started: July 24, 2018
First decision: August 30, 2018
Revised: September 5, 2018
Accepted: October 12, 2018
Article in press: October 12, 2018
Published online: November 6, 2018
Processing time: 105 Days and 22.3 Hours
Tenofovir disoproxil fumarate (TDF) is a potent nucleotide analogue with high barrier to resistance, which is recommended for multi-drug resistant hepatitis B virus (HBV) infection. However, nephrotoxicity has been reported during TDF treatment, and tenofovir alafenamide (TAF), which has comparable efficacy to TDF and improves bone and renal safety, can be used as a replacement strategy. Herein, we describe a clinical case concerning a 60-year-old individual suffering liver cirrhosis and renal dysfunction, and being infected with multidrug-resistant HBV. When failing treatment with TDF, he received TAF as a rescue therapy. TAF effectively inhibited HBV replication without worsening renal function or serum phosphorus abnormality. Furthermore, hepatocellular carcinoma (HCC) occurred during TAF treatment despite controlling the viral load. The risk of HCC could not be eliminated and should be monitored during TAF treatment.
Core tip: Tenofovir alafenamide rescues multidrug resistance and renal dysfunction in an old patient but does not eliminate the risk of hepatocellular carcinoma (HCC) development. Despite controlling the viral load, risk of HCC exists and should be monitored in cirrhotic patients.
- Citation: Lu JC, Liu LG, Lin L, Zheng SQ, Xue Y. Incident hepatocellular carcinoma developing during tenofovir alafenamide treatment as a rescue therapy for multi-drug resistant hepatitis B virus infection: A case report and review of the literature. World J Clin Cases 2018; 6(13): 671-674
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/671.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.671
The emergence of multi-drug resistant hepatitis B virus (HBV) is increasing annually due to the widespread use of antiviral agents with less potency and low genetic barrier to resistance[1]. Tenofovir disoproxil fumarate (TDF) is a potent nucleotide analogue with high barrier to resistance, which is recommended for drug-resistant cases[2]. However, nephrotoxicity has been reported during TDF treatment[3], although it showed a favorable safety and tolerability profile in registration and real-life studies[4].
Tenofovir alafenamide (TAF), which is a prodrug of TDF, has been reported to have comparable efficacy to TDF and improve bone and renal safety in phase III randomized trials[5,6]. Recently, Grossi et al[7] reported the use of TAF as a rescue therapy in a cirrhotic patient with a history of Fanconi syndrome and multi-drug resistance. To our knowledge, hepatocellular carcinoma (HCC) monitoring is lacking in patients switching to TAF due to coexistence of multi-drug resistance and renal dysfunction.
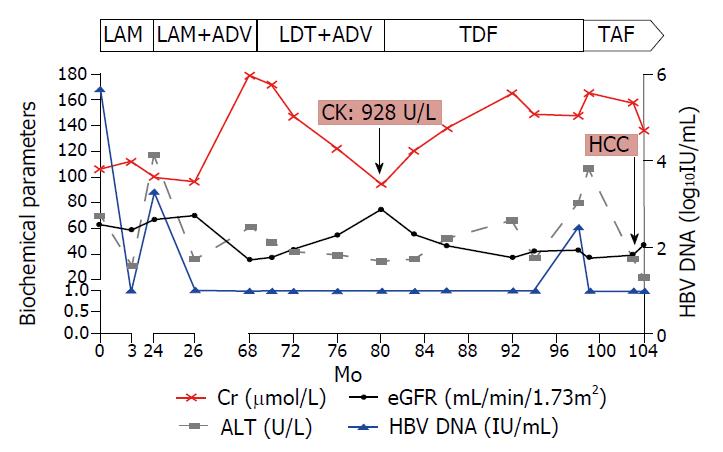
A 60-year-old Chinese man was admitted to our department on October 27, 2009. He was diagnosed with HBeAg negative compensated liver cirrhosis, and lamivudine (LAM) was administered based on the positive viral load (5.68 log10IU/mL, Cobas TaqMan Test, Roche Diagnostic, Basel, Switzerland). Moreover, he had a concomitant comorbidity of arterial hypertension for two years, and took valsartan capsules (80 mg, Novartis Pharma, Beijing, China) every day. Ultrasonic tests showed liver cirrhosis and splenomegaly at admission. Considering that laboratory tests showed normal aspartate aminotransferase (AST, < 40 U/L), total bilirubin (< 17.1 µmol/L) and albumin (> 35 g/L) at admission, we concentrated the parameters of kidney function and levels of HBV DNA. Three months later, HBV DNA was undetectable (lower limit of detection, 20 IU/mL) and alanine aminotransferase (ALT) level returned to normal.
In November 2011, the HBV DNA level increased to 3.28 log10IU/mL, and ALT increased to 117 U/L. Then, the genotypic resistance test was performed, and rtL180M, rtM204V and rtT184A mutations were identified. Based on these results, adefovir dipivoxil (ADV) was added as a rescue therapy according to the Chinese guidelines for prevention and treatment of chronic hepatitis B (2010 version).
On June 2, 2015, biochemical tests showed that the patient’s serum creatinine increased to 179.8 µmol/L, while the estimated glomerular filtration rate (eGFR) declined to 35.71 mL/min/1.73 m2. In addition, the serum phosphorus was lower (0.67 mmol/L). Then, the patient was treated with telbivudine (LdT) and ADV, instead of LAM and ADV, due to the superior nephro-protective effect of LdT. Interestingly, the serum creatinine gradually decreased (from 179.8 µmol/L to 94 µmol/L), whereas the eGFR significantly increased (from 35.71 mL/min/1.73 m2 to 74.72 mL/min/1.73 m2) in the subsequent 12 mo. The serum phosphorus remained normal until the end of the follow-up.
In May 2016, the patient suffered from obvious muscle soreness, and a biochemical test showed that the creatine kinase was 928 U/L, after which LdT and ADV were immediately withdrawn. Given that the patient had a history of LAM resistance, and LAM had overlapping resistance profiles with entecavir (ETV)[8], the patient was at high risk of developing resistance to ETV in subsequent treatment. Thus, TDF, rather than ETV, was chosen to replace LdT and ADV.
Despite good compliance to TDF treatment, the HBV DNA peaked to 2.47 log10IU/mL and ALT increased to 62 U/L in November 2017 (18 mo after initiation of TDF). The genotypic resistance profile could not be tested due to the low viral load. Moreover, the serum creatinine gradually increased and the eGFR declined to < 50 mL/min/1.73 m2 during TDF treatment. Given the renal insufficiency and lack of full viral suppression under TDF, TAF was bought from Japan and started at 25 mg per day with the consent of the patient and approval of the Ethics Committee of our hospital.
Since patients with liver cirrhosis are at risk of HCC, surveillance for HCC continued during the eight-year follow-up of our patient. Unexpectedly, HCC developed in April 2018 (5 mo after the initiation of TAF), and an operation was performed in the Eastern Hepatobiliary Surgery Hospital. A follow-up at six months after the initiation of TAF showed that eGFR was maintained above 35 mL/min/1.73 m2, and that HBV DNA remained undetectable. As shown in Figure 1, the renal function did not worsen and serum phosphorus abnormality was not observed until the last follow-up visit on May 16, 2018, and TAF was continued.
In the present report, the patient was switched from TDF to TAF as a rescue strategy due to coexistence of renal dysfunction and multi-drug resistance. TAF effectively inhibited HBV replication, without worsening renal function or serum phosphorus abnormality. Furthermore, HCC occurred during TAF treatment despite controlling the viral load.
In case of resistance, a more potent antiviral drug that does not share cross-resistance should be started after ascertaining compliance to antiviral treatment. TDF monotherapy or combination of TDF and ETV was found to be effective and safe as a rescue therapy in several clinical trials[2,9]. However, TDF was associated with a higher risk of renal dysfunction as compared to ETV[10,7]. The nephrotoxicity of TDF remains a concern, especially in elderly patients or those with a history of exposure to ADV[7]. Due to a lack of reliable biomarkers for evaluating the kidney function, eGFR is the most widely used parameter in clinical practice.
As TAF is safer than TDF in terms of nephrotoxicity and hypophosphatemia, antiviral drug resistance in patients with renal dysfunction appears to be manageable, but clinical studies are ongoing[7]. The present study implies that TAF could be used in place of TDF in older patients with renal insufficiency, for which a prospective study is needed in clinical practice. Consistent with Grossi et al[7], no significant improvement in eGFR was observed during TAF treatment. It is suspected that older age and arterial hypertension may also contribute to renal insufficiency, which is difficult to rescue in a short duration.
It is true that successful antiviral therapy is beneficial in preventing cirrhosis progression and HCC development. The pathogenesis of HCC is thought to be multifactorial, and liver cirrhosis is an important risk factor for HCC. Even though a potent nucleoside analogue (NA) can maintain HBV suppression, it reduces but does not eliminate the risk of HCC development. Our patient developed HCC during TAF treatment, this emphasizes the findings of Grossi et al[7] that the risk of HCC could not be eliminated despite HBV suppression. This is the first case report of HCC, which suggests that surveillance for HCC should be continued during TAF treatment, especially in patients with liver cirrhosis.
We could not assess the long-term safety of TAF in our patient with HCC, due to the relatively short duration of TAF therapy in this case. Another limitation is that testing of some relevant parameters, such as urine phosphorus and bone mineral density, are unavailable at our hospital.
In summary, HBV replication was suppressed and the renal function did not worsen during TAF treatment. Despite controlling the viral load, risk of HCC exists and should be monitored in cirrhotic patients.
A 60-year-old individual suffered liver cirrhosis and renal dysfunction, and was infected with multidrug-resistant hepatitis B virus (HBV) before tenofovir alafenamide (TAF) treatment.
He was diagnosed with HBeAg negative compensated liver cirrhosis at admission.
The differential diagnosis included alcoholic cirrhosis and hepatocellular carcinoma.
Laboratory tests showed that HBV DNA peaked to 2.47 log10IU/mL and the estimated glomerular filtration rate declined to < 50 mL/min/1.73 m2 before TAF treatment.
Ultrasonic tests showed liver cirrhosis and splenomegaly at admission.
Hepatocellular carcinoma was diagnosed according to liver pathology during follow-up.
Given the renal insufficiency and lack of full viral suppression under tenofovir disoproxil fumarate, TAF was started at 25 mg per day.
Grossi et al[7] reported the use of TAF as a rescue therapy in a cirrhotic patient with a history of Fanconi syndrome and multi-drug resistance, while in our case, the patient received TAF treatment and hepatocellular carcinoma (HCC) occurred during follow-up.
The pathogenesis of HCC is thought to be multifactorial, and liver cirrhosis is an important risk factor for HCC. Even though a potent NA can maintain HBV suppression, it reduces but does not eliminate the risk of HCC development.
The surveillance for HCC should be continued during TAF treatment.
CARE Checklist (2013) statement: The manuscript was prepared according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Azzaroli F, Raghow R S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Zhang HY, Liu LG, Ye CY, Chen CH, Hang SX, Zhu Z, Shen HY, Huang ZY, Chen WY, Xue Y. Evolution of drug-resistant mutations in HBV genomes in patients with treatment failure during the past seven years (2010-2016). Virus Genes. 2018;54:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Lim YS, Yoo BC, Byun KS, Kwon SY, Kim YJ, An J, Lee HC, Lee YS. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: results of a randomised trial. Gut. 2016;65:1042-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Wong GL, Seto WK, Wong VW, Yuen MF, Chan HL. Review article: long-term safety of oral anti-viral treatment for chronic hepatitis B. Aliment Pharmacol Ther. 2018;47:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Marcellin P, Zoulim F, Hézode C, Causse X, Roche B, Truchi R, Pauwels A, Ouzan D, Dumortier J, Pageaux GP. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year, Prospective, Real-World Study in France. Dig Dis Sci. 2016;61:3072-3083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 6. | Buti M, Riveiro-Barciela M, Esteban R. Tenofovir Alafenamide Fumarate: A New Tenofovir Prodrug for the Treatment of Chronic Hepatitis B Infection. J Infect Dis. 2017;216:S792-S796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Grossi G, Loglio A, Facchetti F, Borghi M, Soffredini R, Galmozzi E, Lunghi G, Gaggar A, Lampertico P. Tenofovir alafenamide as a rescue therapy in a patient with HBV-cirrhosis with a history of Fanconi syndrome and multidrug resistance. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593-1608.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 9. | Zoulim F, Białkowska-Warzecha J, Diculescu MM, Goldis AE, Heyne R, Mach T, Marcellin P, Petersen J, Simon K, Bendahmane S. Entecavir plus tenofovir combination therapy for chronic hepatitis B in patients with previous nucleos(t)ide treatment failure. Hepatol Int. 2016;10:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Viganò M, Brocchieri A, Spinetti A, Zaltron S, Mangia G, Facchetti F, Fugazza A, Castelli F, Colombo M, Lampertico P. Tenofovir-induced Fanconi syndrome in chronic hepatitis B monoinfected patients that reverted after tenofovir withdrawal. J Clin Virol. 2014;61:600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |