Published online Sep 16, 2016. doi: 10.12998/wjcc.v4.i9.258
Peer-review started: March 28, 2016
First decision: June 16, 2016
Revised: June 22, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: September 16, 2016
Processing time: 163 Days and 14.6 Hours
Subcutaneous panniculitis-like T cell lymphoma (SPTCL) is a very rare variant of non-Hodgkin’s lymphoma. Currently, there is no standard imaging method for staging of SPTCL nor for assessment of treatment response. Here, we describe our use of fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for staging and monitoring of treatment response in 3 cases of SPTCL. Primary staging by PET/CT showed that all 3 patients had multiple foci in the subcutaneous fat tissue, with SUVmax from 10.5 to 14.6. Involvement of intra-abdominal fat with high SUVmax was identified in 2 of the patients. Use of the triple drug regimen of gemcitabine, cisplatin and methylprednisolone (commonly known as “GEM-P”) as first-line therapy or second-line therapy facilitated complete metabolic response for all 3 cases. FDG PET/CT provides valuable information for staging and monitoring of treatment response and can reveal occult involvement of the intra-abdominal visceral fat. High FDG uptake on pre-treatment PET can identify patients with aggressive disease and help in selection of first-line therapy.
Core tip: We used fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for staging and monitoring of treatment response in 3 cases of subcutaneous panniculitis-like T cell lymphoma (SPTCL), a very rare variant of non-Hodgkin’s lymphoma. FDG PET/CT provided valuable information for SPTCL staging and monitoring of treatment response in the patients. It can reveal occult involvement of the intra-abdominal visceral fat and identify patients with aggressive SPTCL disease.
- Citation: Gorodetskiy VR, Mukhortova OV, Aslanidis IP, Klapper W, Probatova NA. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography evaluation of subcutaneous panniculitis-like T cell lymphoma and treatment response. World J Clinical Cases 2016; 4(9): 258-263
- URL: https://www.wjgnet.com/2307-8960/full/v4/i9/258.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i9.258
Subcutaneous panniculitis-like T cell lymphoma (SPTCL) was first defined as a clinical entity in 1991, and described as a cytotoxic T cell lymphoma that preferentially infiltrates the subcutaneous tissue[1]. Patients present with multiple subcutaneous nodules, usually exclusively, with no other involved sites[2-5]. The original description of SPTCL was further refined in 2008 by the World Health Organization, which restricted the classification to exclude γδT cell lymphoma[2]. To date, SPTCL remains a very rare variant of non-Hodgkin’s lymphoma, and the hematologist faces several unresolved issues in the management of patients with SPTCL. The best imaging method for staging of SPTCL and assessment of response to treatment in patients is controversial.
We report here 3 cases of SPTCL that were examined by positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose (FDG) combined with computed tomography (CT) in order to determine their disease stage and to monitor their treatment response.
All 3 cases of SPTCL were diagnosed in the V.A. Nasonova Research Institute of Rheumatology (Moscow, Russia). On admission to the Institute, each patient underwent laboratory testing for complete blood count, urinalysis, blood chemistry and immunological analysis, the latter of which included measurement of antinuclear antibodies by indirect immunofluorescence test and of antibody titer for double-stranded DNA, Sm, anti-Ro and anti-La. In addition, histological and cytological examination of the bone marrow was performed. Formalin-fixed and paraffin-embedded skin biopsy specimens were also reviewed by 2 pathologists working independently (Wolfram Klapper and Natalya A Probatova), which was followed by immunohistochemical staining and molecular analyses. The immunohistochemical study of paraffin-embedded sections was performed using a wide panel of antibodies, including CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD20, CD56, CD68, CD138, VS38c, Ki67 (proliferation marker, expressed as %), granzyme B, TIA1, perforin, βF1 and TdT. The T cell receptor (TCR) C gamma M1-antibody (gamma 3.20; Pierce Biotechnology, Rockford, IL, United States) was used for staining of TCRγ.
Determination of T cell clonality by investigating rearrangements of the genes encoding the gamma, beta and delta chains of TCRs was carried out on paraffin-embedded tissue blocks using the polymerase chain reaction (PCR) followed by fragment analysis as described previously[6,7].
All patients underwent whole-body FDG PET/CT, with calculation of the maximum standardized FDG accumulation in pathological foci (standard uptake value, SUVmax). FDG PET/CT studies were performed before treatment, during the treatment, and 1 mo after completion of chemotherapy.
The complete resolution of FDG uptake at sites of initial disease and the absence of new uptake areas were considered to indicate complete metabolic response (CMR), and were treated as a complete remission of lymphoma regardless of presence of nodules in the subcutaneous tissue found upon physical examination or of residual tumor signs found on CT.
All 3 cases showed the characteristic panniculitis-like infiltration with obvious rimming of subcutaneous fat cells. The observed atypical CD8-positive lymphoma cells were small and medium-sized. Tumor infiltration did not affect the dermis and epidermis. The lymphoma cells were positive for TCRβ, CD3 and CD8, and expressed the cytotoxic markers TIA1, granzyme B and perforin. Proliferation rate of the neoplastic T cells was 50%-70% (as indicated by Ki-67) for all 3 cases. T cell clonality was confirmed in case 1, which showed incomplete (Dβ-Jβ) clonal rearrangement of the TCR β chain, and case 3, which showed complete clonal rearrangement of the TCR β and γ chains.
Table 1 shows the clinical features of the 3 patients (2 females and 1 male). Two patients had autoimmune disorders, with case 2 showing IgG antibodies to erythrocytes and case 3 showing autoimmune thyroiditis and 2-fold increase of antibody titer to the La/SS-B. None of the patients showed evidence of lupus erythematosus. All 3 patients suffered from multifocal disease with red to purple, non-ulcerated, sometimes painful nodules or plaques involving the face, trunk and extremities. All 3 patients also had B-symptoms and elevated lactate dehydrogenase (LDH) level.
| Case | Age in years/sex | Localization | Autoimmune disorders | B-symptoms | LDH, IU/mL1 | SUVmax | Chemotherapy | Outcome, FU in mo |
| 1 | 27/F | Head, neck, upper extremities, chest, breasts, mesocolon | - | + | 1.165 | 14.6 | PD®6 × CHOP®6 × GEM-P | CR, 29 |
| 2 | 22/M | Right cheek, upper and lower extremities, trunk | Positive direct Coombs test | + | 1.741 | 10.5 | 7 × GEM-P | CR, 26 |
| 3 | 53/F | Head, trunk, upper and lower extremities, epiploon in the left mesogastric area | Autoimmune thyroiditis, 2-fold increase of anti-La/SS-B level | + | 274 | 13.8 | 3 × FCM®6 × GEM-P | CR, 8 |
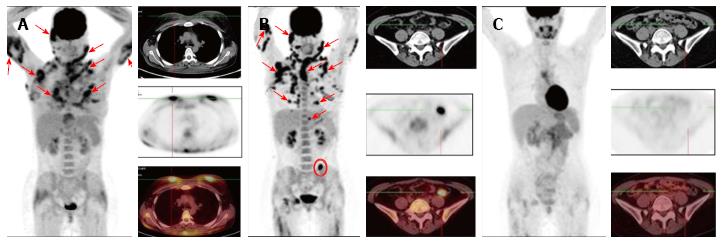
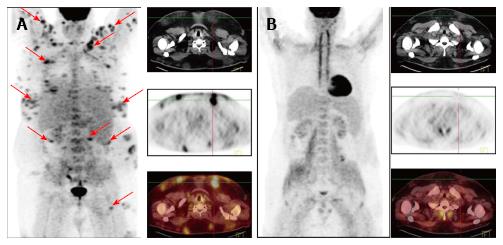
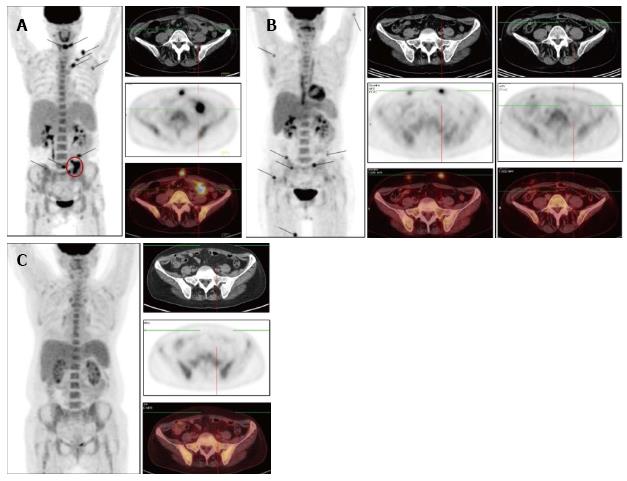
Figure 1, Figure 2 and Figure 3 show results of the PET/CT primary staging and monitoring of treatment response in the 3 patients, respectively. Primary staging by PET/CT showed multiple foci of increased uptake in the subcutaneous fat of all patients, with the SUVmax ranging from 10.5 to 14.6. Involvement of intra-abdominal fat with high SUVmax was identified in two patients; specifically, case 1 showed progression of lymphoma at interim PET, and case 3 showed presence at primary staging.
Initial steroid monotherapy was unsuccessful in case 1. All 3 patients received multi-agent chemotherapy. In cases 1 and 3, the lymphoma was refractory to the therapy; case 1 received the cyclophosphamide, doxorubicine, vincristine and prednisolone (CHOP) regimen and case 3 received the fludarabine, mitoxantrone and cyclophosphamide (FCM) regimen. The gemcitabine, cisplatin and methylprednisolone (GEM-P) regimen was used as first-line therapy for case 2 and as second-line therapy for cases 1 and 3, and led to CMR in all. All 3 of the patients remained in remission at the time of last follow-up (range, 8-29 mo).
The extent of lymphoid tumors and their associated pathologic characteristics play an important role in the choice of therapeutic algorithm, and quantification of disease burden during the therapy is crucial for decision-making of whether to continue the ongoing treatment regimen or to change it. Despite the fact that FDG PET has been used increasingly for staging and response assessment for different types of lymphomas, the experience of its use in SPTCL is limited[8-14]. Analysis of published data on 7 patients with SPTCL revealed FDG-avid tumors in 71%[8]. Feeney et al[11] studied 9 SPTCL patients, all of who were PET-positive, and the average SUVmax was 5.7 (range, 1.5-13.1).
All 3 of our patients showed FDG-avid lesions in the pre-treatment PET, which correlated with a high index of proliferative activity of lymphoid cells. In addition, generalized distribution of subcutaneous fat foci with high SUVmax were identified in the mesenteric fat in 2 of the patients. Involvement of intra-abdominal fat in SPTCL has been described in recent years[15,16]. Our results suggest that PET/CT may therefore be useful in detecting occult extracutaneous involvement in SCPTL. However, the prognostic value of intra-abdominal dissemination of SCPTL is unknown.
To evaluate the effectiveness of chemotherapy, we focused on the FDG PET/CT data in addition to the clinical picture of the cases. In case 1, following 6 cycles of the CHOP regimen, a marked improvement was noted, which manifested as normalization of temperature and LDH level, and a significant reduction in the size and density of the subcutaneous nodules. However, the FDG PET/CT data revealed preservation of previously defined and developing new lesions, which led to a change in the chemotherapy regimen to that of GEM-P. After 6 cycles of the GEM-P chemotherapy regimen, CMR was obtained. In case 2, following 7 cycles of the GEM-P chemotherapy regimen, palpable subcutaneous nodules and elevated LDH was still present but the FDG PET/CT data revealed CMR; as a result, the therapy was discontinued and the risks associated with overtreatment were avoided. In case 3, following 3 cycles of the FCM chemotherapy regimen, an ambivalent picture was observed, with the disappearance of previously palpable nodes and the emergence of new, fast-growing, subcutaneous nodules. For this last case, the FDG PET/CT data confirmed development of new lesions, with SUVmax of 12.9, and a lack of FDG accumulation in the previous nodules. Three cycles of the GEM-P chemotherapy regimen produced CMR, after which 3 consolidating cycles of GEM-P were added.
Recently, SPTCL was classified as a lymphoid tumor with indolent clinical course[17]. However, in each of our 3 patients, despite the lack of hemophagocytic syndrome, the SPTCL course progressed rapidly. B-symptoms and LDH increase were observed, and the index of proliferative activity of the tumor was about 50%-70%, which is comparable with the proliferative activity of diffuse large B cell lymphoma. Additionally, the PET/CT findings in our cases also suggested aggressive behavior of the tumors.
The GEM-P regimen is accepted as a first-line therapy for treatment of peripheral T cell lymphomas, as well as for lymphoma cases of recurrence or primary resistance[18-20]. Our review of the literature found only a single SPTCL case for which the GEM-P regimen was used as treatment. That patient had been refractory to the CHOP regimen but responded well to GEM-P[21]. Our results suggest that the GEM-P regimen is efficacious for the treatment of SPTCL, including in those patients who are refractory to the CHOP or FCM regimens.
It is possible that SPTCL is an inherently biologically heterogeneous tumor or acquires heterogeneity in the course of tumor progression. It would then be assumed that some SPTCL cases are indolent, have a low index of proliferative activity, with low FDG accumulation, and respond well to immunosuppressive therapy. Alternatively, a rapidly progressive disease course would then be observed in some cases, accompanied by constitutional symptoms and with a high index of proliferative activity and high level of FDG uptake. These more aggressive lymphomas would presumably require multi-agent chemotherapy.
Although there is need for further study, the findings from our 3 cases suggest that FDG PET/CT provides valuable information towards detecting occult lesions in SPTCL and may be useful in disease staging and monitoring of treatment response. Moreover, high FDG uptake on pre-treatment PET could identify patients with aggressive disease and help in choosing first-line therapy.
Three patients (two females aged 27 and 53, and one male aged 22) presented with multiple subcutaneous nodules and accompanying fever.
Panniculitis was the provisional diagnosis.
The morphological differential diagnosis included atypical lymphocytic lobular panniculitis, lupus profundus, natural killer (NK) cell and NK-like T cell lymphomas involving subcutis.
All 3 patients in this study had elevated lactate dehydrogenase level.
Positron emission tomography combined with computed tomography (PET/CT) showed multiple foci of increased uptake of fluorine-18 fluorodeoxyglucose in the subcutaneous fat of all 3 patients; the SUVmax values ranged from 10.5 to 14.6. Involvement of intra-abdominal fat was identified in 2 of the patients.
Subcutaneous panniculitis-like T cell lymphoma (SPTCL).
Use of the gemcitabine, cisplatin and methylprednisolone regimen as first-line therapy or second-line therapy was followed by achievement of complete metabolic response for all 3 cases.
SPTCL is a very rare variant of non-Hodgkin’s lymphoma, commonly confused with a non-neoplastic process due to its unusual location; in histological analysis, it can mimic panniculitis.
SPTCL is a malignant neoplasm belonging to the non-Hodgkin’s lymphomas.
Fluorine-18 fluorodeoxyglucose PET/CT provides valuable information for staging of SPTCL and monitoring of treatment response in patients.
The article is well written, clear and concise. The topic and the results are interesting. Methods are sound.
Manuscript source: Invited manuscript
Specialty type: Medicine
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mehdi I, Mocellin S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Gonzalez CL, Medeiros LJ, Braziel RM, Jaffe ES. T-cell lymphoma involving subcutaneous tissue. A clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 289] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Jaffe ES, Gaulard P, Ralfkiaer E, Cerroni L, Meijer CJLM. Subcutaneous panniculitis-like T-cell lymphoma. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon: IARC 2008; 294-295. |
| 3. | Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, Canninga-van Dijk MR, Carlotti A, Geerts ML, Hahtola S. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 424] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Oschlies I, Simonitsch-Klupp I, Maldyk J, Konovalov D, Abramov D, Myakova N, Lisfeld J, Attarbaschi A, Kontny U, Woessmann W. Subcutaneous panniculitis-like T-cell lymphoma in children: a detailed clinicopathological description of 11 multifocal cases with a high frequency of haemophagocytic syndrome. Br J Dermatol. 2015;172:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, García-Sanz R. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2284] [Cited by in RCA: 2379] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 7. | Sandberg Y, van Gastel-Mol EJ, Verhaaf B, Lam KH, van Dongen JJ, Langerak AW. BIOMED-2 multiplex immunoglobulin/T-cell receptor polymerase chain reaction protocols can reliably replace Southern blot analysis in routine clonality diagnostics. J Mol Diagn. 2005;7:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 1151] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 9. | Kako S, Izutsu K, Ota Y, Minatani Y, Sugaya M, Momose T, Ohtomo K, Kanda Y, Chiba S, Motokura T. FDG-PET in T-cell and NK-cell neoplasms. Ann Oncol. 2007;18:1685-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Rodriguez VR, Joshi A, Peng F, Rabah RM, Stockmann PT, Savaşan S. Positron emission tomography in subcutaneous panniculitis-like T-cell lymphoma. Pediatr Blood Cancer. 2009;52:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010;195:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Babb A, Zerizer I, Naresh KN, Macdonald D. Subcutaneous panniculitis-like T-cell lymphoma with extracutaneous dissemination demonstrated on FDG PET/CT. Am J Hematol. 2011;86:375-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Uematsu T, Kasami M. 3T-MRI, elastography, digital mammography, and FDG-PET CT findings of subcutaneous panniculitis-like T-cell lymphoma (SPTCL) of the breast. Jpn J Radiol. 2012;30:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kim JS, Jeong YJ, Sohn MH, Jeong HJ, Lim ST, Kim DW, Kwak JY, Yim CY. Usefulness of F-18 FDG PET/CT in subcutaneous panniculitis-like T cell lymphoma: disease extent and treatment response evaluation. Radiol Oncol. 2012;46:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Mitsuhashi K, Momose M, Masuda A, Tsunemi Y, Motoji T. Positron emission tomography revealed diffuse involvement of the lower legs and occult extracutaneous lesions in subcutaneous panniculitis-like T-cell lymphoma. Clin Nucl Med. 2013;38:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Lester L, Ewalt M, Warnke R, Kim J. Systemic panniculitis-like T-cell lymphoma with involvement of mesenteric fat and subcutis. J Cutan Pathol. 2015;42:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Quintanilla-Martinez L, Jansen PM, Kinney MC, Swerdlow SH, Willemze R. Non-mycosis fungoides cutaneous T-cell lymphomas: report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:491-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Ng M, Waters J, Cunningham D, Chau I, Horwich A, Hill M, Norman AR, Wotherspoon A, Catovsky D. Gemcitabine, cisplatin and methylprednisolone (GEM-P) is an effective salvage regimen in patients with relapsed and refractory lymphoma. Br J Cancer. 2005;92:1352-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Arkenau HT, Chong G, Cunningham D, Watkins D, Sirohi B, Chau I, Wotherspoon A, Norman A, Horwich A, Matutes E. Gemcitabine, cisplatin and methylprednisolone for the treatment of patients with peripheral T-cell lymphoma: the Royal Marsden Hospital experience. Haematologica. 2007;92:271-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Yim KL, Ashley S. Assessment of gemcitabine, cisplatin and methylprednisolone (GEM-P) combination treatment for non-Hodgkin T cell lymphoma. Med Oncol. 2012;29:3535-3539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Velez NF, Ishizawar RC, Dellaripa PF, Saavedra AP, Laga AC, Murphy GF, Fisher DC, Kupper TS, Vleugels RA. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-e236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |