Published online Aug 16, 2015. doi: 10.12998/wjcc.v3.i8.736
Peer-review started: September 29, 2014
First decision: December 17, 2014
Revised: January 10, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: August 16, 2015
Processing time: 326 Days and 14 Hours
The evidence in the medical literature on the efficacy and safety of rituximab therapy for primary glomerulonephritis is limited and controversial. We describe two male Caucasian patients with rapidly progressive kidney failure due to primary proliferative glomerulonephritis. Both of them received high-dose intravenous corticosteroids and oral cyclophosphamide with limited benefit. The first patient (hepatitis C virus-negative mixed cryoglobulinemia) underwent plasma-exchange with intravenous immunoglobulins; he showed significant benefit on kidney function (he became dialysis independent with serum creatinine going back to 1.6 mg/dL) after one rituximab pulse even if urinary abnormalities were still present. No improvement in renal function or urinary changes occurred in the second patient. Both these individuals developed sepsis over the follow-up, the first patient died two months after rituximab therapy. This report is in keeping with the occurrence of severe infections after rituximab therapy in patients with renal impairment at baseline and concomitant high-dose steroids.
Core tip: A small but growing body of evidence is emerging on the efficacy and safety of rituximab therapy for primary glomerulonephritis. Various authors have claimed that rituximab for glomerular diseases is effective and has minimal adverse effects. We report on two male Caucasian patients who were refractory to conventional immunosuppressive therapy; each of them received one rituximab pulse and developed sepsis over the follow-up, the first patient died two months after rituximab therapy. The risks (and the predictive factors) of severe infections in kidney patients on rituximab therapy are unclear and appear an area of active research.
- Citation: Fabrizi F, Cresseri D, Fogazzi GB, Moroni G, Passerini P, Martin P, Messa P. Rituximab therapy for primary glomerulonephritis: Report on two cases. World J Clin Cases 2015; 3(8): 736-742
- URL: https://www.wjgnet.com/2307-8960/full/v3/i8/736.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i8.736
Primary glomerulonephritis (GN) remains an important cause of end-stage kidney disease. Preliminary trials have recently shown the efficacy of rituximab for adult-onset primary GN[1]; rituximab being a genetically chimeric monoclonal antibody directed to CD20 antigen, a B-cell-specific transmembrane found on immature and mature cells, as well as on malignant B cells. Following treatment with rituximab (RTX), B-cells are prevented from proliferating, and undergo apoptosis and lysis through complement-dependent and - independent mechanisms. B-cell depletion usually persists for 6-9 mo in around 80% of patients, but the degree of depletion is greatly variable. Rituximab is currently approved for treating various malignancies including B cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia; also, it has been licensed for refractory rheumatoid arthritis, granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis. Rituximab was expected to inhibit the production of autoantibodies involved in the pathogenesis of the disease without the toxicity of nonspecific immunosuppression. It has been the first monoclonal employed for the treatment of glomerular diseases and has been initially used for patients with membranous nephropathy but its use has rapidly spread to other glomerular diseases[1]. Membranous nephropathy and membranoproliferative GN are characterized by glomerular deposition of immune complexes; a crucial role of B cells in membranous (MN) and membranoproliferative glomerulonephritis pathogenesis through autoantibody production and antigen presentation has been mentioned. A small but growing body of literature is emerging on the benefits of rituximab in MN and membranoproliferative glomerulonephritis as primary treatment or as treatment of lesions refractory to other immunomodulatory regimens. In this setting, the drug appears to be well tolerated with small adverse events (Table 1)[2-8].
| Admission | Discharge | Middle follow-up | Final follow-up | |
| Creatinine (0.5-1.2, mg/dL) | 2.8 | 1.59 | 1.76 | 0.8 |
| Blood urea nitrogen (8-20, mg/dL) | 147 | 104 | 86 | 85 |
| AST (5-32, IU/L) | 12 | 24 | 27 | 23 |
| ALT (5-31, IU/L) | 9 | 43 | 39 | 7 |
| γGT (5-36, IU/L) | 12 | 44 | 41 | 69 |
| Cholinesterasis (5300-12900, IU/L) | 3833 | 2160 | 2920 | 3173 |
| Total bilirubin (0.2-1.1, mg/dL) | 0.2 | 0.25 | 0.22 | 0.23 |
| Direct bilirubin (0-0.3, mg/dL) | 0.09 | 0.07 | 0.08 | 0.16 |
| Total protein (6.6-8.7, g/dL) | 3.9 | 4.6 | 4.6 | 4.5 |
| Albumin (3.4-4.8, g/dL) | 2.4 | 3 | 2.5 | 3 |
| Prothrombin time (0.88-1.16) | 1.08 | 1.07 | 1.06 | 1.08 |
| Partial thromboplastin time (0.85-1.18) | 1.01 | 1 | 1.03 | 1.19 |
| C3 (90-180) | 20 | 56 | 59 | 99 |
| C4 (10-40) | 0 | 1 | 3 | 2 |
| Cryoglobulins | Present | Absent | Absent | Present |
| Leucocytes (4.8-10.8, 103/mmc) | 10670 | 3440 | 3400 | 3730 |
| Hemoglobin (12-16, g/dL) | 10.8 | 9.7 | 10.2 | 10.5 |
| Platelets (130-400, 103/mmc) | 313000 | 159000 | 153000 | 73000 |
We report here our experience on rituximab use in two patients with progressive kidney failure due to primary proliferative GN. Both of them received conventional immunosuppressive therapy with limited benefit on urinary and biochemical abnormalities; then, they underwent one RTX pulse but developed sepsis over the follow-up. A brief review on the safety and efficacy of rituximab for primary GN has been also added.
A 51-year-old Caucasian male patient was admitted to hospital for two-week’s duration of abdominal pain with vomiting and diarrhoea. His medical history included arterial hypertension and symptomatic hepatitis C virus (HCV)-negative mixed cryoglobulinemia (since three years) with recurrent purpura and peripheral neuropathy at the lower extremities. Skin biopsy had shown leukocytoclastic vasculitis whereas neurological evaluation had revealed mono-neuritis at the left foot with axonal ischemic damage, probably related to cryoprecipitable immune complexes in the vasa nervorum. He had received low dose oral corticosteroids and azathioprine with partial control of cutaneous and neurological abnormalities. A bone marrow biopsy had reported no evidence of malignant lymphoma, and a small expansion of B lymphocytes (10%-15%).
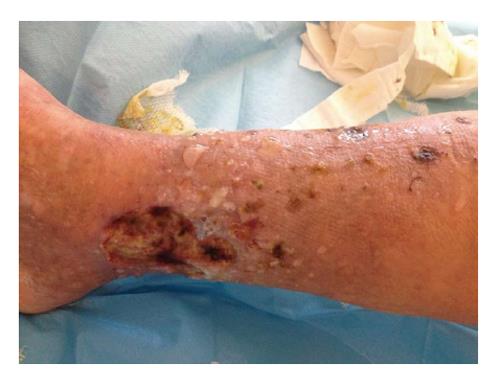
A physical examination showed bilateral edema and hypertension (180/100 mmHg), purpuric rash with ulcers at the legs (Figure 1); no bowel movements were apparent from the clinical standpoint, this being confirmed by an abdomen X-ray. An ultrasound scan of the abdomen showed normal sized kidneys bilaterally, with normal echotexture. At presentation (Table 1), abnormal laboratory results included serum creatinine level of 2.5 mg/dL, proteinuria, 3.6 g/24 h, and hypoalbuminemia (2 g/L). Other pertinent chemistries were: positive cryoglobulins, with a cryocrit of 3% (polyclonal IgG and monoclonal IgMk), elevated rheumatoid factor (148 IU/mL) and hypocomplementemia. Serology was negative for hepatitis B virus (HBV), HCV and human immuno-deficiency virus (HIV) markers, polymerase chain reaction tested negative for HCV RNA. Repeat urine sediment, analyzed by phase-contrast microscopy, showed severe microscopic hematuria (> 50 erythrocytes/microscopic field), many dysmorphic erythrocytes and casts (ialine, granular and red cell casts). Bence Jones proteinuria (kappa type) was positive. The search for anti-neutrophil cytoplasmic antibody (proteinase 3 and myeloperoxidase), anti-glomerular basement membrane antibody, extractable nuclear antigen antibody, antinuclear and anti-double stranded DNA tested negative.
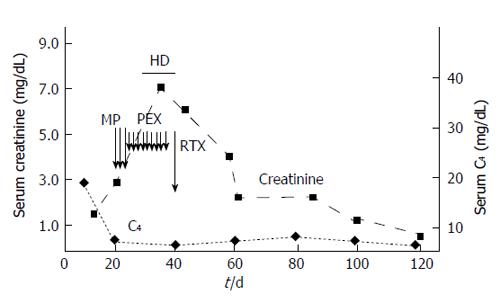
Renal biopsy was not performed due to anatomic reasons, and a diagnosis of essential MC with rapidly progressive renal failure due to nephritic/nephrotic syndrome was made. Treatment was initiated with intravenous methylprednisolone (600 mg/d for three days), oral prednisone (50 mg/d on taper), and oral cyclophosphamide (100 mg daily). The progressive deterioration of kidney function (serum creatinine raised to 6.3 mg/dL, blood urea nitrogen to 279 mg/L) led us to make sequential plasma-exchange (nine sessions) and high-dose intravenous immunoglobulins (five procedures); in addition, hemodialysis was started. We observed healing of skin ulcers and improvement of neuropathic pain; GI disorders disappeared but immunosuppressive therapy was complicated by Clostridium Difficile-positive diarrhea, which was successfully treated with oral vancocine. Due to the persistence of severe renal failure (serum creatinine of 4.2 mg/dL), he received one infusion of RTX (375 mg/m2) off-label (Figure 2); by day 6 of the RTX dose, an improvement of urine output occurred. Since then, serum creatinine went back to 1.6 mg/dL, and blood urea nitrogen to 104 mg/dL. At discharge from the hospital, his medications included oral steroids, rabeprazole, amlodipine, furosemide, calcium carbonate, gabapentine, and darboepoetin.
Two weeks after hospital discharge, serum creatinine was 1.76 mg/dL, blood urea nitrogen, 86 mg/dL, serum albumin 2.5 g/dL; severe and dysmorphic hematuria and non-nephrotic proteinuria (1.43 g/L) being still present. Low white blood cell count after RTX administration (3.4 × 103/mmc) occurred. According to flow cytometry, CD20+ B cells were 19% of total peripheral blood lymphocytes (before RTX) and fell to 1% (after RTX, since day 6) with no increase over the following weeks.
One month later, he became pyrexial with a temperature around 38 °C and was admitted again to the hospital. Pertinent biochemistry included serum creatinine (0.8 mg/dL), cryoglobulins and rheumatoid factor tested positive. Complete blood count gave the following features: white blood cells 2.67 × 103/mmc, erythrocytes 3.57 × 106/mmc, platelets 209 × 103/mmc. Flow cytometry: lymphocytes 169/mm3, CD3+ cells 142 (83.9%), CD19+ 0 (0%), natural killer cells 25 (14.9%). Gamma globulins were 0.16 g/dL (3.9%, 11%-18.8%). IgA < 4 mg/dL (70-400), IgG 84 mg/dL (700-1600), IgM 108 mg/dL (40-230). Monoclonal component by serum electrophoresis (IgMk + oligoclonal Ig) was again detected. An active urinary sediment with non-nephrotic proteinuria (1.21 g/d) was still present. Sepsis from Enterococcus Spp. was identified whereas the chest radiograph reported multiple pneumoniae. The culture of the broncho-alveolare lavage fluid was positive for Candida albicans, thus, we initiated intravenous imipenem and antifungal medications. Medical and supportive therapy was unsuccessful and the patient ultimately expired due to septic shock (two months after RTX pulse).
A 49-year-old Caucasian male underwent kidney biopsy for evaluation of serum creatinine 1.35 mg/dL [estimated glomerular filtration rate (eGFR) 56 mL/min per 1.73 m2 by MDRD equation], 3.2 g of proteinuria on 24-h urine collection and active urinary sediment (severe microscopic hematuria with red blood cell casts). Renal biopsy showed global ialinosis in some glomeruli (5 out of 14); the others had intense glomerular hypercellularity (mainly due to mesangial proliferation), a limited number of mesangial immune deposits and segmental thickening of glomerular basement membrane were also present. Final diagnosis was mesangial proliferative GN with immune deposits of unclear significance. Other pertinent chemistries were: negative cryoglobulins, normal rheumatoid factor and complement fractions; serum protein electrophoresis in the normal range. Serology tested negative for HBV, HCV and HIV markers, polymerase chain reaction was negative for HCV RNA. The search for anti-neutrophil cytoplasmic antibody (proteinase 3 and myeloperoxidase), anti-glomerular basement membrane antibody, extractable nuclear antigen antibody, antinuclear and anti-double stranded DNA tested negative. At discharge from the hospital, his medications included oral steroids, rabeprazole, amlodipine, doxazosine, furosemide, calcium carbonate, and darboepoetin. Partial remission of nephritic/nephrotic syndrome with improvement of kidney function (serum creatinine going back to 1.1 mg/dL) was obtained with intravenous methylprednisolone pulses, oral cyclophosphamide, azathioprine, and mycophenolate mofetil in variable associations. Eight years later, he was again admitted to our unit, showing bilateral lower-extremity edema, arterial hypertension and serum creatinine of 2.9 mg/dL (eGFR 23 mL/min per 1.73 m2 by MDRD equation). Nephrotic proteinuria was demonstrated (proteinuria of 9.2 g/d) with active urinary sediment. A repeat kidney biopsy revealed intracapillary/extracapillary glomerular proliferation with several crescents and fibrinoid necrosis, diffuse arteriolosclerosis, in addition to uniform and diffuse thickening of the glomerular basement membrane. Immunofluorescence demonstrated sporadic and granular deposition of C1q/C3 in the mesangium and capillary walls; fibrinogen in the Bowman space. Renal biopsy was complicated by perirenal hematoma and a few units of red packed cells were given. He received high-dose intravenous diuretic therapy with edema resolution and body weight loss, and intravenous methylprednisolone pulse therapy (500 mg daily intravenous for three alternate days) with low-dose oral steroids was not effective. Thus, one infusion of RTX (375 mg/m2) off-label was administered. One month after rituximab administration he was again hospitalized (acute pulmonary insufficiency with septic shock); serum creatinine of 4.56 mg/dL (eGFR, 11 mL/min per 1.73 m2). Active urinary sediment and nephrotic proteinuria persisted. Pulmonary aspergillosis was documented- medical plus supportive therapy was initiated and the patient recovered in a few weeks; however, he developed irreversible kidney failure and initiated dialysis acutely. He is currently doing well on maintenance hemodialysis (thrice weekly) treatment.
We report here on two patients with rapidly progressive renal failure due to idiopathic proliferative GN who were resistant to conventional immunosuppressive therapy. Both the patients underwent rituximab treatment in off-label condition, RTX infusion was well tolerated by both the patients but sepsis developed over the follow-up, fatal course occurring in patient 1. Numerous case reports and case series have suggested that the addition of rituximab to standard chemotherapy for malignant lymphoma increases the risk of viral infections such as varicella zoster[9], cytomegalovirus[10], HBV[11], parvovirus[12], and enteroviral encephalitis[13]. The risk of HBV reactivation has been added to the existing Boxed Warning of the rituximab label by the Food and Drug Administration in 2013[14]. Impaired immunity against non-viral pathogen agents such as Pneumocystis jirovecii[15] or cryptococcus[16] after rituximab therapy has been also noted.
A recent systematic review and meta-analysis has shown that rituximab plus standard chemotherapy for malignant lymphoma increases the incidence of severe leucopenia (RR = 1.24; 95%CI: 1.12-1.37) and granulocytopenia (RR = 1.07; 95%CI: 1.02-1.12) even if the overall risk of severe infections has not been increased (RR = 1.0; 95%CI: 0.87-1.14)[17]. We have already reported on a case of cholestatic hepatitis C after rituximab therapy for gastric cancer in a renal transplant recipient[18]. On the other hand, various authors have claimed that rituximab use for glomerular diseases is effective and has minimal adverse effects (Table 2)[1,19,20].
| Ref. | n | Rituximab treatment dose | Follow-up period | Concomitant therapy | Response to RTX | Side-effects after RTX |
| Fervenza et al[2] | 15 | 1 g × 2, on days 1 and 15 | 12 mo | ACE-I + ARB | Complete (n = 2) or partial remission (n = 6) | Nonserious transient AE (n = 10) pneumonia (n = 1) |
| Segarra [3] | 13 | 375 mg/m2 once weekly × 4 | 30 mo | Tac (n = 10), CyA (n = 3), CCS (n = 3) | Partial remission (n = 13) | None |
| Fervenza et al[4] | 20 | 375 mg/m2 once weekly × 4 | 24 mo | ACE-I + ARB | Complete (n = 4) or partial remission (n = 12) | Nonserious transient AE (n = 11) pneumonia (n = 1) |
| Michel et al[5] | 28 | 375 mg/m2 once weekly × 2 or 3 or 4 (n = 27) 1 g × 2, on days 1 and 15 (n = 1) | 12 mo | ACE-I + ARB, CCS (n = 1), Tac (n = 1) | Complete (n = 6) or partial remission (n = 13) | Nonserious transient AE (few) |
| Ruggenenti et al[6] | 100 | 375 mg/m2 once weekly × 4 | 29 mo | CCS | Complete (n = 27) or partial remission (n = 38) | Nonserious transient AE (n = 28) |
| Dillon et al[7] | 6 | 1 g × 2, on days 1 and 15 | 12 mo | ACE-I + ARB | Complete (n = 2) or partial remission (n = 3) | None |
| Kong et al[8] | 13 | 500 mg × 1 (n = 6) | 31.5 mo | CCS (os) (n = 9) | Remission (n = 19) | Nonserious transient AE (n = 8) |
| 500 mg × 2 (n = 3) | CyA (n = 2) | Pneumonia (n = 1) | ||||
| 500 mg × 4 (n = 4) | CCS (iv) (n = 2) |
Our first patient presented idiopathic cryoglobulinemic vasculitis which has undefined therapeutic management[21]. There is some evidence on the efficacy and tolerance of RTX in patients with HCV-associated mixed cryoglobulinemia vasculitis who were naïve, resistant or intolerant to antiviral therapy[22-25]. Two randomized controlled trials have compared RTX with conventional immunosuppressive therapy for HCV-related mixed cryoglobulinemia vasculitis[26,27]. As listed in Table 3, evidence in the medical literature on RTX use among patients with non-infectious cryoglobulinemia vasculitis targeting kidneys is extremely limited, and a total of 16 cases were retrieved[28-37]. Patient 1 gives emphasis on the efficacy of RTX, as one RTX pulse made possible the control of renal disease: kidney function normalized, nephrotic syndrome disappeared and only nephritic urinary changes persisted. However, RTX use was complicated by sepsis a few weeks after RTX pulse. On the basis of the evidence reported in Table 3, severe infections after RTX treatment are not uncommon [35% (6/17)].
| Ref. | n | Age (yr)/gender | Treatment prior to RTX | Features | Response to RTX | Side-effects after RTX |
| Arzoo et al[28] | 1 | 71/F | CS | C, N, R | Remission | None |
| Ghijsels et al[29] | 1 | 44/M | CS, CPH, CHL | C, Ca, R | Remission | None |
| Koukoulaki et al[30] | 1 | 48/F | CS, CPH | GI, P, R | Partial remission | None |
| Bryce et al[31] | 1 | NA | NA | R | No response | None |
| Ruch et al[32] | 1 | 64/M | CS | R | Remission | Cold agglutinine disease, sepsis |
| Annear et al[33] | 1 | 42/F | CS | C, R | Remission | None |
| Terrier et al[34] | 7 | 73 ± 5/M (n = 4) | CS (n = 4) | C (n = 6), N (n = 2), A (n = 2), R (n = 7) | Remission (n = 3), partial remission (n = 1), NA (n = 3) | Severe infections (n = 4) |
| Wink et al[35] | 1 | 72/F | CS, Aza | C, P, R | Remission | None |
| Choudhry et al[36] | 1 | 61/F | CS, CPH | C, P, R | Remission | None |
| Kamel et al[37] | 1 | 77/F | CS | C, A, R | Remission | None |
| Own case | 1 | 51/M | CS, Aza | C, GI, N, R | Remission | Severe infection |
RTX therapy in patients with nonviral cryoglobulinemia vasculitis or membranoproliferative GN raises various questions such as the role of RTX as first-line or rescue therapy, the efficacy/safety of maintenance therapy with RTX, and the tolerance to RTX. In the absence of randomized controlled trials, such questions remain unanswered; as an example, the poor tolerance of our patients after RTX administration remains unclear. The French multicenter CryoVas survey retrospectively evaluated 242 patients with non-infectious mixed cryoglobulinemia vasculitis, RTX plus corticosteroids had greater therapeutic efficacy compared with corticosteroids alone and corticosteroids plus alkylating agents[38]. However, RTX plus corticosteroids was associated with more frequent infections than corticosteroids alone (HR = 9; 95%CI: 3.1-20, P < 0.001). Prospective data from the AIR (AutoImmunity and Rituximab) registry, which includes data on patients treated with rituximab off-label, have shown that among patients (n = 23) with nonviral cryoglobulinemia vasculitis on RTX, side-effects occurred in almost half of the patients (n = 11), including severe infections[34]. Infectious episodes were mostly reported in a patient subgroup (age > 70 years, essential type II MC, GFR < 60 mL/min, and high-dose steroids) and were fatal in many (n = 3)[34]. Both our patients had important kidney impairment at baseline and concomitant therapy with intravenous high-dose corticosteroids, among other immunosuppressive agents.
The current study calls for further research on the RTX-based treatment of essential cryoglobulinemic vasculitis or membranoproliferative GN but the low frequency of patients in individual centers would make randomised controlled trials extremely difficult. Rituximab has surfaced as potential treatment option for some primary glomerular diseases and the HCV KDIGO Study Group[39] had already included rituximab among the recommended drugs (steroids, and cyclophosphamide) for the immunosuppressive treatment of HCV-associated kidney disease. The risks (and the predictive factors) of infections in kidney patients on RTX-therapy are not yet understood and are an area of active research. These patients should be monitored over the follow-up to avoid the occurrence of infectious episodes.
Two male Caucasian patients with progressive kidney failure.
Arterial hypertension, bilateral lower-extremity edema.
Progressive kidney failure due to secondary glomerular disease.
At presentation serum creatinine ranged between 2.5 and 2.9 mg/dL and proteinuria 3.6 and 9.2 g/d, microscopic haematuria with dysmorphic erythrocytes and red cell casts.
Computed tomography scan revealed normal sized kidneys bilaterally with normal echotexture in both the patients.
Renal biopsy (patient 2) showed intracapillary/extracapillary glomerular proliferation with several crescents and fibrinoid necrosis, in addition to uniform diffuse thickening of the glomerular basement membrane.
Both the patients received one infusion of rituximab (375 mg/m2) off-label.
Various authors have claimed that rituximab use for glomerular diseases is effective and has minimal adverse effects.
Phase-contrast microscopy is a microscopy technique to analyze the morphology of urine erythrocytes.
The risks and the predictive factors of severe infections in kidney patients on rituximab therapy are still unclear and appear an area of active research.
It is a good article.
P- Reviewer: Fujita T S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Manrique J, Cravedi P. Role of monoclonal antibodies in the treatment of immune-mediated glomerular diseases. Nefrologia. 2014;34:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Segarra A, Praga M, Ramos N, Polanco N, Cargol I, Gutierrez-Solis E, Gomez MR, Montoro B, Camps J. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin J Am Soc Nephrol. 2009;4:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Michel PA, Dahan K, Ancel PY, Plaisier E, Mojaat R, De Seigneux S, Daugas E, Matignon M, Mesnard L, Karras A. Rituximab treatment for membranous nephropathy: a French clinical and serological retrospective study of 28 patients. Nephron Extra. 2011;1:251-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Dillon JJ, Hladunewich M, Haley WE, Reich HN, Cattran DC, Fervenza FC. Rituximab therapy for Type I membranoproliferative glomerulonephritis. Clin Nephrol. 2012;77:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kong WY, Swaminathan R, Irish A. Our experience with rituximab therapy for adult-onset primary glomerulonephritis and review of literature. Int Urol Nephrol. 2013;45:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Bermúdez A, Marco F, Conde E, Mazo E, Recio M, Zubizarreta A. Fatal visceral varicella-zoster infection following rituximab and chemotherapy treatment in a patient with follicular lymphoma. Haematologica. 2000;85:894-895. [PubMed] |
| 10. | Suzan F, Ammor M, Ribrag V. Fatal reactivation of cytomegalovirus infection after use of rituximab for a post-transplantation lymphoproliferative disorder. N Engl J Med. 2001;345:1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001;344:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Song KW, Mollee P, Patterson B, Brien W, Crump M. Pure red cell aplasia due to parvovirus following treatment with CHOP and rituximab for B-cell lymphoma. Br J Haematol. 2002;119:125-127. [PubMed] |
| 13. | Quartier P, Tournilhac O, Archimbaud C, Lazaro L, Chaleteix C, Millet P, Peigue-Lafeuille H, Blanche S, Fischer A, Casanova JL. Enteroviral meningoencephalitis after anti-CD20 (rituximab) treatment. Clin Infect Dis. 2003;36:e47-e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | US Food and Drug Administration. FDA Drug Safety Communication: Boxed Warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab). Available from: http://www.fda.gov/drugs/drugsafety/ucm366406.htm. |
| 15. | Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, Ikeda K, Tanimoto M, Hatake K. Increased incidence of interstitial pneumonia by CHOP combined with rituximab. Int J Hematol. 2008;87:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Basse G, Ribes D, Kamar N, Mehrenberger M, Sallusto F, Esposito L, Guitard J, Lavayssière L, Oksman F, Durand D. Rituximab therapy for mixed cryoglobulinemia in seven renal transplant patients. Transplant Proc. 2006;38:2308-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lanini S, Molloy AC, Fine PE, Prentice AG, Ippolito G, Kibbler CC. Risk of infection in patients with lymphoma receiving rituximab: systematic review and meta-analysis. BMC Med. 2011;9:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Fabrizi F, Martin P, Elli A, Montagnino G, Banfi G, Passerini P, Campise MR, Tarantino A, Ponticelli C. Hepatitis C virus infection and rituximab therapy after renal transplantation. Int J Artif Organs. 2007;30:445-449. [PubMed] |
| 19. | Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009;4:734-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Tanna A, Tam FW, Pusey CD. B-cell-targeted therapy in adult glomerulonephritis. Expert Opin Biol Ther. 2013;13:1691-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Zaja F, De Vita S, Mazzaro C, Sacco S, Damiani D, De Marchi G, Michelutti A, Baccarani M, Fanin R, Ferraccioli G. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003;101:3827-3834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Sansonno D, De Re V, Lauletta G, Tucci FA, Boiocchi M, Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti-CD20. Blood. 2003;101:3818-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Roccatello D, Baldovino S, Rossi D, Giachino O, Mansouri M, Naretto C, Di Simone D, Francica S, Cavallo R, Alpa M. Rituximab as a therapeutic tool in severe mixed cryoglobulinemia. Clin Rev Allergy Immunol. 2008;34:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Terrier B, Saadoun D, Sène D, Sellam J, Pérard L, Coppéré B, Karras A, Blanc F, Buchler M, Plaisier E. Efficacy and tolerability of rituximab with or without PEGylated interferon alfa-2b plus ribavirin in severe hepatitis C virus-related vasculitis: a long-term followup study of thirty-two patients. Arthritis Rheum. 2009;60:2531-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Arzoo K, Sadeghi S, Liebman HA. Treatment of refractory antibody mediated autoimmune disorders with an anti-CD20 monoclonal antibody (rituximab). Ann Rheum Dis. 2002;61:922-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Ghijsels E, Lerut E, Vanrenterghem Y, Kuypers D. Anti-CD20 monoclonal antibody (rituximab) treatment for hepatitis C-negative therapy-resistant essential mixed cryoglobulinemia with renal and cardiac failure. Am J Kidney Dis. 2004;43:e34-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Koukoulaki M, Abeygunasekara SC, Smith KG, Jayne DR. Remission of refractory hepatitis C-negative cryoglobulinaemic vasculitis after rituximab and infliximab. Nephrol Dial Transplant. 2005;20:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Bryce AH, Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Inwards DJ, Yasenchak CA, Kumar SK, Gertz MA. Response to rituximab in patients with type II cryoglobulinemia. Clin Lymphoma Myeloma. 2006;7:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Ruch J, McMahon B, Ramsey G, Kwaan HC. Catastrophic multiple organ ischemia due to an anti-Pr cold agglutinin developing in a patient with mixed cryoglobulinemia after treatment with rituximab. Am J Hematol. 2009;84:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Annear NM, Cook HT, Atkins M, Pusey CD, Salama AD. Non-hepatitis virus associated mixed essential cryoglobulinemia. Kidney Int. 2010;77:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Terrier B, Launay D, Kaplanski G, Hot A, Larroche C, Cathébras P, Combe B, de Jaureguiberry JP, Meyer O, Schaeverbeke T. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res (Hoboken). 2010;62:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Wink F, Houtman PM, Jansen TL. Rituximab in cryoglobulinaemic vasculitis, evidence for its effectivity: a case report and review of literature. Clin Rheumatol. 2011;30:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Choudhry M, Rao N, Juneja R. Successful treatment of cryoglobulinaemia with rituximab. Case Rep Nephrol Urol. 2012;2:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Kamel M, Thajudeen B, Bracamonte E, Madhrira M. Idiopathic Nonviral Cryoglobulinemia Treated Successfully With Rituximab. Am J Ther. 2014;Epub ahead of print. [PubMed] |
| 38. | Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, de Saint-Martin L, Quemeneur T, Huart A, Bonnet F. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. 2012;119:5996-6004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;S1-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |