Published online Jul 16, 2017. doi: 10.12998/wjcc.v5.i7.258
Peer-review started: February 7, 2017
First decision: May 8, 2017
Revised: May 17, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: July 16, 2017
Processing time: 159 Days and 8.1 Hours
It is well-known that colonoscopy is considered the gold standard for colon cancer prevention. Although performed by experienced endoscopists, the matter remains of polyps missed during this examination. The reasons may include the size, shape and location of the lesions. Many colorectal cancer screening programs have been proposed to increase the adenoma detection rate. The substantial difference between these methods is whether the improvement in vision, particularly the detection of irregularities of the mucosa, is inside the endoscope electronic components (magnification, wide-angle vision, narrow band imaging, flexible spectral imaging colour enhancement, i-Scan) or outside the same, by the use of specific caps (EndoCuff, EndoVision, EndoRings). Endocuff is a plastic device mounted at the end of the scope with a constant vision field of the entire colon. The aim of this study is to explore the potential clinical and technical benefits of Endocuff.
Core tip: One of the main goals of colonoscopy screening is to identify polypoid lesions, which are precursors of colorectal cancer. Once identified, the polypoid lesions need to be removed whenever possible. Throughout the years, many prototypes of colonoscopes, magnification techniques, and different devices such as caps have been developed for colonoscopy screening. Endocuff is a new device used to improve adenoma detection rates during colonoscopy. Based on the findings of many studies, Endocuff seems to be of great help in increasing the detection of colonic polyps, with no significant complications associated with its use.
- Citation: Zippi M, Hong W, Crispino P, Traversa G. New device to implement the adenoma detection rate. World J Clin Cases 2017; 5(7): 258-263
- URL: https://www.wjgnet.com/2307-8960/full/v5/i7/258.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i7.258
Colorectal cancer (CRC) is one of the most frequently observed cancers, and screening programs, including the adenoma detection rate (ADR), play an important role in reducing its incidence. There are many screening methods such as withdrawal time and technique, second evaluation of the right colon, patient positional changes, gastrointestinal assistant participation during colonoscopy, water-aided technique, optimisation of bowel preparation, and antispasmodic administration[1].
Colonoscopy is globally recognised as the gold standard for CRC screening. A widely used indicator to emphasise “good colonoscopy” is the ADR, which refers to the number of patients out of every 100 undergoing first-time colonoscopy who have at least one adenoma removed[2]. Several studies showed that the prevalence of adenomas in asymptomatic adults vary from 25% to 40%[3-6]. Based on these findings, in 2014, a joint task force of the American College of Gastroenterology and the American Society of Gastrointestinal Endoscopy recommended an ADR benchmark of 25% for all patients (30% for men and 20% for women)[7]. ADR has been considered as the major quality measure predicting subsequent CRC incidence and mortality[8].
Over the years, several accessories have been developed in order to obtain a more accurate visualisation of the colon, facilitating and increasing the identification of polypoid lesions. Recently, one such new device called Endocuff has been developed.
The aim of this review is to identify the studies comparing Endocuff-assisted colonoscopy to standard colonoscopy considering the ADR as the end-point by searching through MEDLINE/PubMed and abstracts presented at international meetings, from January 2014 until January 2017. In particular, the following key-words were searched: “adenoma detection rate”, “Endocuff” and “Endocuff-assisted colonoscopy”.
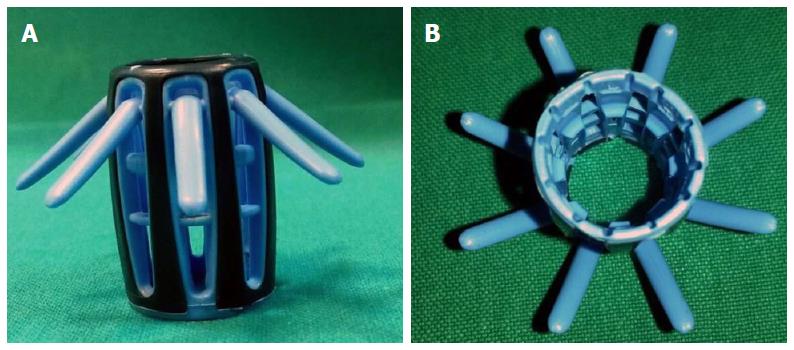
The Endocuff™ Vision (ARC Medical Design and Norgine) is a new device created with the intent to improve the endoscopic view. It is a soft plastic cap of 2 cm in length, consisting of a cylindrical core in propylene endowed with small flexible finger-like projections made of a thermoplastic elastomer fixed to the core[9,10]. The first version of Endocuff™, dated in 2012 with the Food and Drug Administration approval, presented one proximal and one distal row of finger-like projections. On the contrary, the latest version, named Endocuff Vision™, has only one proximal row of more rounded finger-like projections in order to eliminate mucosal lacerations that were observed in the first model[11] (Figure 1). This device presents different colour-coded sizes (blue, green, purple, and orange) depending on the various colonoscopy compatible, both for paediatric than for adults instruments.
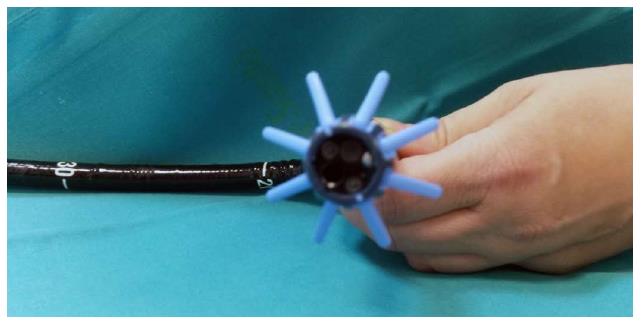
The device is for single use and is not recyclable. The usage is very simple, as it uses the distal end of the endoscope (Figure 2), which virtually coincides with the end of the tip of the colonoscope. Here, lubricants are not used due to their high risk of displacement from the scope during the procedure.
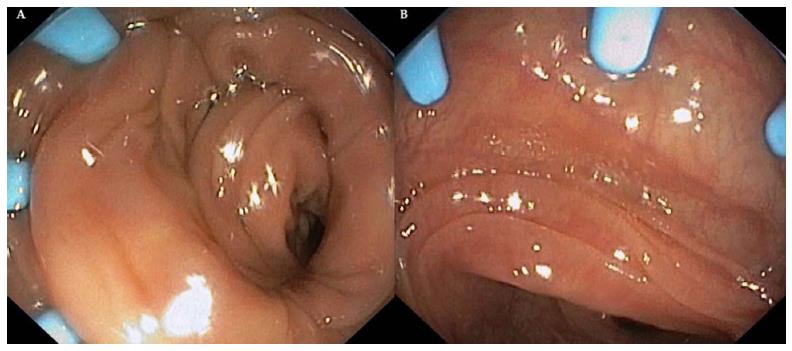
There are two principal indications for use: (1) keeping the suitable depth of endoscope's view field; and (2) helping the endoscope with being inserted into the gastrointestinal tract. During colon intubation, this accessory is practically invisible, and the projections do not interfere with the introduction. On the contrary, during the tool retraction, this device flattens folds, in particular of the sigmoid colon, and flexures of bowels (Figure 3).
Pioche et al[12] conducted a simulated pilot study which included an animal colorectal model used for learning and 32 endoscopists as follows, 16 Japanese and 16 visitors, in order to verify the Endocuoff’s effectiveness in identifying the polypoid lesions. The model was specifically designed with the “packaging” of 13 polyps located in various locations, including those behind the folds. Endoscopists had a different degree of experience and worked randomly, either by performing standard colonoscopies (SC) or Endocuff-assisted colonoscopy (EAC). Their results showed that EAC detected more polyps compared to SC (mean lesions: 9.9 vs 7.5, P = 0.03) and that the use of this device was independent of the various endoscopic medical expertise levels[12].
Reported contraindications in the usage of Endocuff Vision™ are: (1) known colonic strictures; and (2) active inflammatory disorders (acute infective colitis, colonic Crohn’s disease, ulcerative colitis, and acute diverticulitis)[11]. Moreover, this device was not designed with the objective of deep ileal intubation, and it is strongly discouraged for complex sub-mucosal dissection (such as ESD, Endoscopic Submucosal resection).
The first report on the use of this accessory was published in 2012 by Sanders and Tsiamoulos et al[9] of St Mark’s Hospital in London. This was a single-centre, retrospective study with a small number of cases. The authors reported their experience with endoscopic cuff-assisted endoscopic mucosal resection (EMR) (5 patients) and control post scars-EMR (7 patients) for large flat/sessile sigmoid colon polyps. All the lesions were located in the sigmoid sigma, and no adverse events were seen.
Reviewed available studies focusing on EndoCuff-assisted colonoscopy are reported in Table 1. It was excluded from the analysis of the data, an ongoing study, promoted by Bevan et al[11]. It is a is a prospective, multicenter, randomised controlled trial comparing the ADR in patients undergoing EAC with SC. This study will be held at seven hospitals and will include the enrolment of 1772 patients[11].
| Ref. | Year | No. of patients (EAC) | No.of patients (SC) | Adenoma detection rate (%) (EAC) | Adenoma detection rate (%) (SC) | ADR significance |
| Floer et al[14] | 2014 | 238 | 229 | 35.4 | 20.7 | P < 0.0001 |
| Lenze et al[15] | 2014 | 50 | // | 34 | // | // |
| Marsano et al[16] | 2014 | 165 | 153 | 46.6 | 30 | P = 0.002 |
| Tsiamoulos et al[17] | 2014 | 133 | 133 (pre-cuff period) 133 (post-cuff period) | 68.98 | 55.13 61.74 | // |
| Sawatzki et al[18] | 2015 | 104 | // | 47 | // | // |
| Chin et al[19] | 2015 | 93 | 193 | 44.1 | 27.3 | P = 0.01 |
| Van Doorn et al[20] | 2015 | 530 | 533 | 52 | 52 | P = 0.92 |
| Biecker et al[10] | 2015 | 245 | 253 | 56 | 42 | P = 0.001 |
| Cattau et al[21] | 2015 | 329 | 329 | 49.7 | 46.4 | P = 0.392 |
| Shah-Ghassemzadeh et al[22] | 2015 | 219 | 230 | 62.1 | 49.13 | P = 0.0057 |
| Bhattacharyya et al[23] | 2016 | 266 | 265 | 63 | 60.9 | NS |
| Cavallaro et al[24] | 2016 | 445 | 403 | 53 | 46 | P < 0.05 |
| De Palma et al[25] | 2017 | 137 | 137 | 26.9 | 26.3 | P = 0.002 |
As observed in a recent meta-analysis[13], four studies[10,14,17,20] reported complication rates in the EC groups. The most frequent complication was superficial mucosal injury of negligible clinical significance that was found in 27 patients. Patient discomfort resulted in the removal of the cap in 23 cases, following which it was possible to complete the procedure. Another common complication was the loss of the device during the examination of 6 patients. In all these cases, the accessory was removed, and the study was complete. No perforations were reported[13]. Tsiamoulos et al[17] described elective removal in 4 cases due to sigmoid diverticulitis and 1 due to anal discomfort. Cattau et al[21] signalled one loss of the cap and one incomplete examination due to advanced diverticulosis. De Palma et al[25] reported nine complications: 2 cases of device loss during the withdrawal and 7 cases of mucosal erosions, of which in 1 case was necessary sclerosis with adrenaline.
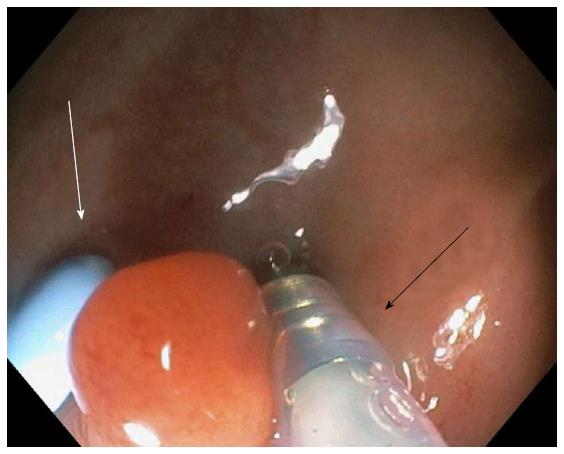
The regional program for the CRC screening is operating at our Hospital. After the adhesion of the population to faecal occult blood test (FOBT), colonoscopy is mandatory. Colonoscopies, all conducted until the cecum, were performed using Endocuff Vision™ by expert operators with conventional colonoscopes (CF-Q165L, CF-H1285L, Olympus Optical, Hamburg, Germany). The bowel preparations used were the standard large-volume polyethylene glycol electrolyte solutions prepared the previous day or the split-dose regimens, depending on the time of the examination. Thirty patients (F 18, M 12) with a mean age of 67 years (range: 50-75 years), who underwent first-time screening colonoscopy, were studied. A total of 45 polyps were removed, 36 sessile (80%) and 9 pedunculated (20%). The sigma was involved in nearly half of the cases (45.7%). During our initial experience, we found polypoid lesions localised especially in the sigmoid colon that could be easily removed (Figure 4). No major adverse events were recorded, except for two cases of superficial “scratch-like” mucosal lesions of no clinical significance that occurred in the case of rigid colon due to inflammation (mild diverticulitis).
Prompt diagnosis of precancerous polyps during colonoscopy is extremely important in order to reduce CRC rate, especially in asymptomatic patients. During colonoscopy, the rate of colonic polyps missed varies from 6% to 27%[26]. It is known that the most effective way to estimate the adenoma miss rate, and consequently improve the ADR, is represented by the “back-to-back colonoscopy” technique performed in two consecutive same-day procedures in the same patient[27]. However, we cannot ignore this may double the potential complications, such as the risk of perforation.
The first study of this method using EndoCuff has been conducted by De Palma et al[25] in a single-centre randomised back-to-back-study. The participants underwent two colonoscopies, with and without the use of the device. The authors concluded that these kinds of examinations allow finding lesions missed by other procedures, but on the other hand, a limitation raised being the endoscopists not blinded for the presence of Endocuff[25]. From these studies emerge that the use of transparent plastic caps attached to the tip of the colonoscope can increase the ADR, with a mechanical mechanism of flattening the folds and the consequent increase of the visual field. This technique is known as cap-assisted colonoscopy (CAC). However, several works show conflicting results with respect to improvement in adenoma detection by CAC. In particular, the ADR was not significantly improved in 6 studies analysed in a meta-analysis including 16 RCTs that compared CAC to standard colonoscopy[28].
As for the CAP, our results were not in agreement in defining the EAC superiority over SC. In fact, in three studies, there was no statistical significance between the two groups (EAC vs SC)[20,21,23]. As the Table 1 shows, this device can enhance the ADR.
The most frequently observed complication was the removal of Endocuff’s due to the discomfort of the patient (24 times), followed by the loss of the device during the examination (9 times). No major complications were reported.
In Italy, CRC is one of the most frequently found cancers. At our local hospital, we started regional screening program for this kind of tumor from January 2012 onwards. In our country, the device has been registered in the database of medical devices of the Ministry of Health on January 29, 2016[29].
Our early experiences with EAC on a small population show that Endocuff can identify and facilitate polypectomy, especially in floppy folds of sigma, allowing better stabilization of endoscope in front of the polyp. Among 30 patients, we found 2 cases (6.6%) of insignificant superficial mucosal lacerations, probably related to the lack of experience with this accessory.
Some major limitations are represented by special circumstances such as sub-colonic strictures and acute inflammation of the mucosa (diverticulitis and inflammatory bowel diseases).
Unfortunately, when a person is subjected to colonoscopy for the first time, it is impossible to know any underlying diseases. Therefore, in some cases, it becomes necessary to remove the device in order to complete the procedure safely.
In conclusion, the results of EAC are still evolving. This accessory appears safe and useful in increasing the detection of the number of polyps and subsequently, the detection rate of adenomas. We recommend that Endocuff should be further investigated in other larger trials.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amiri M, Pellicano R, Vij M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Rondonotti E, Andrealli A, Amato A, Paggi S, Conti CB, Spinzi G, Radaelli F. Technical interventions to increase adenoma detection rate in colonoscopy. Expert Rev Gastroenterol Hepatol. 2016;10:1349-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Anderson JC, Butterly LF. Colonoscopy: quality indicators. Clin Transl Gastroenterol. 2015;6:e77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 4. | Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1203] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 5. | Rex DK, Lehman GA, Ulbright TM, Smith JJ, Pound DC, Hawes RH, Helper DJ, Wiersema MJ, Langefeld CD, Li W. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol. 1993;88:825-831. [PubMed] |
| 6. | Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, Avalos JC, Buck JL, Kooyman G, Cattau EL. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990;85:969-974. [PubMed] |
| 7. | Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG, Park WG, Rizk MK, Sawhney MS. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 836] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 8. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1468] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 9. | Tsiamoulos ZP, Saunders BP. A new accessory, endoscopic cuff, improves colonoscopic access for complex polyp resection and scar assessment in the sigmoid colon (with video). Gastrointest Endosc. 2012;76:1242-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Biecker E, Floer M, Heinecke A, Ströbel P, Böhme R, Schepke M, Meister T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Bevan R, Ngu WS, Saunders BP, Tsiamoulos Z, Bassett P, Hoare Z, Rees CJ. The ADENOMA Study. Accuracy of Detection using Endocuff Vision™ Optimization of Mucosal Abnormalities: study protocol for randomized controlled trial. Endosc Int Open. 2016;4:E205-E212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Pioche M, Matsumoto M, Takamaru H, Sakamoto T, Nakajima T, Matsuda T, Abe S, Kakugawa Y, Otake Y, Saito Y. Endocuff-assisted colonoscopy increases polyp detection rate: a simulated randomized study involving an anatomic colorectal model and 32 international endoscopists. Surg Endosc. 2016;30:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Chin M, Karnes W, Jamal MM, Lee JG, Lee R, Samarasena J, Bechtold ML, Nguyen DL. Use of the Endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J Gastroenterol. 2016;22:9642-9649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 14. | Floer M, Biecker E, Fitzlaff R, Röming H, Ameis D, Heinecke A, Kunsch S, Ellenrieder V, Ströbel P, Schepke M. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One. 2014;9:e114267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Lenze F, Beyna T, Lenz P, Heinzow HS, Hengst K, Ullerich H. Endocuff-assisted colonoscopy: a new accessory to improve adenoma detection rate? Technical aspects and first clinical experiences. Endoscopy. 2014;46:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Marsano J, Tzimas D, Mckinley M, Robbins DH, Mammen A, Sun E, Chugh P, Razavi F, Hasan N, Buscaglia J. Endocuff assisted colonoscopy increases adenoma detection rates: a multi-center study. Gastrointest Endosc. 2014;79:AB550. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tsiamoulos ZP, Mirsa R, Bourikas LA, Rajaratnam R, Patel KP, Thomas-Gibson S, Haycock A, Suzuki N, Beintaris I, Saunders BP. Endocuff-vision: impact on colonoscopist performance during screening. Gastrointest Endosc. 2015;81 (Suppl. 5): AB209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Sawatzki M, Meyenberger C, Marbet UA, Haarer J, Frei R. Prospective Swiss pilot study of Endocuff-assisted colonoscopy in a screening population. Endosc Int Open. 2015;3:E236-E239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Chin M, Chen CL, Karnes WE. Improved polyp detection among high risk patients with Endocuff. Gastrointest Endosc. 2015;81:AB283. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | van Doorn SC, van der Vlugt M, Depla A, Wientjes CA, Mallant-Hent RC, Siersema PD, Tytgat K, Tuynman H, Kuiken SD, Houben G. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2017;66:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Cattau EL, Leal RK, Ormseth EJ, Aycock R, Ward JD, Thompson BF, Dragutsky M, Towne C. The effect of Endocuff-assisted colonoscopy on adenoma detection rate: a randomized trial in community ambulatory surgical centers. Am J Gastroenterol. 2015;110 (Suppl.1): S602. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Shah-Ghassemzadeh M, Baek M, Jackson C, Lunn J, Nguyen C, Serrao S, Juma D, Strong R. Endocuff-assisted colonoscopy increases sessile serrated adenoma/polyp detection and adenoma detection rates: a quality improvement study. Am J Gastroenterol. 2015;110:S666. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bhattacharyya R, Chedgy F, Kandiah K, Fogg C, Higgins B, Gadeke L, Thursby-Pelham F, Ellis R, Goggin P, Longcroft-Wheaton G. The First Randomised Controlled Trial of Endocuff Vision® Assisted Colonoscopy Versus Standard Colonoscopy for Polyp Detection in Bowel Cancer Screening Patients (E-Cap Study). Gastroenterology. 2016;150:S1270. [DOI] [Full Text] |
| 24. | Cavallaro LG, Lecis PE, Galliani E, Dal Pont E, Giacomin A, Mel R, Di Camillo S, Soppelsa F, Roldo C, Iuzzolino P. Higher Adenoma Detection Rate With Endocuff- Assisted Colonoscopy in a Screening Population. Gastrointest Endosc. 2016;84:AB380. [DOI] [Full Text] |
| 25. | De Palma GD, Giglio MC, Bruzzese D, Gennarelli N, Maione F, Siciliano S, Manzo B, Cassese G, Luglio G. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc. 2017;pii:S0016-5107(17)30004-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The Miss Rate for Colorectal Adenoma Determined by Quality-Adjusted, Back-to-Back Colonoscopies. Gut Liver. 2012;6:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 28. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |