Published online Aug 16, 2015. doi: 10.12998/wjcc.v3.i8.743
Peer-review started: August 25, 2014
First decision: November 27, 2014
Revised: May 26, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: August 16, 2015
Processing time: 370 Days and 17.3 Hours
A 59-year-old nursing home patient with Down syndrome was brought to the internal medicine department of our hospital due to fever, cough without expectorate, and dyspnea. A thoracic computed tomography revealed the presence of bilateral basal parenchymal opacities. Her condition deteriorated after admission and troponin reached a peak serum concentration of 16.9 ng/mL. The patient was in cardiogenic shock. In addition to fluid resuscitation, vaso-active amine infusion was administered to achieve hemodynamic stabilization. The differential diagnosis investigated possible pulmonary embolism, myocardial infarction, and myocarditis. Furthermore, a second transthoracic echocardiogram suggested Tako-Tsubo syndrome. This is a septic patient. The purpose of this manuscript is to review studies which formerly examined the possible association between high levels of troponin and mortality to see if it can be considered a positive predictive factor of fatal prognosis as the case of thrombocytopenia, already a positive independent predictive factor of multiple organ failure syndrome, and generally to characterize risk profile in a septic patient.
Core tip: The importance of cardiac involvement during sepsis, when occurs, worsens prognosis. However, as myocardial dysfunction is reversible, an early diagnosis and treatment to improve the survival. The awareness of risk profile to develop a severe myocardial dysfunction in a septic patient would be suitable in order to enforce careful resources in this subset of patients. Moreover, other research are needful to perform the best therapeutic strategy of haemodynamic stay which, sometimes, (e.g., when Tako-Tsubo syndrome occurs) can call for intra-aortic balloon pump counter pulsation.
- Citation: Clemente G, Tuttolomondo A, Colomba D, Pecoraro R, Renda C, Della Corte V, Maida C, Simonetta I, Pinto A. When sepsis affects the heart: A case report and literature review. World J Clin Cases 2015; 3(8): 743-750
- URL: https://www.wjgnet.com/2307-8960/full/v3/i8/743.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i8.743
Sepsis is a syndrome caused by the inefficiency of the mechanisms of control and containment of the infection. It is characterized by symptoms and signs of systemic inflammatory reaction to infection and manifestations of organ dysfunction resulting from alterations in the microcirculation.
It is the second most common cause of death in non-coronary intensive units, and the tenth in high-income countries, with a mortality rate between 15% and 50%. Approximately 150000 deaths per year are caused by sepsis in Europe. The number of cases is expected to increase at a rate of 1.5% per year from the current prevalence of 3 cases for every 1000 inhabitants[1].
The most common pathogenic Gram positives (whose incidence is progressively increasing) are Staphylococcus aureus and Streptococcus pneumoniae, whereas among the most frequent Gram negatives it is possible to include Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa[2].
In a smaller percentage of cases, sepsis can be caused by mycobacteria, mycetes, protozoa (Plasmodium Falciparum) and viruses[1].
A 59-year-old nursing home patient with Down syndrome had high fever unresponsive to paracetamol, and unproductive cough for 4 d. Accordingly, cefriaxone was administered with some improvement (defervescence and reduction of cough). After the reappearance of fever associated with dyspnea, acrocyanosis, and her deteriorating condition, she was brought to the emergency room, and on initial evaluation she was admitted to the internal medicine department of our hospital. She had a ventricular (pacing), ventricular (sensing), inhibition (response) (and) rate-adaptive holder pacemaker because of a third degree atrioventricular block. Furthermore, her past medical history included chronic cerebrovascular disease due to previous ischemic strokes complicated by vascular dementia and epilepsy. The latter was possibly due to Alzheimer-like disease as is often seen in down syndrome patients.
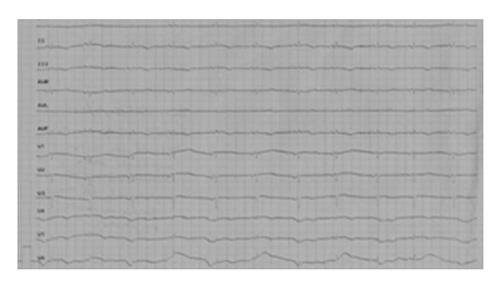
On arrival in the internal medicine department the patient was drowsy, tachypneic, tachycardic, low blood pressure (80/50 mmHg) and hypoxemic (PaO2 57 mmHg). The thoracic computed tomography (CT) revealed the presence of bilateral basal parenchymal opacities. The patient was treated empirically with piperacillin/tazobactam, levofloxacin, and vancomycin according to protocol for health care-associated pneumonia. An initial bed-side echocardiogram evaluation revealed severe left ventricular dysfunction with an ejection fraction of 36%, and dilatation of the right ventricle with medium-apical akinesis. In addition to fluid resuscitation, dopamine, dobutamin, and norepinephrine infusion were administred. At times simultaneous administration of two vaso-active amines was necessary to achieve hemodynamic stabilization and adequate diuresis. The first electrocardiogram showed regular activation of the pacemaker and subsequent evaluations revealed repolarization abnormalities of probable hypoxic nature in the inferior wall only (Figure 1).
The results of blood tests are shown in Table 1, reporting thrombocytopenia (80000 103/μL) and the peak serum concentration of troponin I (16.9 ng/mL - reference range < 0.012 ng/mL). Blood and urine cultures showed no growth.
| Admission | Discharge | Referencerange | |
| Aspartate aminotrasferase (U/L) | 94 | 17 | < 37 |
| Alanine aminotrasferase (U/L) | 66 | 37 | < 41 |
| Calcaemia (mg/dL) | 7.5 | 7.4 | 8.4-10.2 |
| Gamma-glutamyltranspeptidase (U/L) | 155 | 171 | 8-61 |
| C-reactive protein (mg/dL) | 14.6 | 3.2 | 0-0.5 |
| Alkaline phosphatase (U/L) | 163 | 47 | 40-129 |
| Lactate dehydrogenase (UI/L) | 755 | 511 | 240-480 |
| Ferritin (ng/mL) | 2440 | 15-150 | |
| D-dimer (ng/mL) | 478 | 338 | 10-250 |
| RB count (× 106/μL) | 3.72 | 3.53 | 4.5-5.5 |
| Hemoglobin (g/dL) | 12.5 | 11.7 | 12-18 |
| Platelet count (× 103/μL) | 92 | 217 | 150-450 |
| Myoglobin (ng/mL) | 1031 | 99 | 0-62 |
| Troponin I (ng/mL) | 6.43 | 1.36 | 0-0.034 |
A second transthoracic echocardiogram showed akinesis of medium-apical segments of both ventricles with moderate systolic dysfunction (E.F. 45%). This evidence does not rule out an acute ischemic event, but could be seen as suggesting Tako-Tsubo syndrome.
The differential diagnosis also concerned pulmonary embolism, myocardial infarction and myocarditis. The former was excluded through execution of CT angiography. In relation to myocardial infarction and myocarditis, it was not possible to perform coronary angiography or a myocardial biopsy. However, the absence of persistent regional abnormalities ruled out acute coronary syndrome. The third transthoracic echocardiogram showed complete remission of the regional abnormalities (E.F. 50%). The patient was discharged after gradual weaning from vaso-active amines in adequate clinical condition. Therefore, our patient had survived, in spite of severe cardiac involvement and possible Tako-Tsubo syndrome.
In our case there was a significant cardiac involvement associated with sepsis due to pneumonia, up to hearth failure which presented itself as an out-and-out cardiogenic shock. The “fluid resuscitation”, the administration of vasoactive amines and early antibiotic therapy were needed to restore the hemodynamic stability, until the complete recovery of cardiac function, as indeed typically happens in Tako-Tsubo syndrome. The latter, in our patient, was induced by septic injury and characterized initially by hypokinesia of intermediate and apical segments of left ventricle and at a later stage by akinesis of the same with hyperkinesis of basal segment that typically characterizes the disease.
Nevertheless, the absence of head trauma, cerebral hemorrhage, pheochromocytoma, hypertrophic cardiomyopathy made the diagnosis of Tako-Tsubo syndrome plausible. Instead, it was ruled out obstructive atherosclerosis of coronary epicardial artery, since coronary angiography has not been carried out within 48 h, as suggested by Mayo Clinic’s diagnostic criteria[3]. However, the disappearance of the alterations of the segmental kinesis at echocardiographic final assessment, allowed us to exclude this diagnosis. Endomyocardial biopsy would have been necessary for ruling out a myocarditis, in which the predominant involvement of right ventricle is quite, as it has been at any rate[4] initially in our case. The clinical presentation, at last, was not suggestive of Guillain-Barrè syndrome[5] nor electrocardiographic monitoring of recurrent ventricular tachycardia[6], conditions in which cases of reversible left ventricular dysfunction have been observed[7]. Therefore, differential diagnosis about Tako-Tsubo syndrome has been ruled out after analyzing anamnesis and clinical presentation. The latter (hypotension, tachycardia, hypoxiemia) also was suggestive of pulmonary embolism, excluded by CT angiography.
The principal cardiovascular manifestation of severe sepsis and septic shock is hypotension and myocardial dysfunction is often associated with them. Myocardial dysfunction does not seem to be caused by myocardial hypoperfusion[8,9] (coronary circulation is maintained or even intensified, although to observe disfunctions in the microcirculation is probable)[10] but rather by the action of depressant factors such as alpha tumor necrosis factor and beta interleukin 1 and does persist despite fluid resuscitation, as Court et al[11] have already shown. In addition to the effects of host’s immuno-inflammatory responses (e.g., cytokines and mechanisms related to nitric oxide)[12] circulating substances released by pathogens (e.g., endotoxins) also seem to play an important role in provoking myocardial depression. In this sense, the first-line therapy is causal and consists of antibiotic therapy associate with the possible surgical excision of the infectious focus[13]. However, the restoration of hemodynamic stability is an important goal for the survival of the patient. Fluids remain a first-step therapy in clinical management of the cardiovascular failure in sepsis but it is arguable which of them would be the gold standard. Recent results indicate that albumin also might be used with advantage in some specific subgroups of patients pending for the results of the ongoing trials on new generation starches[14]. Furthermore, thanks to its electrostatics properties, albumin reduces the endothelial permeability (sealing effect)[15-20]. Its efficacy is still now matter of debate. In patients with severe sepsis, treated with albumin and crystalloids compared with ones treated with crystalloids only, an increase in survival to 28 and 90 d was not observed[21]. As regards the methods of liquids’ administration, according to Surviving Sepsis Campaign 2012, an initial fluid challenge in patients with tissue hypoperfusion and suspected hypovolemia, up to achieve ≥ 30 mL of crystalloids per kilogram of body weight. It would be needed to continue with the fluid-challenge technique until an actual hemodynamic improvement. Yet, a particular attention in balancing the fluids is necessary, inasmuch a positive fluid balance and elevated central venous pressure are associated with increased mortality[22,23].
With ongoing sepsis, advantageous effects, especially as for cardiac output, could be gained with administration of hypertonic saline solutions, as Oliveira et al[24] already suggested in their review. For the first time, this kind of therapy was employed in the treatment of hemorrhagic and traumatic shock, with quick restoration of central and peripheral blood flow[25]. Intravenous infusion of hypertonic saline solution summons fluids into vascular compartment and determines a redistribution of blood flow which, as for that matter our team has shown, in refractory heart failure enhances myocardial performance[26]. The proposed mechanism to explain these effects suggests a direct action on myocardial functionality and a decreased sympathetic tone[27]. Hence, infusion of hypertonic saline could be an alternative to early volume resuscitation of a patient with sepsis[28].
Furthermore, in a multicentric trial conducted in a tertiary care setting, protocol-based resuscitation of patients with septic shock diagnosed in the emergency department, does not improve the outcomes[29].
Even more complex is the pathogenesis of heart failure that could occur during sepsis and which can provoke a significant increase of troponin.
The increase of troponin in sepsis is an event to be rationally expected. Its dosage, therefore, should not be taken for granted. Considering the heart’s fundamental cardiovascular adaptation role in sepsis, a significant metabolic-inflammatory impairment can occur with high levels of troponin, associated with severe myocardial dysfunction. Moreover, a meta-analysis in “Intensive Care Medicine” a year ago evaluated the prognostic role of troponin in sepsis, showing that its elevated serum concentration was associated with a subset of patients at higher risk of death. Nonetheless, further studies are needed to determine an optimal troponin cut-off value[30]. B-type natriuretic peptides could also have a role in alerting clinicians to myocardial dysfunction. Their low serum values could exclude severe myocardial impairment. Yet echocardiography is the gold standard method to reveal cardiac dysfunction. Heart rate has also been proposed in the prognostic evaluation of septic patients. A rate of < 106 bpm on presentation suggested a favorable prognosis[31]. Concerning the latter, it is still debatable whether the use of β-blockers in septic tachycardial patients improves the survival. It has been observed that patients being in chronic treatment with β-blockers and later developed sepsis, and were admitted to the intensive care unit (ICU), could have advantages in terms of survival. However, physicians’ doubts about using β-blockers in early stages of sepsis are licit[32]. Among other things, it is not still clear enough if in septic shock the increased cardiac rate is pathological or simply an expression of sympathetic hyperactivation. Instead, tachycardia is associated with a worse prognosis. In a prospective observational study in an ICU, esmolol’s titrated administration for 24 h, maintaining a cardiac rate between 80 and 94 bpm in selected adult patients in septic shock after 24 h of hemodynamic stabilization, was able to maintain the microvascular blood flow and reduced the demand for epinephrine. However, patients with severe myocardial disfunction had been excluded from the study[33]. Recent results suggest that β-blockers’ effects on metabolism, glucidic homeostasis, inflammatory feedback and cardiac function might be advantageous for septic patients[34,35]. In regard to the anti-inflammatory, antioxidant, immunomodulatory and anti-apoptotic actions, statins also might fall within preventing and treating patients with severe sepsis and septic shock[36,37].
However, beyond the value of troponin, B-type natriuretic peptides, and heart rate, the presence of myocardial dysfunction in sepsis is associated with higher mortality. It has been shown that cardiovascular disablement increased mortality from 70% to 90%, compared to 20% in septic patients without myocardial impairment[38].
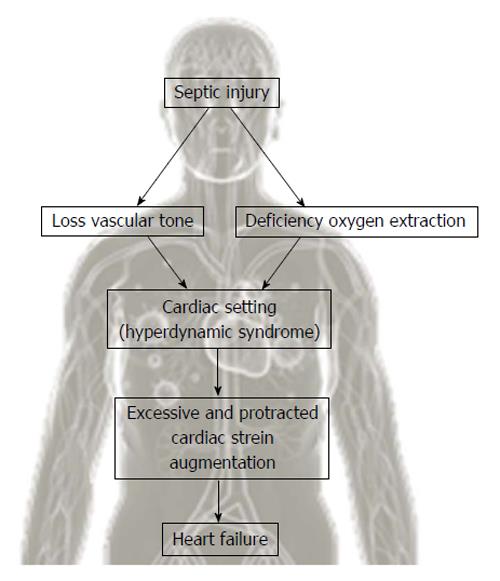
Therefore, cardiac dysfunction in sepsis has prognostic value and coincides with its severity. Hence it’s mandatory to know the pathophysiology of cardiovascular disease in sepsis. Microcirculatory disfunctions and mitochondrial derangement occurring in septic shock reduce the cellular energetic production[39]. Septic injury triggers a reaction in the cardiovascular setting that aims to increase the peripheral availability of oxygen and reduce the cellular effects of oxygen deficiency[40]. The cellular deficiency of oxygen and reduction in systemic vascular resistance give rise to hyperdynamic syndrome (increased stroke volume, heart rate, usage of oxygen)[41] (Figure 2). Alteration of cellular energetic production following to mitochondrial imbalances might be of great relevance in determining tissue injury and sepsis-associated multi organ failure. Future studies should focus on mitochondrial disfunction in order to comprehend the pathophysiological mechanisms of apoptosis and cellular protection to achieve a increasingly accurate treatment[42].
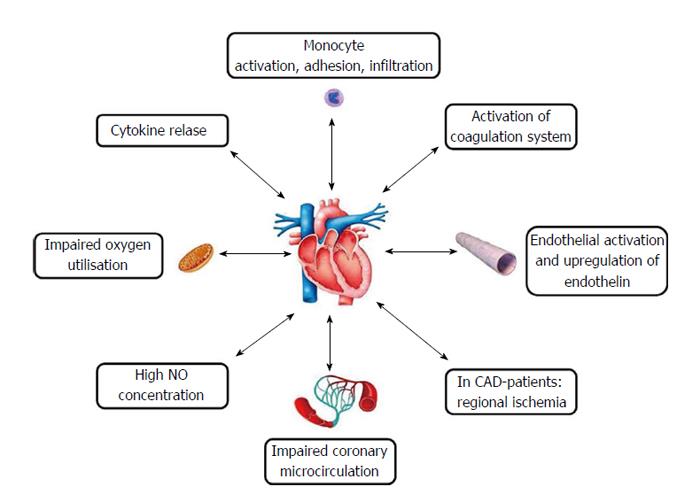
Such a hyperdynamic reaction, favoring adrenergic stimulation, can be hidden by hypovolemia due to insufficient fluid contribution or a mechanism of myocardial impairment[43] (Figure 3). The most frequent occurrence is heart failure with high cardiac index, which is in a phase of unbalance, but it is insufficient to increase metabolic requirements. Hyperdynamic syndrome endows an organism with the possibility to reduce septic injury and survival derives largely from that[44]. Hence the usefulness of administering inotropic positive drugs and to correct hypovolemia because a possible condition of circulatory failure, in the presence of increased oxygen requirements, is linked to a fatal prognosis[45].
The management of myocardial dysfunction sepsis-induced encompasses fluids’s administration until the optimization of preload and, among positive inotropic agents, norepinephrine is the first choice[39]. The administration of dopamine should be reserved to carefully selected patients (those with a low risk of arrhythmias and either known grave left ventricular systolic dysfunction or low heart rate). The Surviving Sepsis Campaign guidelines 2012 promote either norepinephrine or dopamine as the first-choice vasopressor agent to maintain adequate perfusion tissue in septic shock[46,47]. Dobutamine also can be used in the early stages of sepsis in order to increase cardiac output. It has a certain selectivity for β1-receptors[48]. Anyway, β1-agonists can be less effective in case of septic shock. It has been shown that its infusion improves the left ventricular ejection fraction more than 10% in 35% of patients affected by septic shock[49]. Its use requires careful clinical and instrumental monitoring for risk of tachycardia or arrhythmias and hypotension through beta2-adrenergic receptors activation[50]. However, dobutamine is endorsed as the care’s fundamental element of sepsis-related cardiovascular failure in international guidelines. Furthermore, it has been demonstrated that dobutamine enhances liver function and hepatic perfusion after experimental hemorrhagic shock[51]. Since one of the mechanisms of sepsis-induced myocardial dysfunction is the alteration of intracellular transport of calcium, a possibility of therapy might be represented by levosimendan[52], inotrope and peripheral vasodilator which is employed in acute congestive heart failure[53]. Levosimendan, acting with a mechanism of calcium-sensibilization in randomized studies comparing it with dobutamine in patients with severe heart failure with low cardiac output, has been observed as emodynamically more effective than dobutamine[54-56].
Another kind of heart failure associated with sepsis is Tako-Tsubo syndrome. For this reason it has been supposed that sepsis-induced systemic inflammation could have a role in starting the pathogenesis of the syndrome[57-59]. Myocardial dysfunction in sepsis could be a consequence of the direct action of different mediators of flogosis (cathecolamines responsible for hyperdynamic syndrome included) and of products of microbial derivation[13]. On the other hand, another pathogenic hypothesis for Tako-Tsubo syndrome is cardiac cathecolamine toxicity, as it could occur in sepsis, which would constitute the trigger[60].
Our case shows that exogenous support of vasoactive amines can be essential in facilitating hyperdynamic syndrome which characterizes sepsis in the pre-clinical phase. As for Tako-Tsubo syndrome, even though β-agonist agents have often been used, the results are conflicting[7], so intra-aortic balloon pump counter pulsation remains the first-line treatment if, after medical therapy (dopamine) and volume resuscitation, hypotension endures[61]. However, the disappearance of segmental kinesis’s alterations and complete resolution of myocardial dysfunction, as in our case, if Tako-Tsubo syndrome is actually diagnosed, offer new perspectives that could improve our understanding of the physiopathology of this illness. Randomized clinical trials could demonstrate the possible efficacy of the treatment.
A 59-year-old nursing home patient with down syndrome presented fever, cough and dyspnea.
Main clinical findings were tachypnea, tachycardia and hypotension.
Computed tomography (CT) angiography, thoracic CT, transthoracic echocardiogram were executed and differential diagnosis concerned pulmonary embolism, myocardial infarction, myocarditis, Tako-Tsubo syndrome and sepsi with severe myocardial involvement.
The results of blood tests showed alterations of liver function (aspartate aminotrasferase: 94 U/L; alanine aminotrasferase: 66 U/L; gamma-glutamyltranspeptidase: 155 U/L; alkaline phosphatase: 163 U/L); ferritin: 2240 ng/mL; myoglobin: 1031 ng/mL; C-reactive proteinmg: 14.6 mg/dL; lactate dehydrogenase: 755 UI/L; calcaemia: 75 mg/dL; D-Dimer: 478 ng/mL; thrombocytopenia (92000 × 10 3/μL); myoglobin: 1031 ng/mL; troponin: I 643 with peak serum concentration of 169 ng/mL.
Echocardiogram revealed severe left ventricular dysfunction with an ejection fraction of 36% and dilatation of the right ventricle with medium-apical akinesis.
The thoracic CT showed the presence of bilateral basal parenchymal opacities but blood cultures showed no growth.
The patient was treated with piperacillin/tazobactam, levofloxacin, and vancomycin according to protocol for health care-associated pneumonia in add to fluid resuscitation and infusion of dopamine, dobutamin and norepinephrine.
The sepsis-induced systemic inflammatory response syndrome can produce myocardial dysfunction that sometimes defines Tako-Tsubo syndrome.
The dosage of troponin and B-type natriuretic peptides, the monitoring cardiac rate can be helpful to identify setting risk of myocardial dysfunction during sepsis.
This article points out the importance of early haemodinamic support with fluid resuscitation, vaso-active amine and catecholamines in sepsis-induced myocardial dysfunction, trying at the same time to define a risk profile of a septic patient with cardiac involvement whose mortality is high.
In spite of richness of literature about the cardiac involvment during sepsis and management of sepsis-induced myocardial dysfunction, other research to identify the more suitable therapeutic strategy is necessary.
P- Reviewer: Najafi M, Weber V S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Ferrari AM, Barletta C. Medicina di emergenza-urgenza. Il sapere e il saper fare del medico di emergenza tra linee-guida, percorsi clinico assistenziali e rete dell’emergenz. Milano: Elsevier 2012; 318. |
| 2. | Angus DC, van der Poll T. Severe Sepsis and Septic Shock. N Engl J Med. 2013;369:840-851. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2315] [Cited by in RCA: 2563] [Article Influence: 213.6] [Reference Citation Analysis (0)] |
| 3. | Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1020] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 4. | Mancio J, Bettencourt N, Oliveira M, Pires-Morais G, Ribeiro VG. Acute right ventricular myocarditis presenting with chest pain and syncope. BMJ Case Rep. 2013;2013:pii: bcr2012007173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Pillière R, Mansencal N, Digne F, Lacombe P, Joseph T, Dubourg O. Prevalence of tako-tsubo syndrome in a large urban agglomeration. Am J Cardiol. 2006;98:662-665. [PubMed] |
| 6. | Iga K, Hori K, Matsumura T. Reversible left ventricular dysfunction induced by recurrent ventricular tachycardia. Chest. 1992;102:1897-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Novo S, Akashi Y, Arbustini E, Assennato P, Azzarelli S, Barbaro G, Fazio G, Fedele F, Giordan M, Mazzarotto P. La cardiomiopatia takotsubo: documento di consenso. G Ital Cardiol. 2008;9:785-797. |
| 8. | Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE. The coronary circulation in human septic shock. Circulation. 1986;73:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 284] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Dhainaut JF, Huyghebaert MF, Monsallier JF, Lefevre G, Dall’Ava-Santucci J, Brunet F, Villemant D, Carli A, Raichvarg D. Coronary hemodynamics and myocardial metabolism of lactate, free fatty acids, glucose, and ketones in patients with septic shock. Circulation. 1987;75:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 232] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 11. | Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Zhang T, Feng Q. Nitric oxide and calcium signaling regulate myocardial tumor necrosis factor-α expression and cardiac function in sepsis. Can J Physiol Pharmacol. 2010;88:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Paoli G, Valente S, Ardissino D, Gensini GF. [Myocardial dysfunction during sepsis: epidemiology, prognosis and treatment]. G Ital Cardiol (Rome). 2011;12:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | De Backer D, Scolletta S. Clinical management of the cardiovascular failure in sepsis. Curr Vasc Pharmacol. 2013;11:222-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Verheij J, van Lingen A, Beishuizen A, Christiaans HM, de Jong JR, Girbes AR, Wisselink W, Rauwerda JA, Huybregts MA, Groeneveld AB. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med. 2006;32:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 700] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 17. | Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 529] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 19. | Gondos T, Marjanek Z, Ulakcsai Z, Szabó Z, Bogár L, Károlyi M, Gartner B, Kiss K, Havas A, Futó J. Short-term effectiveness of different volume replacement therapies in postoperative hypovolaemic patients. Eur J Anaesthesiol. 2010;27:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 1880] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 21. | Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 738] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 22. | Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1008] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 23. | Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med. 2014;29:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Oliveira RP, Velasco I, Soriano FG, Friedman G. Clinical review: Hypertonic saline resuscitation in sepsis. Crit Care. 2002;6:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Kreimeier U, Brueckner UB, Schmidt J, Messmer K. Instantaneous restoration of regional organ blood flow after severe hemorrhage: effect of small-volume resuscitation with hypertonic-hyperoncotic solutions. J Surg Res. 1990;49:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Tuttolomondo A, Pinto A, Parrinello G, Licata G. Intravenous high-dose furosemide and hypertonic saline solutions for refractory heart failure and ascites. Semin Nephrol. 2011;31:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Monteiro Pacheco A, Martins Coimbra RS, Kreimeier U, Frey L, Messmer K. Hypertonic volume therapy: feasibility in the prevention and treatment of multiple organ failure and sepsis. Sao Paulo Med J. 1995;113:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Singh A, Carlin BW, Shade D, Kaplan PD. The use of hypertonic saline for fluid resuscitation in sepsis: a review. Crit Care Nurs Q. 2009;32:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1314] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 30. | Bessière F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987;15:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 333] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Macchia A, Romero M, Comignani PD, Mariani J, D’Ettorre A, Prini N, Santopinto M, Tognoni G. Previous prescription of β-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis. Crit Care Med. 2012;40:2768-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Koenig KL. Beta-Blockers in Septic Shock: A New Indication? Crit Care Med. 2013;Epub ahead of print. |
| 34. | Novotny NM, Lahm T, Markel TA, Crisostomo PR, Wang M, Wang Y, Ray R, Tan J, Al-Azzawi D, Meldrum DR. beta-Blockers in sepsis: reexamining the evidence. Shock. 2009;31:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Hamzaoui O, Teboul JL. The role of beta-blockers in septic patients. Minerva Anestesiol. 2015;81:312-319. [PubMed] |
| 36. | Kouroumichakis I, Papanas N, Proikaki S, Zarogoulidis P, Maltezos E. Statins in prevention and treatment of severe sepsis and septic shock. Eur J Intern Med. 2011;22:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Fraunberger P, Drexel H, Walli AK. [Pathophysiology of sepsis and possible influence of statins]. Dtsch Med Wochenschr. 2010;135:2128-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 820] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 39. | Rudiger A, Singer M. The heart in sepsis: from basic mechanisms to clinical management. Curr Vasc Pharmacol. 2013;11:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | da Silva Ramos FJ, Azevedo LC. Hemodynamic and perfusion end points for volemic resuscitation in sepsis. Shock. 2010;34 Suppl 1:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Shoemaker WC, Appel PL, Kram HB, Bishop M, Abraham E. Hemodynamic and oxygen transport monitoring to titrate therapy in septic shock. New Horiz. 1993;1:145-159. [PubMed] |
| 42. | De Kock I, Van Daele C, Poelaert J. Sepsis and septic shock: pathophysiological and cardiovascular background as basis for therapy. Acta Clin Belg. 2010;65:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 45. | Baue AE, Faist E, Fry D. Multiple Organ Failure: Pathophysiology, Prevention and Therapy. British Journal of Surgery. 2001;1:712. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 46. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 3979] [Article Influence: 331.6] [Reference Citation Analysis (0)] |
| 47. | Póvoa P, Carneiro AH. Adrenergic support in septic shock: a critical review. Hosp Pract (1995). 2010;38:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Silverman HJ, Penaranda R, Orens JB, Lee NH. Impaired beta-adrenergic receptor stimulation of cyclic adenosine monophosphate in human septic shock: association with myocardial hyporesponsiveness to catecholamines. Crit Care Med. 1993;21:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Jozwiak M, Persichini R, Monnet X, Teboul JL. Management of myocardial dysfunction in severe sepsis. Semin Respir Crit Care Med. 2011;32:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Fink T, Heymann P, Taha-Melitz S, Taha A, Wolf B, Rensing H, Volk T, Mathes AM. Dobutamine pretreatment improves survival, liver function, and hepatic microcirculation after polymicrobial sepsis in rat. Shock. 2013;40:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 53. | Tsao CM, Li KY, Chen SJ, Ka SM, Liaw WJ, Huang HC, Wu CC. Levosimendan attenuates multiple organ injury and improves survival in peritonitis-induced septic shock: studies in a rat model. Crit Care. 2014;18:652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Innes CA, Wagstaff AJ. Levosimendan: a review of its use in the management of acute decompensated heart failure. Drugs. 2003;63:2651-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 730] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 56. | De Luca L, Colucci WS, Nieminen MS, Massie BM, Gheorghiade M. Evidence-based use of levosimendan in different clinical settings. Eur Heart J. 2006;27:1908-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Geng S, Mullany D, Fraser JF. Takotsubo cardiomyopathy associated with sepsis due to Streptococcus pneumoniae pneumonia. Crit Care Resusc. 2008;10:231-234. [PubMed] |
| 58. | Eitel I, Lücke C, Grothoff M, Sareban M, Schuler G, Thiele H, Gutberlet M. Inflammation in takotsubo cardiomyopathy: insights from cardiovascular magnetic resonance imaging. Eur Radiol. 2010;20:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 59. | Morel O, Sauer F, Imperiale A, Cimarelli S, Blondet C, Jesel L, Trinh A, De Poli F, Ohlmann P, Constantinesco A. Importance of inflammation and neurohumoral activation in Takotsubo cardiomyopathy. J Card Fail. 2009;15:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Y-Hassan S, Settergren M, Henareh L. Sepsis-induced myocardial depression and takotsubo syndrome. Acute Card Care. 2014;16:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Reeder GS, Prasad A. Stress (takotsubo) cardiomyopathy uptodate. [Accessed August 4, 2014]. Available from: http://www.uptodate.com/contents/stress-takotsubo-cardiomyopathy. |