Published online Jun 16, 2017. doi: 10.12998/wjcc.v5.i6.247
Peer-review started: February 12, 2017
First decision: March 8, 2017
Revised: March 23, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: June 16, 2017
Processing time: 136 Days and 6.3 Hours
Glioblastoma-multiforme (GBM), the most aggressive glial tumor, has a worldwide age-adjusted incidence ranging from 0.59-3.69/100000 persons. Despite current multimodal-treatment approach, median-survival time and progression-free survival (PFS) remains short. Glioblastomas display a variety of molecular alterations, which necessitates determining which of these have a prognostic significance. This is a case of a 45-year-old patient who presented with progressive slurring of speech and features of raised intracranial pressure. Computed tomography (CT) scan revealed a large heterogeneously enhancing lesion in the left front-temporal-perisylvian region with solid, cystic areas, suggestive of malignant glioma. Partial tumor-excision was followed by concurrent chemo-radiotherapy. Histopathologically, the tumor was astrocytoma grade-IV. Patient had an extended PFS of 12 mo, with an overall survival of 26 mo. Primary-GBM was confirmed using molecular markers and the immunophenotypic signature was defined by evaluating systemic expression of human telomerase reverse transcriptase, interleukin-6, neutrophil-lymphocyte ratio, tissue inhibitor of metalloproteinases-1, human chitinase-3-like-protein-1 (YKL-40) and high mobility group-A1. Current findings suggest that this signature can identify worst outcomes, independent of clinical criteria.
Core tip: Delineating the immunophenotypic signature for glioblastoma with reference to disease progression is of clinical importance. This case report presents for the first time a panel of 6 systemic molecular markers in circulation namely; human telomerase reverse transcriptase, interleukin-6, neutrophil-lymphocyte ratio, tissue inhibitor of metalloproteinases-1, human chitinase-3-like-protein-1 and high mobility group-A1 representing the mechanistic of inflammation, proliferation and invasion of the tumor. Their expression is suggested to be linked to progression-free survival in glioblastoma-multiforme.
- Citation: Gandhi P, Khare R, Garg N, Sorte S. Immunophenotypic signature of primary glioblastoma multiforme: A case of extended progression free survival. World J Clin Cases 2017; 5(6): 247-253
- URL: https://www.wjgnet.com/2307-8960/full/v5/i6/247.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i6.247
The most common and fatal glioma subtype in adults, glioblastoma-multiforme (GBM) has a worldwide age-adjusted incidence ranging from 0.59 to 3.69 per 100000 persons[1]. Despite the current multimodal treatment approach, the median survival time remains in the range of 15-18 mo[2]; while progression-free survival (PFS) is of 6 mo[3]. Glioblastoma display a variety of molecular alterations, which necessitates determining which of these have a diagnostic and/or prognostic significance[4]. According to the latest World Health Organization guidelines (WHO 2016), a molecular classification of tumors of the central nervous system has been established, which defines glioblastoma on the basis of IDH-mutant and wild type. In primary glioblastoma, the absence of IDH mutation, along with over-expression of p53 and epidermal growth factor receptor (EGFR) are distinct molecular signatures[5], associated with enhanced therapeutic response and longer survival[6].
The clinico-patholoical profile of GBM is not clearly defined because of the extensive variability of tumor histologies. Conventionally, a glioma is confirmed as GBM, based on positive expression of glial fibrillary acidic protein (GFAP) and Ki-67 proliferation index. At the molecular level, as per 2016 WHO guideline; screening for IDH-1, TP53 and EGFR expression is to be carried out to confirm the case as primary GBM. In lieu of this molecular scenario, delineating the molecular signature of glioblastoma with reference to progression and survival is of clinical importance.
Current research points to a strong relation between inflammatory mechanism and glioma tumor proliferation, stemness and invasion. Expression of human telomerase reverse transcriptase (hTERT) and high mobility group-A1 (HMGA1) as proliferation and stemness markers in glioma subtype has been recently established by Gandhi et al[7]. Similarly, human chitinase -3-like-protein-1 (YKL-40), an acute phase glycoprotein is secreted by activated macrophages and neutrophils in gliomas[8] and its involvement is documented in inflammation[9], angiogenesis[10], cell proliferation and invasion[11] of glial tumor. Longitudinal changes in plasma levels of tissue inhibitor of metalloproteinases-1 (TIMP-1) have also been seen to be associated with prognosis of GBM (grade-IV) patients[12], reflecting the role of TIMP-1 in invasive growth pattern resulting from degradation of extracellular matrix.
Neutrophils and lymphocytes too have been recently acknowledged to play an important role in uncontrolled inflammation; driving tumor proliferation[13]. There are several studies associating high pre-treatment neutrophil-lymphocyte ratio (NLR) with GBM[14,15]. Likewise, in a study by Samaras et al[16], IL-6 secretion levels in peripheral blood mononuclear cells of glioma patients were found to be significantly higher compared to controls indicating a role of inflammation in tumor proliferation.
Keeping in view the poor prognosis of glioblastoma, this case report undertook to define for the first time the systemic immunophenotypic molecular signature of a case of primary GBM in terms of extended PFS.
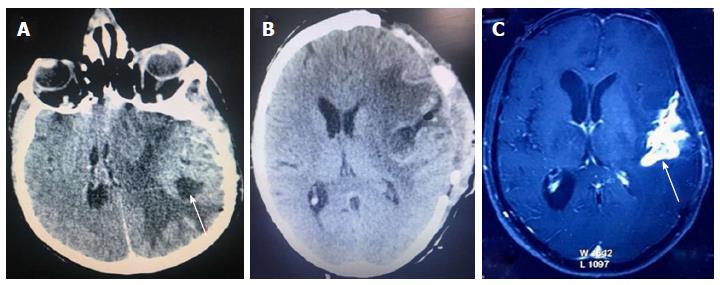
A 45-year-old patient presented with progressive slurring of speech since 2 mo and features of raised intracranial pressure since a few days. On examination, he was drowsy, obeying commands but confused; with irrelevant verbalization and irritability. Computed tomography (CT) scan revealed a large heterogeneously enhancing lesion in left front-temporal-perisylvian region with solid and cystic areas. There was significant perilesional edema surrounding the lesion in frontal and temporal lobes with mass effect (Figure 1A). Possibility of malignant glioma was considered.
Patient underwent left front-tempo-parietal decompressive craniotomy and tumor decompression, three months after initial diagnosis. Intra-operatively, tumor was soft to firm in consistency, moderately vascular with both solid and cystic areas. Partial tumor excision was done; with tumor adherent to perisylvian vessels left behind (Figure 1B). The brain was still full; hence bone flap was not repositioned but placed in the abdomen. Patient recovered well from surgery. Histo-pathological report stated sample to be glioblastoma.
Patient was administered concurrent chemo-radiotherapy and he had an extended 12 mo of PFS. After 18 mo, he developed progressive weakness of the right upper and lower limbs along with seizures. Magnetic resonance imaging brain showed features of recurrence of tumor with significant edema (Figure 1C). Steroids and anti-epileptics were administered. The patient continued to have neurological deterioration and expired after few months with an overall survival (OS) of 26 mo.
According to current WHO guideline, diagnosis of GBM was confirmed by tissue based expression of IDH-1, p53 and EGFR. Biomarkers hTERT and HMGA1 were quantified in formalin-fixed paraffin-embedded tissues (FFPE) in areas with the maximum proliferation. Their expression was compared with non-malignant tissues obtained from subjects serving as control, undergoing surgery for reasons other than brain tumor. Venous blood samples were collected from patient before surgery and centrifuged (Beckman Coulter, United States) at 3000 rpm, for 15 min at 4 °C. Plasma and serum were divided into aliquots and stored at -80 °C. This was followed by assessing levels of NLR, TIMP-1, YKL-40 in plasma as well as that of hTERT and HMGA1 in serum. Corresponding samples from 30 healthy subjects were collected to define the threshold values and statistical analysis of the panel of molecular markers mentioned above.
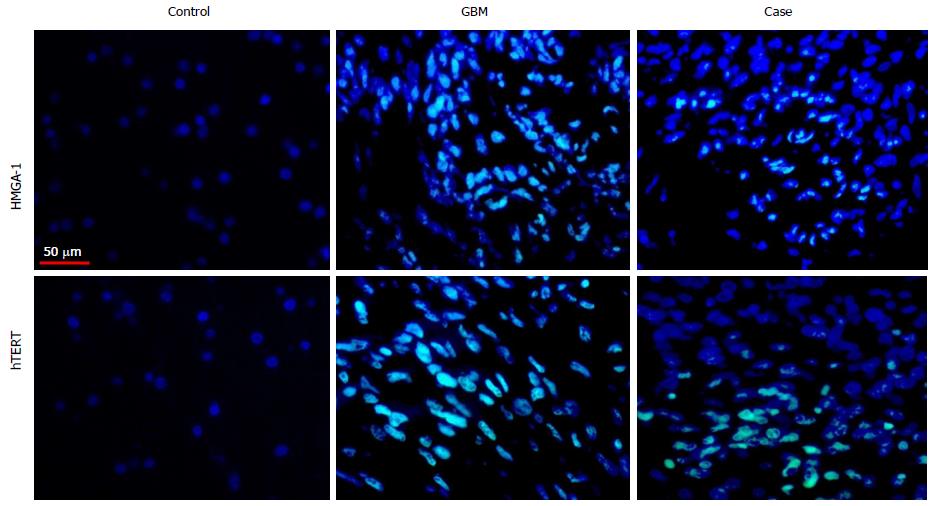
IDH-1, p53, EGFR, hTERT and HMGA1 by immunofluorescence based immuno-histochemistry: To ascertain the expression of IDH-1, p53, EGFR, hTERT and HMGA1 molecules in FFPE tissue, immunofluorescence based immuno-histochemistry (IF-IHC) was performed as per protocol discussed earlier[7]. The slides were incubated overnight at 4 °C with primary antibodies IDH-1 (Santa Cruz Biotechnology, United States, 1:1000 dilutions), p53 (Bethyl Laboratories, United States, 1:1000 dilutions), EGFR (Bethyl Laboratories, United States, 1:1000 dilutions), HMGA1 (Abcam, United Kingdom, 1:1000 dilutions) and hTERT (Abcam, United Kingdom, 1:1000 dilutions), followed by treatment with host specific secondary antibodies (FITC labelled, 1:300 dilutions), washed and mounted.
All images were observed with Plan-Neofluar 40 × 0.75 NA lens. With regard to protein of interest, areas with highest protein labelling were considered and approximately 1000 cells per section were captured with 40-fold magnification followed by digitalization and analysis with the Case Data Manager Expo 4.5 software (Applied Spectral Imaging, Edingen Neckarhausen, Germany). These images were exported as TIFF files and analyzed. Quantification of fluorescence signals of the identified molecular markers hTERT and HMGA1 was carried out using ImageJ software (National Institute of Health, United States).
NLR: The NLR was calculated from a pre-operative whole blood sample count stained with Leishman. The patient did not present any clinical signs of sepsis at that point of time, having been on steroids for the last 24 h.
hTERT, TIMP-1 and YKL-40 by ELISA: Plasma concentrations of TIMP-1 (ng/mL, RD Systems, MN, United States), hTERT (ng/L, MyBiosourse, CA, United States) and plasma YKL-40 (ng/mL, RD Systems, MN, United States) were determined by sandwich ELISA assay using commercially available kits as per the manufacturer’s protocol. All samples were analysed in duplicate. Concentrations were measured as absorbance at 450 nm for hTERT and YKL-40 and for TIMP-1 using correction wavelength of 540 nm in ELISA Reader (Bio-Rad, United States).
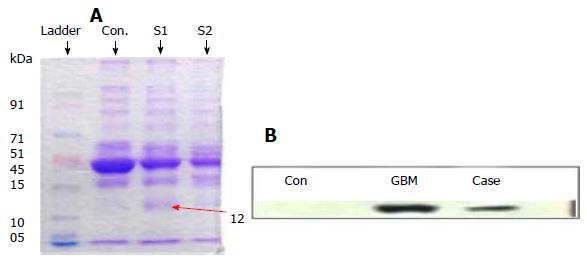
HMGA-1 by Western blot: To estimate circulating HMGA1 protein in serum of the patient using minimal sample volume, 10 μL serum was subjected to protein extraction using a spin column for removing high abundance proteins, yielding ≥ 30 μg/mL of total protein. Extracted sample was then lyophilized to 1/10th volume followed by resolution on 11.5% SDS-PAGE. Western blot analysis was performed following modified method of Ferrín et al[17]. Briefly, nitrocellulose membrane was probed with primary polyclonal HMGA1 antibody (rabbit anti-human HMGA1 antibody, Abcam, United Kingdom; 1:1000 dilution)by incubating overnight at 4 °C; followed by compatible secondary alkaline phosphatase-conjugated antibody (Santa Cruz Biotechnology, United States; 1:5000 dilution) for two hours at room temperature.
According to the institutional ethics committee sanction reference (IEC/21/Res/11), a written informed consent was taken from the patient. Data regarding histo-pathological grade, GFAP, Ki-67 score and IL-6 were obtained from medical records and re-analysed by an independent pathologist.
IF-IHC: Negative expression of IDH-1 and over-expression of p53 and EGFR confirmed the case as primary GBM at the molecular level. Amplified expression of markers hTERT and HMGA1 in tissue was concurrent with GBM grade-IV and Ki-67 proliferation index (Figure 2, Table 1) and also correlated with the OS (Supplementary Table S1). However the values of hTERT and HMGA1 markers in FFPE tissue of the subject in question were found to be lower than the threshold values of these markers established for GBM group (n = 30) in our study (Supplementary Table S2).
| No. | Case/groups | HMGA1 (%) | hTERT (%) | Ki-67 (%) |
| 1 | Case | 2.6 | 11.5 | 45 |
| 2 | controls (n = 15, median with range) | 0.0 (0-0) | 0.0 (0.0-0.05) | 0.0 (0-0) |
| 3 | GBM (n = 30, median with range) | 14.55 (2.3-23.9) | 25.76 (10.5-44.75) | 28 (6.5-80) |
Following this, the five molecular markers identified for systemic immmunophenotypic signature were quantified.
NLR: The pre-operative NLR value of patient was 3.9 while that of the GBM group was 5.58 (Table 2).
ELISA: The inflammatory and proliferation markers hTERT, IL-6, YKL-40 and TIMP-1 for this GBM patient were analyzed (Table 2) and showed significantly higher levels than control samples. But this patient’s values were lower than the median values of the GBM reference group.
Western Blot: The 12 kDa band of HMGA1 was identified and quantified using Image J software. The level of HMGA1 in circulation was found to be lower than the reference GBM group studied (Figure 3, Table 1).
Glioblastomas have extensive and divergent alterations of molecular pathways but morphological differences are insignificant, making conventional histochemical diagnosis unreliable. The immuno-molecular characterization of GBM is therefore, a prerequisite to decision-making regarding diagnosis and prognosis. Herein, a case-based approach is presented to illustrate the prognostic significance of circulating molecular biomarkers: hTERT, HMGA1, IL-6, NLR, YKL-40, and TIMP-1 in a primary GBM with extended PFS of 12 mo.
On reviewing various recent studies on GBM, expression of these markers was seen to be linked to inflammation, increased cell proliferation and invasion, parameters that are directly associated with survival in this grade IV tumor. Molecular basis of cell proliferation is crucial for prognosis when dealing with this malignancy. Our results indicate that increased expression of hTERT in tissue and serum corresponds with GBM status, but are lower in the presented case than the threshold value of this maker in tissue and blood of reference GBM group (Supplementary Table S2) and thus may have contributed to the extended PFS seen in this case.
Experimental findings of a group working with prognosticators in GBM suggest that IL-6 expression is regulated by TERT status[18]. A parallel increase in values of IL-6 with that of hTERT (P = 0.0286) as evident from the reference GBM group, indicates that this inflammatory marker plays a key role in tumor progression and prognosis of GBM. The level of circulating IL-6 being less for our patient than the cut off value set for GBM, positively predicts a longer time to progression (Table 2, Supplementary Table S2). In accordance is a recent study by Hori and Sasayama[19] which correlates levels of IL-6 in cerebro-spinal fluid with PFS in glioblastoma.
The value of NLR (3.9) in this patient positively correlated with extended PFS. In agreement are studies on grade IV patients (glioblastoma) describing NLR (≤ 4.73) to be significantly correlated with longer PFS[20,21].
Blood based studies determining the unfavourable prognostic markers in GBM point towards a positive correlation of lower levels YKL-40 with slow progression of the disease, but are limited in number. The plasma level of YKL-40 in this case was 71.47 ng/mL and TIMP1 plasma level corresponded to 80 ng/mL, values, which were within the range of the control group. In line with this observation, is the study of Hormigo et al[22], which also suggests that GBM patients with persistently normal values of YKL-40 have a longer overall survival as well as disease-free survival. However, no studies correlating levels of TIMP-1 with PFS are available in literature.
It can be inferred from the medical history of the case that tumor re-growth was initiated probably due to the stem cells that were left behind in the tumor mass during surgical intervention. At the molecular level, analysis is suggestive of tumor recurrence due to increased expression of HMGA1, a stemness marker. This can be distinctly linked to recurrence, as evident from IF based quantitative analysis and levels of expression of this protein in patient’s serum (Tables 1 and 2). The results are corroborated by earlier studies, one, which showed a differential IHC expression of HMGA1 in patients with primary and recurrent GBM[23] and also with our previous study, which correlates HMGA1 expression to recurrence and survival [7].
In the present case, the level of all systemic molecular markers was lower than the median values of the GBM reference group which is concomitant with an extended PFS of this patient, than documented for GBM in literature. Establishing optimal cut off for threshold values of this blood-based marker panel with enhanced sensitivity and specificity (Supplementary Table S2, Supplementary Figure S1) indicated that these markers were significantly associated with median survival (Supplementary Table S3, Supplementary Figure S2) in GBM. The current investigation thus provides experimental data-based evidence of the clinical utility of circulating plasma YKL-40, TIMP-1, IL-6 and NLR and serum hTERT and HMGA1 for monitoring primary GBM patients.
The authors are grateful to staff of histopathology division and Dr. Hanni Gulwani for providing tissue sections of the patient. Gratitude is also due to Dr. Jharna Mishra (MD, Pathology) for histo-pathological review of the case.
A 45-year-old male presenting with features of progressive raised intracranial pressure and focal neurological deficits.
Intracranial space occupying lesion presenting with features of raised intracranial pressure and focal neurological deficits.
Malignant glioma, metastasis.
All labs were within normal limits.
Magnetic resonance imaging revealed heterogeneously enhancing variegated intensity lesion suggestive of malignant glioma.
Haematoxylin and eosin stained sections showed fragments of necrotic tissue and small fragments of neoplastic astrocytes moderate nuclear pleomorphism, high endothelial vessel proliferation, at places forming glomeruloid bodies. Peroxidase-immuno-histochemistry (IHC) was used to assess Ki-67, glial fibrillary acidic protein and immunofluorescence based IHC was used to evaluate IDH-1, p53, epidermal growth factor receptor, human telomerase reverse transcriptase (hTERT) and high mobility group-A1 (HMGA1).
Near/sub-total excision of the tumor was done at initial resection. Concomitant chemo-radiotherapy followed.
Predicting progression-free survival in glioblastoma-multiforme (GBM) based on histological findings has limitations and necessitates defining the GBM sub-type based on molecular markers, followed by systemic immunophenotypic signature for monitoring outcome in terms of survival.
hTERT is a ribonucleoprotein polymerase that maintains telomere ends with reverse transcriptase activity, its expression correlates with grade of malignancy. HMGA1 protein is an architectural transcription factor widely expressed during embryonic development and tumor progression. Neutrophil-lymphocyte ratio (NLR) is a marker of subclinical inflammation and a factor of poor prognosis in glioma. Human chitinase-3-like-protein-1 (YKL-40), an acute phase glycoprotein is secreted by activated macrophages and neutrophils in various cancers. Tissue inhibitor of metalloproteinases-1 (TIMP-1) glycoprotein is a natural inhibitor of the matrix metalloproteinases, a group of peptidases involved in degradation of the extracellular matrix. Interleukin 6 (IL-6) is an interleukin that is characterized as a regulator of immune and inflammatory responses and capable of crossing the blood-brain barrier.
The current investigation provides experimental data-based evidence for systemic monitoring of disease progression in GBM in terms of the delineated immunophenotypic signature. Systemic expression of, plasma YKL-40, TIMP-1, IL-6 and NLR and serum hTERT, HMGA1; has clinical utility for predicting progression-free survival.
The case presented by Gandhi et al, is interesting, thoroughly studied and well documented.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shao R, Sotelo J S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15 Suppl 2:ii1-i56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 1161] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 2. | Liu Y, Shete S, Etzel CJ, Scheurer M, Alexiou G, Armstrong G, Tsavachidis S, Liang FW, Gilbert M, Aldape K. Polymorphisms of LIG4, BTBD2, HMGA2, and RTEL1 genes involved in the double-strand break repair pathway predict glioblastoma survival. J Clin Oncol. 2010;28:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 4. | Haque A, Banik NL, Ray SK. Molecular alterations in glioblastoma: potential targets for immunotherapy. Prog Mol Biol Transl Sci. 2011;98:187-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [PubMed] |
| 6. | Dimitrov L, Hong CS, Yang C, Zhuang Z, Heiss JD. New developments in the pathogenesis and therapeutic targeting of the IDH1 mutation in glioma. Int J Med Sci. 2015;12:201-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Gandhi P, Khare R, Niraj K, Garg N, Sorte SK, Gulwani H. Unique case of oligoastrocytoma with recurrence and grade progression: Exhibiting differential expression of high mobility group-A1 and human telomerase reverse transcriptase. World J Clin Cases. 2016;4:296-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Iwamoto FM, Hottinger AF, Karimi S, Riedel E, Dantis J, Jahdi M, Panageas KS, Lassman AB, Abrey LE, Fleisher M. Serum YKL-40 is a marker of prognosis and disease status in high-grade gliomas. Neuro Oncol. 2011;13:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Bhardwaj R. Regulation of YKL-40 in sterile inflammation and its role in glioblastoma in vivo, PhD thesis, Virginia commonwealth university. Richmond: Virginia 2014; . |
| 10. | Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, Yan W, Bentley B, Shao R. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286:15332-15343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Zhang W, Murao K, Zhang X, Matsumoto K, Diah S, Okada M, Miyake K, Kawai N, Fei Z, Tamiya T. Resveratrol represses YKL-40 expression in human glioma U87 cells. BMC Cancer. 2010;10:593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Lin Y, Wang JF, Gao GZ, Zhang GZ, Wang FL, Wang YJ. Plasma levels of tissue inhibitor of matrix metalloproteinase-1 correlate with diagnosis and prognosis of glioma patients. Chin Med J (Engl). 2013;126:4295-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47034] [Article Influence: 3359.6] [Reference Citation Analysis (5)] |
| 14. | Bambury RM, Teo MY, Power DG, Yusuf A, Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Zadora P, Dabrowski W, Czarko K, Smolen A, Kotlinska-Hasiec E, Wiorkowski K, Sikora A, Jarosz B, Kura K, Rola R. Preoperative neutrophil-lymphocyte count ratio helps predict the grade of glial tumor - a pilot study. Neurol Neurochir Pol. 2015;49:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Samaras V, Piperi C, Levidou G, Zisakis A, Kavantzas N, Themistocleous MS, Boviatsis EI, Barbatis C, Lea RW, Kalofoutis A. Analysis of interleukin (IL)-8 expression in human astrocytomas: associations with IL-6, cyclooxygenase-2, vascular endothelial growth factor, and microvessel morphometry. Hum Immunol. 2009;70:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Ferrín G, Rodríguez-Perálvarez M, Aguilar-Melero P, Ranchal I, Llamoza C, Linares CI, González-Rubio S, Muntané J, Briceño J, López-Cillero P. Plasma protein biomarkers of hepatocellular carcinoma in HCV-infected alcoholic patients with cirrhosis. PLoS One. 2015;10:e0118527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Mosrati MA, Malmström A, Lysiak M, Krysztofiak A, Hallbeck M, Milos P, Hallbeck AL, Bratthäll C, Strandéus M, Stenmark-Askmalm M. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6:16663-16673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Hori T, Sasayama T. Tumor associated M2 macrophage infiltration in glioblastoma. Neuro oncol. 2016;49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Alexiou GA, Vartholomatos E, Zagorianakou P, Voulgaris S. Prognostic significance of neutrophil to-lymphocyte ratio in glioblastoma. Neuroimmunol Neuroinflammation. 2014;1:131-134. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | McNamara MG, Lwin Z, Jiang H, Templeton AJ, Zadeh G, Bernstein M, Chung C, Millar BA, Laperriere N, Mason WP. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol. 2014;117:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Hormigo A, Gu B, Karimi S, Riedel E, Panageas KS, Edgar MA, Tanwar MK, Rao JS, Fleisher M, DeAngelis LM. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin Cancer Res. 2006;12:5698-5704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Liang Z. The Expression and Significane of HMGA1, TROP2 in Malignant Gliomas. Shandong Daxue Xuebao: Yixueban. 2013;51:59-61. |