Published online Feb 16, 2016. doi: 10.12998/wjcc.v4.i2.38
Peer-review started: May 25, 2015
First decision: September 14, 2015
Revised: November 19, 2015
Accepted: December 9, 2015
Article in press: December 11, 2015
Published online: February 16, 2016
Processing time: 247 Days and 12.9 Hours
Fine-needle aspiration (FNA) cytology is an important diagnostic tool in patients with thyroid lesions. Several systems have been proposed for the cyropathologic diagnosis of the thyroid nodules. However cases with indeterminate cytological findings still remain a matter of debate. In this review we analyze all literature regarding Thyroid Cytopathology Reporting systems trying to identify the most suitable methodology to use in clinical practice for the preoperative diagnosis of thyroid nodules. A review of the English literature was conducted, and data were analyzed and summarized and integrated from the authors’ perspective. The main purpose of thyroid FNA is to identify patients with higher risk for malignancy, and to prevent unnecessary surgeries for benign conditions. The Bethesda System for Reporting Thyroid Cytopathology is the most widely used system for the diagnosis of thyroid FNA specimens. This system also contains guidelines for the diagnosis and treatment of indeterminate or suspicious for malignancy cases. In conclusion, patients who require repeated FNAs for indeterminate diagnoses will be resolved by repeat FNA in a percentage of 72%-80%.
Core tip: Fine-needle aspiration (FNA) cytology is widely used for the diagnosis of thyroid nodules, although cases with indeterminate results are not rare. We reviewed the English literature regarding Thyroid Cytopathology systems in order to identify the most suitable methodology, taking into account our prospective as well. The Bethesda System for Reporting Thyroid Cytopathology is the most preferred system for the diagnosis of FNA specimens, which also contains guidelines for the diagnosis and treatment of indeterminate cases. Last but not least, repeated FNAs will lead to a diagnosis in 72%-80% of indeterminate cases where repeated FNAs were needed.
- Citation: Misiakos EP, Margari N, Meristoudis C, Machairas N, Schizas D, Petropoulos K, Spathis A, Karakitsos P, Machairas A. Cytopathologic diagnosis of fine needle aspiration biopsies of thyroid nodules. World J Clin Cases 2016; 4(2): 38-48
- URL: https://www.wjgnet.com/2307-8960/full/v4/i2/38.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i2.38
Thyroid nodules is a very usual clinical problem, as it is diagnosed in approximately 60% of the general population in Western countries[1]. However; less than 10% of them represent malignant tumors. Therefore, it is not prudent to remove every thyroid nodule we encounter in our medical practice. Fine-needle aspiration cytology (FNAC) has been widely adopted as a meticulous, secure and cost-effective method for the diagnosis of non-toxic thyroid nodules[1,2]. Its clinical utilization is significant, as it can define whether a recently emerged thyroid nodule should be managed expectantly or surgically, and can assist in selecting the appropriate surgical procedure when necessary[3].
Occasionally FNAC results can be inconsistent and can be a source of dispute among clinicians. The reason is that in approximately 10%-30% of cases, cytology is indeterminate and nondiagnostic[4]. Various diagnostic terminologies, including “indeterminate”, “atypical”, and “suspicious for malignancy,” were used to describe these challenging cases[5]. Until recently there were no uniform criteria for the various diagnostic categories in thyroid cytopathology. This resulted in diagnostic inconsistencies among different laboratories and difficulty in communicating the implications of thyroid fine-needle aspiration (FNA) results both to clinicians (endocrinologists and endocrine surgeons) and laboratory doctors (pathologists and radiologists)[6]. In order to establish a standardized diagnostic terminology/classification system for reporting thyroid FNAC results, the National Cancer Institute (NCI) in the United States sponsored the NCI Thyroid FNA State of the Science Conference with a group of experts at Bethesda, MD, in October 2007[7]. This conference established the Bethesda System for Reporting Thyroid Cytopathology (BSRTC), a 6-tiered diagnostic classification system based on a probabilistic approach[8,9]. Almost simultaneously, in Europe, the British Thyroid Association-Royal College of Physicians and the Italian Society for Anatomic Pathology and Cytopathology-International Academy of Pathology (SIAPEC-IAP) thyroid reporting systems, each comprised of 5 diagnostic classes, have been introduced[10,11]. In several countries the Cytological Communities have adopted the first system or the other, as there is still an ongoing dispute on whether the 5-tiered system or the 6-tiered system is more efficient[12].
In this review we analyze current literature regarding Thyroid Cytopathology Reporting systems trying to identify the most suitable and practical methodology to use in everyday clinical practice.
The thyroid FNAs can be performed either by direct puncture after palpating the thyroid nodule, or more commonly under ultrasound guidance by dedicated thyroid specialists (endocrinologists, radiologists, or pathologists). Palpation-guided FNA can be performed when a thyroid nodule is easily palpable (> 1.0 cm in diameter) and rather solid. Ultrasound guidance is preferable than palpation-guided FNA for small nodules (< 1 cm), cystic lesions and when a prior FNA is nondiagnostic[13].
The thyroid nodules are aspirated 3 to 5 times with a 22-gauge or 25-gauge needle. From each FNA pass one to three smears are prepared and fixed in alcohol for Papanicolaou staining and air dried for Giemsa staining. Liquid-based preparation can also be made after a FNA pass, with the needle been rinsed in normal saline or ThinPrep solutions. As a result, 3 to 15 glass slides from each patient are taken and examined, which can be either Giemsa- or Papanikolaou-stained slides[14]. Regardless the staining method used, all slides with diagnostic material are used for the evaluation and clarification of each case.
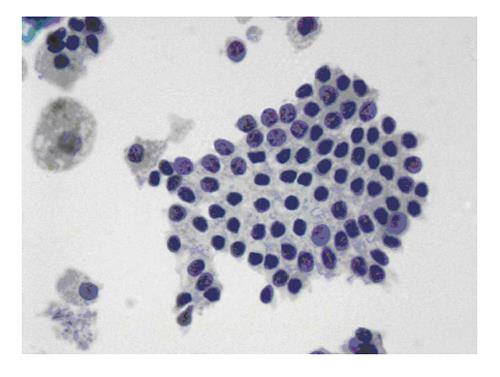
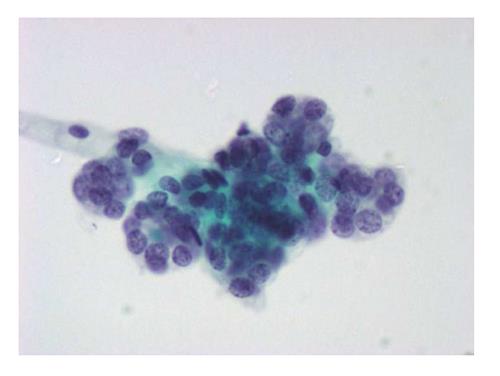
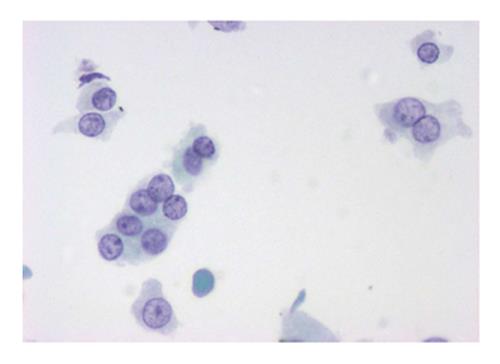
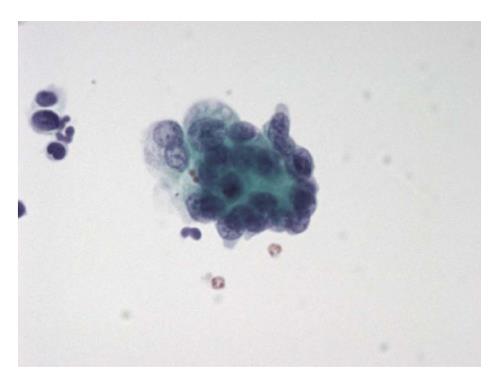
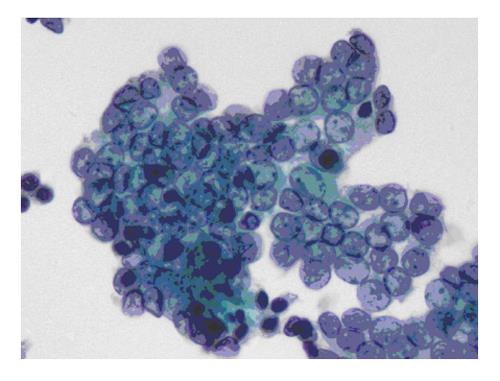
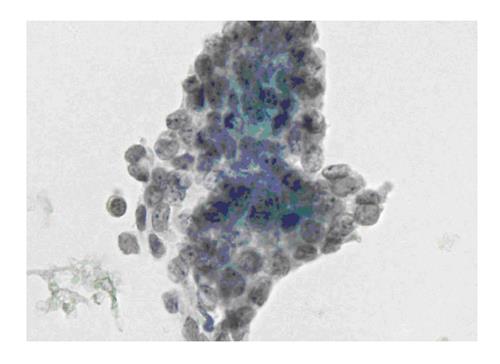
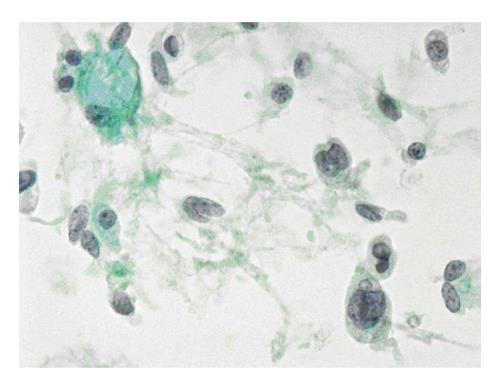
The six-tier diagnostic approach includes the following six categories[8,15]: (1) Disctrict of columbia (DC) I Nondiagnostic or Unsatisfactory. These specimens demonstrate inadequate cellularity, poor fixation and preservation, obscuring blood or ultrasound gel, or a combination of the above factors. Inadequate cellularity is defined as the presence of less than 6 groups of well-preserved follicular cells on each of at least two slides; (2) DC II Benign (Figure 1). This category includes the diagnoses of nodular goiter, nodular goiter with hyperplastic nodules, colloid nodules, cyst contents with/without benign follicular cells, and lymphocytic thyroiditis; (3) DC III Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance (Figure 2). This category is reserved for aspirates with borderline cellularity and is subdivided into two subcategories. One subcategory includes cases with a microfollicular pattern and minimal colloid, that is, follicular lesion of undetermined significance (FLUS). The second subcategory includes cases with nuclear atypia, such as the presence of occasional nuclear grooves, nuclear crowding, and abnormal chromatin pattern, which are characteristics of papillary carcinoma (PTC). This subset of patients could benefit form a repeat FNA; (4) DC IV Follicular Neoplasm or Suspicious for a Follicular Neoplasm. This category refers to cellular specimens with abundant follicular cells arranged in a microfollicular pattern with minimal colloid. The differential diagnosis includes hyperplastic adenomatous nodules, follicular adenoma, follicular carcinoma, and follicular variant of PTC, where the nuclear features remain ill defined. This category also includes cases with a predominant population of Hurthle cells; these cases are labelled Hurthle cell neoplasm (Figure 3). The differential diagnosis for the latter includes hyperplastic adenomatoid nodule with Hurthle cell change, Hurthle cell adenoma, and Hurthle cell carcinoma; (5) DC V Suspicious for malignancy. This category includes specimens with features characteristic of a malignant neoplasm, which are quantitatively or qualitatively insufficient to make a definitive diagnosis of malignancy (Figure 4). These features could be intranuclear inclusions, nuclear grooves, or psammoma calcifications; (6) DC VI Malignant (Figures 5-7). This category includes specimens with unequivocal cytologic evidence of a malignant neoplasm. Herein, all histological types of thyroid carcinoma are included: PTC and its variants, medullary carcinoma, anaplastic carcinoma, lymphoma, and metastatic lesions.
In 2007 the Royal College of Pathologists introduced a new thyroid FNA reporting system, which was based on the existing United Kingdom terminology, but with some alterations, like new subcategories (i.e., “c” for cystic lesions, “a” for atypia, “f” for follicular neoplasm). These alterations were made in order for the British system to be analogous to the BSRTC[11,16], although in other countries these modifications have not be totally embraced. Furthermore, various other thyroid FNA reporting systems have been created, in which the experiences of the pathologists and/or associated risks of malignancy have been taken into account. The most widely known is the SIAPEC-IAP thyroid reporting system, which is also consists of 5 diagnostic classes[12].
Both the European and the American systems are considered as a significant accomplishment and hold the promise for better classification of thyroid FNA results[6,10,11,17,18]. The main difference between the 5-tiered system and the 6-tiered system is that the DC III [atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS)] category is included only in the 6-tier system, a category with considerable prevalence, as it is calculated 6%-7% according to various statistics[14]. Bongiovanni et al[14] analyzed the differences between the 5-tiered and the 6-tiered diagnostic systems for reporting thyroid cytopathology, based in a large series of 7686 thyroid FNA specimens, collected from 3751 patients from several institutions from Italy, Switzerland, and the United States. They found that apart from the TIR III category, for the TIR 1/DC I (unsatisfactory/nondiagnostic) category the percentage of cases in the 5-tiered system was greater than twice the percentage of cases in the 6-tiered system (7.5% vs 3%). There was also a great difference regarding the percentage of the cases classified into the TIR 2/ DC II (benign) category (83.9%) compared with approximately half (55.4%) of the cases in the 6-tiered system. However, the percentage of the cases classified into the TIR 3/DC IV (follicular proliferation/neoplasm) category was substantially smaller (4.6%) in the 5-tiered system compared with the 6-tiered system (23.8%). Moreover, a lower percentage of cases in the European system was placed into the TIR 4 and TIR 5 categories as well, compared with the American system.
The management of each case derives from the category that is classified. Therefore, the DC III (AUS/FLUS) cases are managed conservatively with repeat FNAs, whereas the DC IV, DC V, and DC VI cases, and TIR 3, TIR 4 and TIR 5 cases respectively, are managed operatively, with thyroid lobectomy or total thyroidectomy. In addition, obtaining adequate material at FNA is a very important issue, as the rates of malignancy observed in the nondiagnostic categories of both reporting systems are very high[14].
The majority of the thyroid FNA specimens, in the range of 60% to 70%, are classified as benign, whereas approximately 20% to 30% fall into the 3 categories of suspicious for follicular neoplasm, suspicious for malignancy, and malignant[19]. The remaining 10% of cases represent a significant subset of thyroid specimens with some form of AUS/FLUS. Such atypia may result from a variety of benign cellular changes, but in some cases may reflect an underline malignancy which has been suboptimally sampled or has intermediate diagnostic features[20-22].
The AUS/FLUS category in the Bethesda system, represents aspirates that contain follicular, lymphoid, or other cell types with architectural and/or nuclear atypia that is more pronounced than that observed in benign lesions yet not sufficient to be characterized as suspicious for follicular neoplasm (SFN), or suspicious for malignancy[10]. The authors of the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) recommended that the DC III (AUS/FLUS) category should not exceed 7% of the thyroid FNA diagnoses, and the risk of malignancy in this category should be in the range of 5% to 15%[23].
These indeterminate aspirates may present with architectural atypia or nuclear atypia[21]. Architectural atypia may present in smears with paucity of cells, which contain a few microfollicles, trabeculae, or crowded groups. Several patterns of nuclear atypia may be also present without being quantitatively and/or qualitatively sufficient for the interpretation of “suspicious for malignancy”. These include hypocellular smears with extensive cystic degeneration with rare follicular cells with nuclear atypia indicative of PTC. In some cases more diffuse but mild nuclear changes may exist with nuclear enlargement, crowding, and pallor, but without other characteristics, such as nuclear contour irregularities, grooves and nuclear pseudoinclusions, suggestive of a PTC.
Another pitfall encountered with cystic thyroid nodules are the atypical cyst-lining cells[24]. Benign cyst-lining cells are typically polygonal or fusiform with abundant cytoplasm, well-defined cellular borders, sometimes enlarged, grooved nuclei, and small distinct nucleoli. The isolated cyst-lining cells in thyroid aspirates are often difficult to distinguish from PTC. For that reason the aspirate is then classified as AUS/FLUS to indicate the uncertainty of the findings.
The malignancy rate of the AUS/FLUS category is estimated to be between 5% and 15%[10], which is intermediate between that of the benign category (0%-3%) and that of the SFN category (15%-30%). The most common malignant diagnosis made after surgery in cases initially classified as AUS/FLUS is PTC, usually of the follicular variant (PTC-FV)[24,25]. Since the malignancy rate of this category is quite high, TBSRTC recommends that most patients undergo a repeat thyroid FNA within 3 to 6 mo, in order to define the nature of atypia[24,26]. However, in almost 20% to 28% of AUS/FLUS cases, a repeat thyroid FNA will again be characterized as AUS/FLUS[27,28]. In a study by Teixeira et al[29] the overall incidence of malignancy in the FNA-biopsied nodules characterized as FLUS was 16.2%, a higher value than the suggested 5% to 15%[10,29-31]. In a large study with 1382 cases in a community practice setting, in the United States, Wu et al[32] diagnosed AUS in 27% of cases, ranging from 10% to 47% among pathologists participating in the study. The risk of malignancy of AUS/FLUS was only 6%, a quite lower value than the one reported elsewhere. In this study the AUS category was further subdivided into HCLUS (atypical cells rule out Hurthle cell neoplasm) and FLUS. The risk of malignancy in the HCLUS category was significantly lower than in the other subtypes of AUS. Many of the HCLUS cases did not show any of the above features and were proved to be benign adenomas. Such patients were followed clinically with periodic physical and sonographic examinations. Renshaw noted that a Hurthle cell neoplasm demonstrating one of the following features: Small cell dysplasia, large cell dysplasia, severe nuclear crowding, and dishesive cellular pattern is usually associated with a high risk of malignancy[33].
Most primary thyroid malignancies with the exception of follicular and Hurthle cell carcinomas have unique cytological features which can differentiate primary malignancies from other thyroid lesions. However, there are cases with diagnostic uncertainty due to suboptimal sampling or preservation, and overlapping cytomorphologic features with other thyroid conditions. A specimen is considered as “suspicious for malignancy” (SFM), when some features of malignancy (usually PTC features) exist, but the findings are not sufficient for a definitive diagnosis[9]. These indeterminate results imply surgeons to consider alternative therapies (e.g., thyroid lobectomy with intraoperative frozen section).
In this pattern benign follicular cells are detected, along with cells with nuclear enlargement, nuclear grooves, nuclear membrane irregularity, and/or nuclear molding, usually without any trace of intranuclear inclusions.
In this pattern the nuclear enlargement is generalized in mild-to-moderate degree with evident nuclear grooves and mild nuclear pallor.
In this pattern many features of PTC are found, but it is sparsely cellular.
In this pattern cystic degeneration with hemosiderin-laden macrophages is present. There are also sheets of follicular cells with large pale nuclei and some with nuclear grooves, but without intranuclear inclusions. Moreover, large, atypical, “histiocytoid” cells with enlarged nuclei and abundant vacuolated cytoplasm usually coexist.
Although these nuclear alterations are usually disseminated, they are mild and incomplete. These changes are not pathognomonic, as they are frequently detected in some PTCs, especially in the follicular variant, and in benign lesions as well, such as follicular adenomas. For that reason these findings are best interpreted as SFM.
Cystic degeneration also is often found. Cyst lining cells are usually elongated, containing pale chromatin, with sparsely found intranuclear grooves, large nucleoli, and always associated with hemosiderin-laden macrophages and benign-appearing macrofollicle fragments. The spindle-shaped morphology of these cells is helpful in distinguishing these cells from PTC[24,34].
The cellular sample is typically monomorphic, although some specimens may appear pleomorphic; the cells are usually small or medium-sized, noncohesive, and contain an eccentrically located nuclei[35]. There may be small fragments of amorphous material-colloid vs amyloid. The sensitivity of thyroid FNA for medullary thyroid carcinoma (MTC) is considered high, actually it is higher than the sensitivity of FNA for PTC[36]. However in doubtful cases definitive diagnosis can be made if sufficient material is available for immunocytochemical stains, or if it is known that the patient has an elevated serum calcitonin level.
The sample is composed of numerous monomorphic lymphoid cells. In other cases it is sparsely cellular and contains atypical lymphoid cells. The difficulties in securing diagnosis of a diffuse large B-cell lymphoma derive from the inadequate sampling technique and/or insufficient preservation of the specimen. On the other hand a definitive diagnosis of a low-grade lymphoma (usually a MALT lymphoma) is even more difficult. The diagnosis of a MALT lymphoma of the thyroid requires the use of immunophenotyping by flow cytometry or immunocytochemistry[9,37].
Review of the literature suggests a malignancy rate of 55%-75% for the suspicious category[8]. Therefore the diagnosis “SFM, suspicious for thyroid carcinoma” is an indication for surgery. The contribution of intraoperative frozen section after a suspicious FNA diagnosis is questionable, as Lee et al[38] have demonstrated that preoperative FNA has a higher sensitivity than frozen section in detecting PTC. Another diagnostic option for patients with repeat ultrasonography-guided FNA of thyroid nodule with non-diagnostic cytology results, would be the utilization of ultrasonography-guided core needle biopsy[39]. This technique is conclusive for the majority of cases suspicious for PTC, lymphoma, or follicular neoplasm after previous incomplete FNA results.
For patients with large tumors (> 4 cm), the best approach could be a total thyroidectomy, considering the fact that large tumors have an elevated risk of malignancy[40].
Apart from imaging studies, serological or immunohistochemical studies can be used to secure diagnosis, when the FNA indicated “suspicious for MTC” or “suspicious for lymphoma”. For example, increased serum calcitonin levels and/or strong immunoresponce of chromogranin which is disclosed after multiple FNA tests can indicate the diagnosis of a medullary carcinoma.
PTC accounts for 80% of all thyroid malignancies and occurs more often in women with a 3:1 female-to-male ratio, with a mean age at presentation 30-40 years. It usually behaves as an indolent malignant tumor; however, an aggressive clinical course with decreased survival has been reported in some histologic variants of PTC[41]. PTC most commonly metastasizes via lymphatics. Distant metastases seldom occur, but may develop in 20% of cases in late stage. The most common sites are the lungs, bone, liver and brain. The prognosis of this tumor is good; death due to PTC is rare.
At low magnification, aspirates of PTC are typically cellular, epithelium-rich structures. Papillary structures are not as common as it was believed, because intact papillae are often too large to enter the fine needle or are disrupted during the preparation of the smears. However, some three dimensional structures that resemble the epithelial tips of papillae without the fibrovascular cores can be seen[35].
The cytological diagnosis of PTC is based mainly on the characteristic nuclear morphology. The FNA specimen of this neoplasm is usually cellular and shows neoplastic cells arranged in papillary groups, or clusters, or as single cells in a background of thick colloid, nuclear or calcific debris, macrophages and stromal fragments[41] (Figure 4).
The individual tumor cells are enlarged, oval in shape with eosinophilic cytoplasm; the nuclei show elongation, oval shape, membrane thickening, chromatin clearing, grooves, and inclusions. The nucleoli are usually small and eccentric; however, rare oncocytic variants of PTC can show prominent nucleoli. Nuclear grooves become an important diagnostic feature when associated with an oval, enlarged nucleus with fine chromatin[41]. However, nuclear grooves can be seen also in several thyroid diseases such, as Hashimoto’s thyroiditis, multinodular goiter, Hurthle cell tumors and medullary carcinoma[42,43].
Psammoma bodies are occasionally seen in some aspirates, most possibly arising from calcification of epithelial tips. The presence of true psammoma bodies with concentric laminations is highly suggestive of PTC; however the presence of psammoma bodies in cystic thyroid lesions is not diagnostic. Consequently it is essential to distinguish this form of atypical calcification from true psammomatous calcifications with their concentrically laminated microscopic appearance[35].
This is the most common variant of PTC and is characterized by a predominantly follicular architecture. Since the PTC-FV variant represents one of the most common causes of a false negative diagnosis of PTC, it is important to distinguish this PTC variant from other benign conditions, such as a follicular neoplasm or adenomatous nodule.
FVPTC is characterized cytologically by the paucity of diagnostic nuclear features. The FNA specimens show enlarged follicular cells arranged in monolayer sheets and follicular groups in a background of thin and thick colloid (Figure 6). The tumor cells show nuclear elongation, chromatin clearing, but nuclear grooves and inclusions are rare[40]. Because the nuclear changes of FVPTC are subtle, the majority of cytologic samples are often diagnosed as suspicious for PTC.
Among thyroid malignancies, PTC has the highest propensity to appear cystic, as 10% of the PTC specimens are entirely cystic. In FNA specimens of this variant, the cancer cells appear more profuse, granular or vacuolated compared to regular PTC. The nuclei are enlarged, with usually an oval or irregular shape, and include intense nuclear grooves and inclusions. This variant is sometimes difficult to diagnose, because in some cases the characteristic neoplastic cells are sparsely evident in the mass. As a result they may be not diagnosed through the FNA test, resulting in a false-negative test[44].
This variant of PTC is not common, but it is important to be recognized as it may be confused with a Hurthle cell neoplasm[44]. The specimen is usually cellular with polygonal cells in loose papillary clusters with abundant eosinophilic cytoplasm. The nuclei have conventional PTC nuclear features that distinguish it from Hurthle cell neoplasms[35].
This PTC variant is a circumscribed thyroid tumor with papillary architecture and lymphoid follicles that mimics a Warthin tumor of the parotid gland. FNAs contain oncocytic cells with abundant granular cytoplasm, conventional nuclei, a papillary architecture, and a lymphoplasmacytic background. The neoplastic cells resemble Hurthle cells but have diagnostic nuclear features of PTC. Because of the mixture of oncocytes with lymphocytes on smears, this tumor should be distinguished from Hashimoto thyroiditis or a follicular lesion with oncocytic changes[44].
The tall cell variant of PTC is an important subtype with a potentially aggressive clinical course. It usually affects the elderly population, and often presents as a large and bulky tumor with extrathyroidal extension and metastases. The diagnosis of this variant as a PTC is relatively easy, due to the numerous papillae and the coexisting intranuclear inclusions. The cancer cells are also elongated, with a height-to-weight ratio of at least 3:1. The cells have abundant pink cytoplasm, basally located nuclei and nuclear features of conventional PTC. These cells constitute more than 50% of tumor volume[44].
This is an aggressive variant of PTC characterized by the presence of crowded, stratified clusters of elongated cells resembling cells from a colonic adenoma. The nuclei are hyperchromatic, uniform in size and shape, and with indinstinct nucleoli. The neoplastic cells show a greater cell height than the tall cell variant and lack the obvious nuclear features of PTC. Due to the fact that the nuclei of this variant are darker than those of the regular PTC, the neoplastic cells of this variant may be mistaken for benign respiratory epithelial cells, or a colorectal neoplasm. Additionally an immunohistochemical panel, including thyroglobulin, TTF1, and CDX2 may help in the differential diagnosis of such difficult cases.
It is not widely agreed whether this neoplasm is a variant of PTC or not, although it seems to have the same RET gene rearrangements as PTC. The hyalinizing trabecular tumor is an uncommon malignancy originating from follicular cells, with certain unique features, such as trabecular growth, marked intracellular hyalinization along with nuclear grooves and pseudoinclusions. In some cases psammoma bodies may be present[35,44].
In general, patients diagnosed with FNA test as having PTC, are usually managed operatively, but the final decision of the type of resection (lobectomy vs total thyroidectomy) depends on numerous coexisting factors. Specifically, the ultrasound image of the malignant nodule, as well as the patient’s general condition and age and other comorbidities should be taken into account when planning surgery.
The standard management of PTCs greater than 1 cm is total, or near-total thyroidectomy followed by radioactive iodine (131I) therapy to ablate residual thyroid tissue. After this therapy the patient’s serum thyroglobulin levels should fall to undetectable levels. Since recurrent PTC typically secretes thyroglobulin, serum monitoring of thyroglobulin serves as a useful tumor marker for recurrent PTC[35].
The management of cases with papillary microcarcinomas, i.e., tumors less than 1.0 cm in diameter, is still controversial. These small tumors may be incidentally discovered in glands removed for other reasons, they are treated with thyroidectomy; these patients usually do not need systemic 131I therapy and do not require a second-stage completion thyroidectomy.
MTC was first described by Horn et al[45] in 1951, and it was first recognized as a unique clinicopathological entity by Hazard et al[46], in 1959. In 1966 Williams demonstrated that this tumor derives from the parafollicular cells, known also as calcitonin-producing C cells, which have an ectodermal neural crest origin[47].
MTC represents 3%-12% of thyroid cancers, the majority of which are sporadic. However, in almost 25%-30% of cases, MTC is inherited, and is associated with one of three familial syndromes: Multiple endocrine neoplasia (MEN) syndrome type 2A (Sipple’s syndrome), MEN type 2B (mucosa neuroma syndrome or Gorlin’s syndrome), and familial MTC[35]. The inherited forms are characterized by an autosomal dominant mode of inheritance and are associated with point mutations in the RET proto-oncogene on chromosome 10.
Patients with the sporadic forms of MTC or the familial MTC are most often middle-aged (mean age 50 years old), except in familial cases, in which they are relatively younger. Patients with sporadic MTC present with a solitary, circumscribed thyroid nodule, usually in the middle to upper-outer half of the thyroid gland. Medullary carcinoma is highly metastatic, as tumor cells can be disseminated through hematogenous and metastatic routes to numerous sites, including cervical lymph nodes, liver, lung, bone, and adrenal glands. Almost all patients with MTC have a significantly elevated serum calcitonin level, and in some cases these tumors can produce substances that can lead to paraneoplastic syndromes[35,44].
The FNA aspirates of an MTC are usually composed of numerous cells, either presenting in cell aggregates or as a mixture of non-cohesive cells. Characteristically, distinct granules (calcitonin granules) are spotted in the cytoplasm of the cancer cells, as well as eccentric nuclei, indicating a plasmacytoid appearance to the tumor cells. The nuclear chromatin is similar to that seen in other neuroendocrine tumors, i.e., “salt and pepper type” (Figure 7). Intranuclear inclusions and multinucleated cells have been reported. Therefore this tumor may mimic other thyroid tumors, such as Hurthle cell neoplasms, PTCs, anaplastic carcinomas, and metastatic tumors. Immunohistochemistry test for specific biomarkers (i.e., calcitonin, thyroglobulin) will easily distinguish MTC from other thyroid malignancies. Amyloid can be observed in close association with tumor cells, and can be distinguished from the thick colloid of PTC by performing a Congo-red stain. The diagnosis of MTC can be confirmed by simply measuring serum calcitonin levels, which are markedly elevated in the majority of cases (> 10 pg/mL)[48].
Undifferentiated (anaplastic) thyroid carcinoma (UTC) is an extremely aggressive thyroid malignancy with a very poor prognosis. It generally affects elderly patients presenting as a firm mass rapidly growing in the neck infiltrating extrathyroidal tissues, such as muscle, trachea, esophagus, skin, bone and cartilage[49]. Half of patients present with significant compression of the upper respiratory and the digestive tract in the neck, resulting in dyspnea, hoarseness, dysphagia, and pain. Lymphadenopathy is also present in one quarter to half of patients, whereas the lungs is the most common site of metastases[49,50]. For most cases surgical resection is not an effective treatment and only palliative therapies are used. Prognosis is dismal with a mean survival of 2.5 to 6 mo and an overall 5-year survival of 0% to 14%.
The aspirates from anaplastic carcinoma do not pose any diagnostic difficulties. They can be sparsely cellular, because of the marked fibrosis and hyalinization encountered in some cases[19,51]. They can be readily classified as malignant due to nuclear pleomorphism, chromatin clumping, necrosis, atypical mitoses and other malignant features[40]. Pan-keratin is the most reliable positive immunostain in UTCs, acquiring an expression ranging from 50% to 100%. Vimentin immunoexpression is also a common finding[52]. When evaluating an undifferentiated carcinoma using immunocytochemistry a basic immunopanel should include cytokeratins, calcitonin, leucocyte common antigen, carcinoembryonic antigen, thyroglobulin, chromogranin, and TTF-1. Some cases may present with diagnostic difficulty if the specimen consists mainly of necrotic debris or if the tumor is extremely sclerotic (the paucicellular variant)[40,53].
Because of its aggressive, infiltrative nature, patients with an undifferentiated carcinoma often require a tracheostomy as an emergency procedure. If the tumor is small and confined to the thyroid, thyroidectomy may be feasible; however, in most cases the tumor extends outside the thyroid gland preventing adequate resection[35]. Chemotherapy or radiotherapy usually cannot change the dismal prognosis of this cancer.
Since there is a considerable proportion of patients with a thyroid nodule who remain undiagnosed with FNA, molecular biology could be very helpful at that point. Research is directed to the identification of molecular markers that, in conjunction with FNA, can identify patients with a malignant nodule.
BRAF mutation has become a specific marker for PTC and its variants[54]. BRAF testing has been coupled successfully with the Bethesda Thyroid FNA classification system to offer molecular quality assurance on positive samples, as well as a diagnostic upgrade on samples of indeterminate diagnostic categories, such as AUS/FLUS and SFN/SHN[54]. The rate of malignancy in FNA-BRAF positive nodules has been shown to be 99.8%[55]. BRAF is not usually found in the follicular variant of papillary thyroid carcinoma, but is increasingly detectable in each step of dedifferentiation, including tall cell tumors and anaplastic cancer. It is a point of great significance that Ohori et al[56] found a greater percentage of BRAF-mutated (V600E, K601E, and others) cases in the AUS/FLUS and SFN/SFN categories, rendering BRAF mutational testing a useful predictor of PTC diagnosis in these indeterminate cases. While the V600E and K601E mutations were almost equally observed in the AUS/FLUS category, there was a slight predominance of K601E mutation in SFN/SHN category. In these SFN/SFN and AUS/FLUS cases with the K601E mutation, the cytomorphology of the PTC specimens prevented a more definitive diagnosis, in contrast to cases where the V600E mutation was observed, whether the diagnosis resolved to a classic (CL) subtype, tall cell variant (TCV) subtype, or a solid (SD) PTC diagnosis. The high sensitivity rate, as well as the high negative prognostic value of BRAF testing in AUS/FLUS and SFN/SFN categories have been also demonstrated by Alexander et al[57].
A full molecular panel of BRAF, RAS, RET/PTC and PAX8PPARγ offer additional diagnostic value[58]. However, this requires additional FNA passes or residual cellular material from the cytologic sample. Cantara et al[59] evaluated this panel of tumor-associated mutations in thyroid FNA samples. The above panel correctly identified cancer in 78.2%, whereas cytology identified 58.9% of the thyroid cancers. It also predicted cancer in the majority of indeterminate samples, as well as of the suspicious for cancer samples. Interestingly all predicted cancer proved to be papillary thyroid carcinoma in the final histology[59]. Moses et al[60] also examined the clinical utility of the above panel in thyroid FNA biopsies. When this panel was used for specimens with indeterminate cytology, sensitivity was 27%, specificity was 95%, positive predictive value was 66%, and negative predictive value was 78%[60]. In addition, Ohori et al[61] investigated the utility of the above panel in specimens classified as FLUS. The molecular testing proved to have a high specificity, although the sensitivity was quite low (60%). Despite the fact that not all PTC were detected by this panel, a positive molecular test helped to refine the FLUS cases into high-risk and low-risk categories[61].
Extensive research is going on in this field; an important step for the introduction of new molecular markers in the diagnosis of molecular tumors could be the clinical testing of FNA samples in large multicenter trials.
Thyroid FNA is a well established procedure used in the preoperative diagnosis of thyroid nodules. It allows classification of nodules as benign or malignant, and patients with malignant nodules are scheduled for surgery. The main purpose of thyroid FNA is to stratify higher risk patients for surgery, and to prevent unnecessary surgeries for benign conditions.
TBSRTC provides a uniform 6-tier system on thyroid FNA for pathologists to communicate with clinicians. Each diagnostic category is associated with a specific risk of malignancy and a recommendation for management.
The TBSRTC classifies thyroid follicular lesions with microfollicle predominance and lack of colloid into the suspicious for follicular neoplasm category. This system allows patients with FNAs showing focal atypia to undergo repeat aspiration prior to surgery. Therefore, in the majority of patients in the AUS/FLUS category (72%-80%) the diagnosis will be resolved by repeat FNA, although 20%-28% of them will have AUS/FLUS on the repeat aspirate and thus require surgery.
A malignant thyroid FNA diagnosis accounts for 4%-8% of all thyroid FNAs, the majority of which are PTCs, and these patients will require thyroidectomy[53]. The use of molecular markers can further increase the diagnostic value of FNA samples for the detection of thyroid cancer.
P- Reviewer: Eilers SG, Li XL S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553-559. [PubMed] |
| 2. | Bukhari MH, Niazi S, Hanif G, Qureshi SS, Munir M, Hasan M, Naeem S. An updated audit of fine needle aspiration cytology procedure of solitary thyroid nodule. Diagn Cytopathol. 2008;36:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Cibas ES. Fine-needle aspiration in the work-up of thyroid nodules. Otolaryngol Clin North Am. 2010;43:257-271, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Jing X, Michael CW, Pu RT. The clinical and diagnostic impact of using standard criteria of adequacy assessment and diagnostic terminology on thyroid nodule fine needle aspiration. Diagn Cytopathol. 2008;36:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Baloch ZW, LiVolsi VA. Fine-needle aspiration of thyroid nodules: past, present, and future. Endocr Pract. 2004;10:234-241. [PubMed] |
| 6. | Redman R, Yoder BJ, Massoll NA. Perceptions of diagnostic terminology and cytopathologic reporting of fine-needle aspiration biopsies of thyroid nodules: a survey of clinicians and pathologists. Thyroid. 2006;16:1003-1008. [PubMed] |
| 7. | Abati A. The National Cancer Institute Thyroid FNA State of the Science Conference: “Wrapped up”. Diagn Cytopathol. 2008;36:388-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, Abati A. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal. 2008;5:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes. New York: Springer 2010; . |
| 10. | British Thyroid Association. Royal College of Physicians. Guidelines for management of thyroid cancer. Report of the Thyroid Cancer Guidelines Update Group. 2nd ed. London: Royal College of Physicians 2007; . |
| 11. | Fadda G, Basolo F, Bondi A, Bussolati G, Crescenzi A, Nappi O, Nardi F, Papotti M, Taddei G, Palombini L. Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica. 2010;102:405-408. [PubMed] |
| 12. | Kocjan G, Cochand-Priollet B, de Agustin PP, Bourgain C, Chandra A, Daneshbod Y, Deery A, Duskova J, Ersoz C, Fadda G. Diagnostic terminology for reporting thyroid fine needle aspiration cytology: European Federation of Cytology Societies thyroid working party symposium, Lisbon 2009. Cytopathology. 2010;21:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Layfield LJ, Cibas ES, Gharib H, Mandel SJ. Thyroid aspiration cytology: current status. CA Cancer J Clin. 2009;59:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Bongiovanni M, Crippa S, Baloch Z, Piana S, Spitale A, Pagni F, Mazzucchelli L, Di Bella C, Faquin W. Comparison of 5-tiered and 6-tiered diagnostic systems for the reporting of thyroid cytopathology: a multi-institutional study. Cancer Cytopathol. 2012;120:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Cross PA, Poller D. The Bethesda thyroid terminology and progress towards international agreement on thyroid FNA cytology reporting. Cytopathology. 2010;21:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Piana S, Frasoldati A, Ferrari M, Valcavi R, Froio E, Barbieri V, Pedroni C, Gardini G. Is a five-category reporting scheme for thyroid fine needle aspiration cytology accurate? Experience of over 18,000 FNAs reported at the same institution during 1998-2007. Cytopathology. 2011;22:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Agarwal A, Kocjan G. FNAC thyroid reporting categories: value of using the British Thyroid Association (Thy 1 to Thy 5) thyroid FNAC reporting guidelines. Cytopathology. 2009;20:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Cibas ES, Ali SZ. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1033] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 20. | Krane JF, Vanderlaan PA, Faquin WC, Renshaw AA. The atypia of undetermined significance/follicular lesion of undetermined significance: malignant ratio: a proposed performance measure for reporting in The Bethesda System for thyroid cytopathology. Cancer Cytopathol. 2012;120:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Bongiovanni M, Krane JF, Cibas ES, Faquin WC. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol. 2012;120:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Rabaglia JL, Kabbani W, Wallace L, Holt S, Watumull L, Pruitt J, Snyder WH, Nwariaku FE. Effect of the Bethesda system for reporting thyroid cytopathology on thyroidectomy rates and malignancy risk in cytologically indeterminate lesions. Surgery. 2010;148:1267-1272; discussion 1272-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Faquin WC, Cibas ES, Renshaw AA. “Atypical” cells in fine-needle aspiration biopsy specimens of benign thyroid cysts. Cancer. 2005;105:71-79. [PubMed] |
| 25. | Marchevsky AM, Walts AE, Bose S, Gupta R, Fan X, Frishberg D, Scharre K, Zhai J. Evidence-based evaluation of the risks of malignancy predicted by thyroid fine-needle aspiration biopsies. Diagn Cytopathol. 2010;38:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Renshaw AA. Should “atypical follicular cells” in thyroid fine-needle aspirates be subclassified? Cancer Cytopathol. 2010;118:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | VanderLaan PA, Marqusee E, Krane JF. Clinical outcome for atypia of undetermined significance in thyroid fine-needle aspirations: should repeated fna be the preferred initial approach? Am J Clin Pathol. 2011;135:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Baloch Z, LiVolsi VA, Jain P, Jain R, Aljada I, Mandel S, Langer JE, Gupta PK. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol. 2003;29:203-206. [PubMed] |
| 29. | Teixeira GV, Chikota H, Teixeira T, Manfro G, Pai SI, Tufano RP. Incidence of malignancy in thyroid nodules determined to be follicular lesions of undetermined significance on fine-needle aspiration. World J Surg. 2012;36:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010;134:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 31. | Broome JT, Solorzano CC. The impact of atypia/follicular lesion of undetermined significance on the rate of malignancy in thyroid fine-needle aspiration: evaluation of the Bethesda System for Reporting Thyroid Cytopathology. Surgery. 2011;150:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: An experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012;40:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Renshaw AA. Hürthle cell carcinoma is a better gold standard than Hürthle cell neoplasm for fine-needle aspiration of the thyroid: defining more consistent and specific cytologic criteria. Cancer. 2002;96:261-266. [PubMed] |
| 34. | Weber D, Brainard J, Chen L. Atypical epithelial cells, cannot exclude papillary carcinoma, in fine needle aspiration of the thyroid. Acta Cytol. 2008;52:320-324. [PubMed] |
| 35. | Clark DP, Faquin WC. Papillary thyroid carcinoma. Thyroid cytopathology. 2nd ed. Springer: New York 2010; 125-150. |
| 36. | Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol. 2000;22:351-358. [PubMed] |
| 37. | Lerma E, Arguelles R, Rigla M, Otal C, Cubero JM, Bagué S, Carreras AM, Eulalia E, Gonzalez-Campora R, Galera H. Comparative findings of lymphocytic thyroiditis and thyroid lymphoma. Acta Cytol. 2003;47:575-580. [PubMed] |
| 38. | Lee TI, Yang HJ, Lin SY, Lee MT, Lin HD, Braverman LE, Tang KT. The accuracy of fine-needle aspiration biopsy and frozen section in patients with thyroid cancer. Thyroid. 2002;12:619-626. [PubMed] |
| 39. | Hahn SY, Shin JH, Han BK, Ko EY, Ko ES. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol. 2013;86:20130007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109-142. [PubMed] |
| 41. | Baloch ZW, LiVolsi VA. Fine-needle aspiration of the thyroid: today and tomorrow. Best Pract Res Clin Endocrinol Metab. 2008;22:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Baloch ZW, LiVolsi VA. Cytologic and architectural mimics of papillary thyroid carcinoma. Diagnostic challenges in fine-needle aspiration and surgical pathology specimens. Am J Clin Pathol. 2006;125 Suppl:S135-S144. [PubMed] |
| 43. | Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocr Pathol. 2006;17:1-18. [PubMed] |
| 44. | Auger M, Stelow EB, Yang GCH. Papillary thyroid carcinoma and variants. The Bethesda System for Reporting Thyroid cytopathology. Springr Science: LLC 2010; 91-116. |
| 45. | Horn RC. Carcinoma of the thyroid. Description of a distinctive morphological variant and report of 7 cases. Cancer. 1951;4:697-707. |
| 46. | Hazard JB, Hawk WA, Crile G. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J Clin Endocrinol Metab. 1959;19:152-161. [PubMed] |
| 47. | Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:114-118. [PubMed] |
| 48. | Filie AC, Asa SL, Geisinger KR, Logani S, Merino M, Nikiforov YE, Clark DP. Utilization of ancillary studies in thyroid fine needle aspirates: a synopsis of the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Aldinger KA, Samaan NA, Ibanez M, Hill CS. Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41:2267-2275. [PubMed] |
| 50. | Agrawal S, Rao RS, Parikh DM, Parikh HK, Borges AM, Sampat MB. Histologic trends in thyroid cancer 1969-1993: a clinico-pathologic analysis of the relative proportion of anaplastic carcinoma of the thyroid. J Surg Oncol. 1996;63:251-255. [PubMed] |
| 51. | Deshpande AH, Munshi MM, Bobhate SK. Cytological diagnosis of paucicellular variant of anaplastic carcinoma of thyroid: report of two cases. Cytopathology. 2001;12:203-208. [PubMed] |
| 52. | Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66:321-330. [PubMed] |
| 53. | Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306-315. [PubMed] |
| 54. | Theoharis C, Roman S, Sosa JA. The molecular diagnosis and management of thyroid neoplasms. Curr Opin Oncol. 2012;24:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 56. | Ohori NP, Singhal R, Nikiforova MN, Yip L, Schoedel KE, Coyne C, McCoy KL, LeBeau SO, Hodak SP, Carty SE. BRAF mutation detection in indeterminate thyroid cytology specimens: underlying cytologic, molecular, and pathologic characteristics of papillary thyroid carcinoma. Cancer Cytopathol. 2013;121:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, Friedman L, Kloos RT, LiVolsi VA, Mandel SJ. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 782] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 58. | Cerutti JM. Employing genetic markers to improve diagnosis of thyroid tumor fine needle biopsy. Curr Genomics. 2011;12:589-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, Di Santo A, Caruso G, Carli AF, Brilli L. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 60. | Moses W, Weng J, Sansano I, Peng M, Khanafshar E, Ljung BM, Duh QY, Clark OH, Kebebew E. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;34:2589-2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Ohori NP, Nikiforova MN, Schoedel KE, LeBeau SO, Hodak SP, Seethala RR, Carty SE, Ogilvie JB, Yip L, Nikiforov YE. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance”. Cancer Cytopathol. 2010;118:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |