Published online Mar 16, 2015. doi: 10.12998/wjcc.v3.i3.210
Peer-review started: August 29, 2014
First decision: October 14, 2014
Revised: November 1, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: March 16, 2015
Processing time: 196 Days and 9.2 Hours
Hepatitis C virus (HCV) genotypes 4, 5 and 6 are mainly present in Africa, the Middle East and Asia and they have been less extensively studied with respect to epidemiology, natural disease history and therapeutic endpoints. Response rates to a 48-wk combined peginterferon/ribavirin treatment range to 40%-69% for HCV 4, 55%-60% for HCV 5 and 60%-90% for HCV 6. Response-guided schedules are recommended to optimize the outcomes of peginterferon/ribavirin treatment in HCV 4 and, in form of preliminary data, for HCV 6, but no data are yet available to support such an individualization of therapy for HCV 5. Recently, the direct-acting antivirals (DAAs) with pan-genotypic activities simeprevir, sofosbuvir and daclatasvir have been recommended in triple regimens with peginterferon/ribavirin for the treatment of HCV genotypes 4 to 6 infections. In the future, DAA-based interferon-free therapies are awaited to drastically improve treatment outcomes in HCV. However, efforts to improve treatment outcomes with peginterferon/ribavirin should continue, as the HCV 4-6 infected population is mainly based in resource-limited settings with restricted access to the costly DAAs.
Core tip: Hepatitis C virus (HCV) 4, 5 and 6 are lesser known genotypes mainly encountered in Africa, the Middle East and Asia. Studies, mostly retrospective, have reported response rates to a 48-wk peginterferon/ribavirin combination ranging to 40%-69% for HCV-4, 55%-60% for HCV-5 and 60%-90% for HCV-6. Increasing evidence has supported a response-guided approach for HCV-4, whereas no robust data are yet available concerning tailoring of treatment duration for HCV-5 and HCV-6. Direct-acting antivirals may significantly improve treatment outcomes in HCV, but use of these agents in countries endemic for HCV 4-6 is currently precluded by the very high costs.
- Citation: Papastergiou V, Karatapanis S. Current status and emerging challenges in the treatment of hepatitis C virus genotypes 4 to 6. World J Clin Cases 2015; 3(3): 210-220
- URL: https://www.wjgnet.com/2307-8960/full/v3/i3/210.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i3.210
Hepatitis C virus (HCV) remains a major health problem worldwide with over 170 million persons chronically infected and a burden of 300000 deaths annually[1,2]. Phylogenetic analyses of viral genomic sequences have identified at least 6 major HCV genotypes (and more than 70 subtypes), each with a distinct geographical distribution and sensitivity to antiviral treatment[3,4]. HCV 1, 2 and 3 are widely disseminated genotypes and have been thoroughly assessed with regard to epidemiology, natural disease history and treatment outcomes. Conversely, HCV 4, 5 and 6 have a restricted geographical distribution, mainly based in countries with limited resources and research facilities, thus epidemiological reports and treatment advances for these genotypes have been generally deficient. Prevalence of HCV genotypes 4 to 6 across different countries is summarized in Table 1. Genotype 4 is encountered throughout Middle East and Africa[5-12], whereas a spread of the infection has been described in other countries[13-15], particularly in Southern Europe[16-20]. HCV 5 is rare outside South Africa[21-23], but its sporadic presence has been reported in different parts of the world[11,15,24-28], including a pocket of the infection in Southeast Greece[29]. Lastly, HCV 6 and its subtypes are found mainly in Asia[30-35]. Crucially, due to the phenomenon of globalization, the prevalence of the HCV 4 to 6 genotypes outside of these “typical” areas is awaited to increase in forthcoming years.
| Genotype 4 | Genotype 5 | Genotype 6 | ||||
| Country | Prevalence | Country | Prevalence | Country | Prevalence | |
| Africa | Egypt[10] | 91% | South Africa[22,23] | 40% | ||
| Gabon[12] | 71% | |||||
| Cameroon[7] | 76% | |||||
| Nigeria[8] | 60% | |||||
| Middle East | Saudi Arabia[11] | 60% | Syria[25] | 10% | ||
| Lebanon[9] | 30% | Saudi Arabia[11] | 1% | |||
| Syria[5] | 30% | |||||
| Iraq[6] | 35.40% | |||||
| Asia | China[33] | 0-1.7% | Hong Kong[33-35] | 10%-30% | ||
| Vietnam[30] | 14% | |||||
| South Korea[31] | 1.40% | |||||
| China[32,35] | 0-50% | |||||
| Europe | France[15] | 4%-10% | France[15,26] | 3%-14.2% | ||
| Spain[17,20] | 1.4%-14% | Belgium[28] | 1%-5% | |||
| Italy[16,19] | 1.4%-3.1% | Spain[27] | 0-10.3% | |||
| Greece[18] | 13.2%-15.2% | Italy[24] | 0-0.1% | |||
| Greece[18,29] | 0.4%-1.9% | |||||
| America | United States[13,14] | 0-2% | ||||
During the past decade, a dual combination of pegylated interferon (PegIFN) and ribavirin (RBV) has represented the standard of care (SOC) for treating chronic hepatitis C (CHC). In 2011, introduction of first generation direct-acting antivirals (DAAs), the NS3/4A protease inhibitors (PIs) boceprevir and telaprevir, has boosted rates of sustained viral response (SVR; i.e., negative HCV-RNA at 6 mo or more after cessation of treatment) in both naïve and treatment-experienced patients, although this only regarded the most difficult-to-treat CHC genotype 1[36]. Latter, in 2013, approval of the second generation DAA, NS5B polymerase inhibitor (PI) sofosbuvir, has been a further step forward due to its pangenotypic effect on HCV, better pharmacokinetics and improved resistance profiles[37]. In the light of the rapidly changing paradigm of treating CHC, new drugs were recently approved or await approval. However, in the era of DAAs, optimal treatment of HCV genotypes 4 to 6 remains, more than ever before, to be defined. Indeed, most treatment data rely on retrospective studies, extrapolations using other HCV genotypes as reference, and expert opinions.
Herein, we aimed to a concise overview on the treatment of HCV 4 to 6, including recent proposals for a response-guided treatment approach as well as the available data and future perspectives on the use of DAAs with respect to these lesser known HCV genotypes.
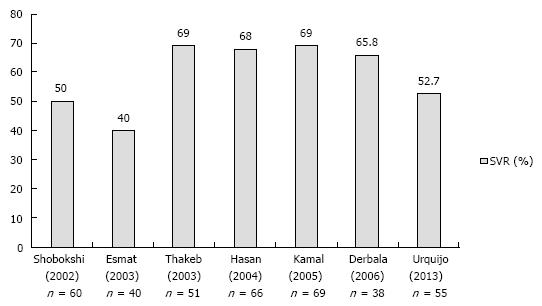
Hepatitis C virus genotype 4: HCV 4 has been traditionally considered a difficult to treat genotype, mainly because of the disappointing SVR rates (5%-25%) obtained in the early clinical trials using conventional interferon monotherapy[38,39]. Later introduction of RBV, used in conjunction with PegIFN, has significantly increased the efficacy of treatment, although response rates were still lower as compared to genotype 2 and 3 patients. Figure 1 summarizes results of prospective studies evaluating a fixed 48-wk treatment using standard-dose PEGIFN and RBV (PegIFNα-2a 180 μg or PegIFNα-2b 1.5 mg/kg and RBV 1-1.2 g/d) in HCV 4[40-46]. Overall, SVR rates ranged between 40% and 69%. However, a significant discrepancy could be noted between the SVR rates reported in highly endemic countries (SVR 60%-69% in studies conducted in Egypt and the Middle East)[40,42,43,47-50] and those (generally < 60%) reported in European populations infected with HCV 4; including 55% in Spain[51], 40.3% in France[52] and 43.5% in a cohort from Greece[53]. This difference has prompted the hypothesis of an impact of ethnicity on antiviral response, with 2 French analyses suggesting Egyptian (vs European) origin as a favorable prognostic indicator for SVR[52,54]. However, no solid pathogenetic basis has been provided for this phenomenon, although genetic or immunological ethnic-specific differences have been put forward[55,56]. Unlike the French observations, we could not identify any influence of Greek (n = 101) vs Egyptian (n = 76) origin on treatment outcomes[57], and only age ≥ 45 years [odds ratio (OR) = 0.42, P = 0.01), presence of diabetes (OR = 0.23, P = 0.007), advanced liver fibrosis (Metavir F3-F4; OR = 0.39, P = 0.01) and treatment suspension (OR = 0.17, P = 0.007) were independent negative associations, in line with previous studies assessing predictors of response in HCV 4[40,42,43,52,54,58-63] (Table 2). The importance of metabolic factors has been highlighted by the observation of a beneficial effect of using an insulin-sensitizing agent, such as pioglitazone, in conjunction with antiviral treatment in patients with insulin resistance (homeostasis model assessment index > 2)[64]. Congruently, presence of hepatic steatosis, known to be a poor predictor of treatment in CHC, has been linked to host metabolic factors in HCV 4 rather than to a direct viral steatogenic effect as in the case of HCV 3 infection[65,66]. Other host factors, including the IL-28B TT genotype and high values of the interferon-c inducible protein 10 (IP-10) have been associated with a poor therapeutic outcome[59]. Interestingly, Boglione et al[67] have recently proposed use of the IL-28B polymorphisms upstream as a genuine basis for the identification of patients unlikely to respond to standard dual therapy, and thus candidates for a DAA regimen. This individualized approach merit further robust assessment.
Definition of the optimal treatment duration is paramount to reduce costs and improve treatment tolerability without compromising the therapeutic efficacy. Due to the consistently lower response rates with 24 wk of therapy, a 48-wk treatment duration has been recommended as the SOC for genotype 4, similar to genotype 1[43,48,68]. A further refinement has been use of early viral responses to allow for shorter treatment durations in highly responsive patients (i.e., response-guided approach). In a double-blind randomized study, Kamal et al[43] showed that in patients achieving a complete early viral response (EVR; defined as a negative HCV-RNA at week 12 of treatment), the SVR rate was 86% with a 36-wk therapy and 92% with 48 wk of therapy (P = 0.8), whereas PegIFN dose reductions were significantly more common in the 48-wk group. Two randomized controlled trials, one including exclusively genotype 4[69] and one including a mixture of both genotype 1 or 4 patients[70] have assessed the utility of a response-guided tailoring of treatment. Based on the results of these studies, a 24- and 36-wk treatment duration have been established as sufficient in patients with a rapid viral response (RVR, defined as negative viral load at week 4 of treatment) and EVR respectively. Contrarily, Ferenci et al[71] showed that a 72-wk extended-duration therapy may benefit slow responders; i.e., those non achieving RVR but attaining at least a partial (i.e., a ≥ 2log10 drop in serum HCV-RNA) EVR and a negative HCV-RNA at week 24. Critically, a suboptimal PegIFN alpha-2a dose (135 μg/wk after week 48) may have compromised SVR rates in this study. Patients with detectable viral load at the end of week 24 are unlike to respond to treatment, and therefore assessment based on serum HCV-RNA at this time-point may serve as a futility rule, as indicated by its 92.8% negative predictive value on SVR[53]. To date, no robust conclusions can be drawn regarding efficacy of PegIFN alpha-2a vs alpha-2b in patients infected with HCV 4[72].
Hepatitis C virus genotype 5: Mainly due to its low worldwide prevalence, HCV 5 probably represents the less studied HCV genotype with respect to therapeutic endpoints. Epidemiological reports from France, Belgium, Canada, Syria and Greece argue that patients infected with HCV 5 have specific epidemiological characteristics: they are predominantly females of advanced age and they are characterized by high baseline viremia and advanced hepatic fibrosis[25,29,73-75]. Despite presence of these classical negative predictors of treatment response, SVR rates have been reported to 55%-60%[29,74-81], although most of the therapeutic studies on HCV 5 (Table 3) have had inherent limitations, including the retrospective design, small sample and extreme heterogeneity with respect to treatment modalities[74-76,78] and patient sampling[79]. Currently, a fixed 48-wk course of combined PegIFN and RBV is recommended for patients with HCV 5. However, the intrinsic sensitivity of HCV to combined antiviral therapy, and thus the ideal treatment duration, remains controversial. In a retrospective study by Antaki et al[77], 13/26 patients were treated for 24 wk due to personal, financial or medical reasons, and no impact of treatment duration (24 wk vs 48 wk) was found on SVR. In support of a 24-wk treatment, some retrospective data[75,78] suggested that response of HCV is similar to that observed for genotypes 2 and 3, although this was disputed in more recent studies[76,79]. Clearly, extrapolations using other HCV genotypes as reference are not an appropriate basis for treatment standardization. In a prospective, open label, single-arm trial we have evaluated 27 patients with HCV 5 and the SVR was 63%, whereas non-response was mainly due to relapse (26.1%)[81]. To our knowledge this is the only prospective therapeutic trial using exclusively a combination of PegIFN and RBV and including only treatment-naïve patients. The most striking finding of our study was the excellent predictive value of early viral responses on SVR: the positive predictive value (PPV) of RVR was 93.8%, whereas the negative predictive value when not achieving EVR was 100%. Based on these data, a response-guided schedule may be a viable option for patients with HCV 5[82]. Thus, it merits appropriate consideration in future trials, possibly conducted on a multi-center basis.
| Author/Country/Year | No. patients | Regimen | SVR |
| Legrand-Abravanel/France/2004[75] | 12 | Standard/Pegylated IFN plus RBV | 63.60% |
| Delwaide/Belgium/2006[74] | 6 | Standard/Pegylated IFN plus RBV | 83% |
| Bonny/France/2006[78] | 87 | Standard/Pegylated IFN plus RBV | 60% |
| 1Antaki/Syria/2008[77] | 26 | Standard/Pegylated IFN plus RBV | 54% |
| D’Heygere/Belgium/2011[79] | 38 | Standard/Pegylated IFN plus RBV | 55.30% |
| Karatapanis/Greece/2012[29] | 10 | Pegylated IFN plus RBV | 60% |
| Antaki/Syria/2012[76] | 49 | Standard/Pegylated IFN plus RBV | 49% |
| Mauss/Germany/2012 | 24 | Pegylated IFN plus RBV | 58% |
| Papastergiou/Greece/2014[81] | 27 | Pegylated IFN plus RBV | 63% |
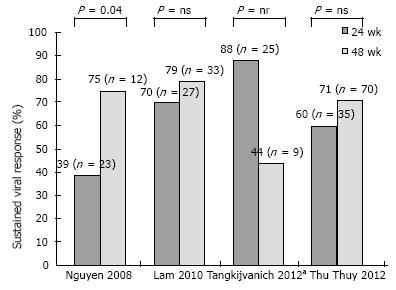
Hepatitis C virus genotype 6: Small studies, using a combination of either standard interferon[83,84] or PegIFN[85,86] and RBV have examined treatment outcomes in HCV 6. Overall, SVR rates have ranged to 60%-90%, indicating a more favorable response in comparison to HCV 1 and comparable to that of HCV 2 and 3. Crucially, a favorable IL28B status among Asians patients may have contributed significantly to these good results[87]. Apart from baseline viral load and the degree of hepatic fibrosis, which represent classical predictors of treatment response in CHC, other host factors such as age, BMI and adherence to treatment schedule have been identified as relevant in cohorts of patients with HCV 6[88]. To date, optimal treatment duration for HCV 6 has been a matter of controversy, with studies comparing a 48- vs a 24-wk regimen providing equivocal results (Figure 2)[86,89-91]. Most studies investigating therapeutic outcomes in HCV 6 have applied 48-wk treatment duration[83-85]. Moreover, in a retrospective analysis by Nguyen et al[86], the SVR rates were significantly lower with a 24-wk treatment schedule (39% vs 75%, P = 0.044). However, two randomized controlled trials including 60 and 105 patients have shown non-inferiority with a 24-wk (vs a 48-wk) regimen of PegIFN alpha/2a and RBV[89,91]. As in case of other HCV genotypes, individualization of treatment duration based on early viral dynamics may allow for reduction to drug exposure and in treatment costs. In an open-label randomized study by Thu Thuy et al[91], RVR occurred in about 80% of patients and had a 75%-86% PPV on SVR irrespective of treatment duration; nonetheless, among those with no RVR, even a 48-wk treatment achieved low SVR rates (8%). Efficacy of a response-guided approach based on RVR has been evaluated by Tangkijvanich et al[90]: in a pilot study including 34 patients with CHC genotype 6, the SVR rate in patients with RVR who underwent a 24-wk treatment was 88%. Based on these data, patients with a RVR may benefit with 24 wk of therapy. On the other hand, interruption of treatment may be the most reasonable option in patients with a detectable viral load by week 12 of therapy (non-EVR), as these patients are unlikely to respond to a 48-wk course[89,91,92] whereas there is no data to support they might benefit from longer treatment duration. Additionally to the use of early viral responses, evaluation of baseline parameters such as age, the degree of liver fibrosis, viral load and BMI may further rationalized choice of the optimum treatment duration[88]. Larger randomized trials are awaited to optimize treatment schedules for HCV 6.
First generation protease inhibitors: Telaprevir and boceprevir are first generation NS3/4 PIs firstly approved in 2011 for patients infected with HCV 1. Although both drugs are not approved for HCV 4, telaprevir has shown a modest activity against this genotype. A phase IIa trial (study C210) has assessed the activity of telaprevir on early viral dynamics[21]. Twenty four patients with HCV 4 were randomized to three groups: telaprevir alone; PegIFN plus RBV; and a triple regimen comprising telaprevir plus PegIFN/RBV. By day 15 of therapy telaprevir monotherapy induced only a 0.77 log10 decline in HCV-RNA levels (vs 4.77 log10 for HCV 1). The viral decline was more pronounced (4.32 log10) when telaprevir was administered together with PegIFN/RBV indicating a synergic effect. A descriptive subanalysis of the C210 study showed that the most frequent mutation accounting for the limited antiviral efficacy of telaprevir monotherapy was the T54A/T previously described for HCV 1[93]. Interestingly, this mutation has limited or no impact on the efficacy of subsequent treatment with dual PegIFN/RBV.
Indeed, emergence of resistant variants has generally precluded monotherapy with first generation PIs. However, use of these agents in conjunction with PegIFN/RBV still depends on interferon sensitivity, requires a high pill burden and a complex treatment algorithm. Moreover, both drugs may enhance or induce a set of considerable side effects. Anemia, neutropenia and dysgeusia are the most common side effects with boceprevir, whereas anemia, skin rash and anorectal symptoms are more frequently associated with telaprevir. Anemia, occurring in about 40%-50% of cases, may lead to discontinuation of treatment despite management with ribavirin dose adaptations, use of erythropoietin alpha or blood transfusions. Skin rush specifically related to telaprevir is generally mild and manageable using emollients and topical corticosteroids, although, in about 5% of cases, a severe life-threating cutaneous reaction may lead to treatment discontinuation[94].
Better-tolerated new generation DAAs with improved pharmacokinetics (allowing once-daily administration) and favorable resistance profiles (allowing interferon-free, all-oral regimens) were recently approved or await approval. These agents, with activities against HCV 4 to 6, will be discussed below.
Simeprevir (TMC435): It is a second generation NS3/4A PI, active against genotypes 1, 2, 4, 5 and 6. It is administered as a once-daily tablet orally and has demonstrated a favorable safety profile and limited drug-drug interactions[95]. It was approved in November 2013 by the United States Food and Drug Administration (FDA) and in Japan in September 2013.
RESTORE, a phase III, multicenter, single-arm, open-label study, conducted in France and Belgium, evaluated simeprevir (150 mg once-daily for 12 wk in combination with PegIFN/RBV, followed by 12-36 wk of PegIFN/RBV only) in 107 patients with HCV 4, either naïve or treatment-experienced[96]. Overall, SVR at week 12 was observed in 65.4% of patients, with rates being particularly high among treatment-naïve (82.9%) and prior-relapsers (86.4%). Both these patient groups were eligible to a shorter (totally 24 wk) treatment duration if they achieved a HCV-RNA < 25 IU/mL at week 4 and undetectable at week 12. These response-guide criteria were met by 88.6% of treatment-naïve and 90.9% of prior relapsers; among them 93.5% and 95% respectively achieved a SVR at week 12. Notably, none patient had a detectable Q80K substitution in the NS3 protease sequence at baseline, associated with decreased efficacy in patients with HCV 1a.
Overall, simeprevir has demonstrated favorable safety profile. By pooling data form phase III clinical trials (QUEST-1, QUEST -2 and PROMISE), discontinuation of treatment due to severe adverse events occurred in 2% of patients receiving a simeprevir plus PegIFN/RBV combination[97-99]. Rates of adverse events, most commonly fatigue, influenza-like illness, headache, nausea and pruritus, were generally similar between simeprevir/PegIFN/RBV and placebo/PegIFN/RBV groups. Incidence of photosensitivity and rush has been slightly higher with simeprevir (vs placebo), although the vast majority of cases were graded 1/2 in severity. Transient moderate bilirubin increases were noted; however, no clinically relevant cases of hepatotoxicity were recorded.
Recent European guidelines have included a 24-48 wk simeprevir plus PegIFN/RBV combination as an option for HCV 4-related compensated liver disease (including cirrhotics), suggesting interruption of treatment if HCV-RNA levels are ≥ 25 IU/mL at week 4, 12 or 24[100].
Sofosbuvir: This is a nucleotide inhibitor of NS5B, with a pan-genotypic effect activity and a high barrier to resistance. It is administered as an oral 400 mg tablet/day with no food effect, whereas it has been proven safe and well-tolerated in phase II and III clinical trial including > 2000 patients. It was approved by FDA for HCV 1 in combination with PegIFN/RBV, and in HCV 2 and 3 in interferon-free regimens in December 2013 and in Europe in January 2014.
NEUTRINO, an open-label, single-arm, phase III trial, evaluated a 12-wk regimen, comprising sofosbuvir plus PegIFN/RBV, in 327 treatment-naïve patients with genotypes 1, 4, 5 and 6[101]. However, the vast majority (89%) of the patient population had HCV 1. Overall, 27 out of the 28 (96%) patients with HCV 4, all 6 patients with HCV 6 and the single patient with HCV 5 achieved an SVR, 12 wk after the end of treatment. Currently, a sofusbuvir-based triple combination for 12 wk appears as the most efficacious and easy-to-use interferon containing option for the treatment of HCV genotypes 4 to 6, without the risk for selecting resistant variants in case of treatment failure[100]. Critically, rates of SVR were relatively lower in cirrhotics in the NEUTRINO trial (80% vs 92% in patients without cirrhosis), whereas no data with this regimen has been presented in treatment-experienced patients. Thus, it remains unknown whether longer treatment duration may be required for these more difficult-to-treat patient populations.
More recently, promising data have emerged on the efficacy of an interferon-free combination of sofosbuvir plus ribavirin in patients with HCV 4. Ruane et al[102] randomized (1:1) 60 patients of Egyptian ancestry (treatment-naïve: 28, treatment-experienced: 32; 23% cirrhotics; 17% with the IL28B CC genotype), stratified by prior treatment status and cirrhosis, to receive 12 or 24 wk of sofosbuvir (400 mg/d) plus RBV (1200 mg/d). After 12 wk of treatment, SVR rates were 11/14 (79%) in treatment-naïve and 10/17 (59%) in treatment-experience patients. However, extending the duration of treatment to 24 wk resulted in higher SVR rates in both treatment-naïve (14/14; 100%) and -experienced (13/15; 87%) groups. Thus, a dual sofosbuvir/ribavirin combination given for 24 wk is currently recommended for HCV 4 patients who are interferon-intolerant or -ineligible[100].
As evident in phase III clinical trials, sofosbuvir in combination with RBV represents a well-tolerated option with rates of treatment discontinuation as low as 1%-2%[103]. Drug-related adverse events attributable to RBV such as fatigue, insomnia and anemia were the most common, and headache was also frequent. Unsurprisingly, incidence of adverse effects commonly associated with interferon, such as influenza-like illness and depression, was significantly lower and hematological abnormalities were less prominent among patients who receive sofosbuvir/RBV than among those who receive the standard PegIFN/RBV combination[101]. Consistently, health-related quality of life and health utilities of patients have been shown to be only minimally affected by sofosbuvir/RBV irrespectively to treatment duration[104,105].
Daclatasvir: This is an HCV NS5A oral PI with a pan-genotypic activity, but a lower barrier to resistance in genotype 1a[106]. A recent phase IIb double-blind, placebo-controlled study evaluated a triple combination comprising daclatasvir plus PegIFN/RBV including treatment-naïve patients with HCV 1 (n = 365) or 4 (n = 30)[107]. Patients were randomly assigned (2:2:1) to daclatasvir 20 mg or 60 mg, or placebo once daily plus PegIFN/RBV. Overall, SVR rates (week 24 post-treatment) were 8/12 (66.7%) in HCV 4 patients receiving 20 mg, 12/12 (100%) in those receiving 60 mg and 3/6 (50%) in patients receiving placebo. Patients on daclatasvir did not have adverse events beyond those typical of PegIFN/RBV.
Based on this preliminary data, a daclatasvir (dose: 60 mg/d) plus PegIFN/RBV regimen has been included as an option for the treatment of genotype 1b and 4 patients[100]. The triple combination should be administered for 12 wk. In those who do not achieve an HCV-RNA level < 25 IU/mL at week 4 and undetectable at week 10, all three drugs should be continued for an additional 12 seeks. Conversely, PegIFN/RBV should be continued alone between week 12 and 24 in those who achieve such response[100].
Other DAAs evaluated in HCV 4: Phase II trials have evaluated other triple or quadruple drug combinations in patients infected with HCV 4. In the DAUPHINE trial, different dosing schedules of danoprevir boosted with ritonavir plus PegIFN/RBV for 12-24 wk achieved up to 100% of SVR in treatment-naïve patients with HCV 4[108]. In yet another phase IIb study, 25 HCV-4 patients were assigned to asunaprevir 200 mg or placebo twice daily plus PegIFN/RBV; the SVR rates were 89% in those receiving asunaprevir vs 43% in the placebo group[109]. Lastly, two randomized placebo-controlled trials have evaluated use of mericitabine in combination with PegIFN/RBV including patients infected with HCV 4[110,111]. In the JUMP-C trial, a 24-wk response-guided combination of mericitabine 1000 mg twice daily plus PegIFN/RBV was well-tolerated and more effective than a standard 48-wk PegIFN/RBV combination[110].
While standardization of dual PegIFN/RBV regimens for HCV 4 to 6 is still pending, interferon-based treatment of HCV has been superseded by the introduction of oral DAAs with pan-genotypic activities. These agents are characterized by improved antiviral efficacy and offer the perspective for short-course, all-oral and interferon-free therapies. However, as it is reasonable, most trials evaluating DAAs have focused on the more prevalent and difficult to treat HCV genotype 1. Given obvious difficulties in patient sampling, multi-centric efforts may be necessary to assess therapeutic sensitivity, optimize DAA schedules and establish cost-effective response-guided approaches for HCV 4 to 6. Crucially, the very high cost of HCV DAAs is a central barrier to their widespread use, thus interferon-based treatments are likely to continue to have a role as cost-containing options in low- or lower-middle income countries. This is particularly relevant in the case of genotypes 4 to 6 which are mainly based in resource-limited countries; but with large HCV epidemics, hence these genotypes represent > 20% of the global HCV burden. Therefore, unless low-cost DAAs become available, a large population of untreated patients will continue to spread HCV in these countries and worldwide. Furthermore, treatment with DAAs of special patient population (e.g., patients with kidney disease, HIV coinfection, patients undergoing solid organ transplantation) remains a challenge, as few or no data are available.
In conclusion, it seems we need to wait for a while until arrangement of both practical and logistic issues will allow for low-cost, all-oral and interferon-free regimens, at a level to dramatically change the global epidemics of HCV 4 to 6. Until then, efforts to further rationalize the use of the traditional PegIFN/RBV treatment should continue.
P- Reviewer: Cho SY, Fourtounas C S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 939] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 2. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [PubMed] [DOI] [Full Text] |
| 3. | McOmish F, Yap PL, Dow BC, Follett EA, Seed C, Keller AJ, Cobain TJ, Krusius T, Kolho E, Naukkarinen R. Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative survey. J Clin Microbiol. 1994;32:884-892. [PubMed] |
| 4. | Robertson B, Myers G, Howard C, Brettin T, Bukh J, Gaschen B, Gojobori T, Maertens G, Mizokami M, Nainan O. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. International Committee on Virus Taxonomy. Arch Virol. 1998;143:2493-2503. [PubMed] |
| 5. | Abdulkarim AS, Zein NN, Germer JJ, Kolbert CP, Kabbani L, Krajnik KL, Hola A, Agha MN, Tourogman M, Persing DH. Hepatitis C virus genotypes and hepatitis G virus in hemodialysis patients from Syria: identification of two novel hepatitis C virus subtypes. Am J Trop Med Hyg. 1998;59:571-576. [PubMed] |
| 6. | Al-Kubaisy WA, Niazi AD, Kubba K. History of miscarriage as a risk factor for hepatitis C virus infection in pregnant Iraqi women. East Mediterr Health J. 2002;8:239-244. [PubMed] |
| 7. | Njouom R, Pasquier C, Ayouba A, Gessain A, Froment A, Mfoupouendoun J, Pouillot R, Dubois M, Sandres-Sauné K, Thonnon J. High rate of hepatitis C virus infection and predominance of genotype 4 among elderly inhabitants of a remote village of the rain forest of South Cameroon. J Med Virol. 2003;71:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Oni AO, Harrison TJ. Genotypes of hepatitis C virus in Nigeria. J Med Virol. 1996;49:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Ramia S, Koussa S, Taher A, Haraki S, Klayme S, Sarkis D, Naman R. Hepatitis-C-virus genotypes and hepatitis-G-virus infection in Lebanese thalassaemics. Ann Trop Med Parasitol. 2002;96:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL. Genetic epidemiology of hepatitis C virus throughout egypt. J Infect Dis. 2000;182:698-707. [PubMed] |
| 11. | Shobokshi OA, Serebour FE, Skakni L, Al-Saffy YH, Ahdal MN. Hepatitis C genotypes and subtypes in Saudi Arabia. J Med Virol. 1999;58:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Xu LZ, Larzul D, Delaporte E, Bréchot C, Kremsdorf D. Hepatitis C virus genotype 4 is highly prevalent in central Africa (Gabon). J Gen Virol. 1994;75:2393-2398. [PubMed] |
| 13. | Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1934] [Cited by in RCA: 1900] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 14. | Zein NN, Rakela J, Krawitt EL, Reddy KR, Tominaga T, Persing DH. Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Collaborative Study Group. Ann Intern Med. 1996;125:634-639. [PubMed] |
| 15. | Payan C, Roudot-Thoraval F, Marcellin P, Bled N, Duverlie G, Fouchard-Hubert I, Trimoulet P, Couzigou P, Cointe D, Chaput C. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millenium: The GEMHEP GenoCII Study. J Viral Hepat. 2005;12:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Argentini C, Dettori S, Villano U, Guadagnino V, Infantolino D, Dentico P, Coppola RC, Rapicetta M. Molecular characterisation of HCV genotype 4 isolates circulating in Italy. J Med Virol. 2000;62:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Katsoulidou A, Sypsa V, Tassopoulos NC, Boletis J, Karafoulidou A, Ketikoglou I, Tsantoulas D, Vafiadi I, Hatzis G, Skoutelis A. Molecular epidemiology of hepatitis C virus (HCV) in Greece: temporal trends in HCV genotype-specific incidence and molecular characterization of genotype 4 isolates. J Viral Hepat. 2006;13:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Matera G, Lamberti A, Quirino A, Focà D, Giancotti A, Barreca GS, Guadagnino V, Liberto MC. Changes in the prevalence of hepatitis C virus (HCV) genotype 4 in Calabria, Southern Italy. Diagn Microbiol Infect Dis. 2002;42:169-173. [PubMed] [DOI] [Full Text] |
| 20. | Sánchez-Quijano A, Abad MA, Torronteras R, Rey C, Pineda JA, Leal M, Macias J, Lissen E. Unexpected high prevalence of hepatitis C virus genotype 4 in Southern Spain. J Hepatol. 1997;27:25-29. [PubMed] [DOI] [Full Text] |
| 21. | Benhamou Y, Moussalli J, Ratziu V, Lebray P, De Backer K, De Meyer S, Ghys A, Luo D, Picchio GR, Beumont M. Telaprevir activity in treatment-naive patients infected hepatitis C virus genotype 4: a randomized trial. J Infect Dis. 2013;208:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Ohno T, Mizokami M, Tibbs CJ, Ohba K, Suzuki K, Wu RR, Nouri-Aria KT, Williams R. New genotype of hepatitis C virus in South Africa. J Med Virol. 1994;42:409-413. [PubMed] |
| 23. | Smuts HE, Kannemeyer J. Genotyping of hepatitis C virus in South Africa. J Clin Microbiol. 1995;33:1679-1681. [PubMed] |
| 24. | Ansaldi F, Bruzzone B, Salmaso S, Rota MC, Durando P, Gasparini R, Icardi G. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J Med Virol. 2005;76:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Antaki N, Haddad M, Kebbewar K, Abdelwahab J, Hamed O, Aaraj R, Alhaj N, Haffar S, Assil M, Ftayeh M. The unexpected discovery of a focus of hepatitis C virus genotype 5 in a Syrian province. Epidemiol Infect. 2009;137:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Henquell C, Cartau C, Abergel A, Laurichesse H, Regagnon C, De Champs C, Bailly JL, Peigue-Lafeuille H. High prevalence of hepatitis C virus type 5 in central France evidenced by a prospective study from 1996 to 2002. J Clin Microbiol. 2004;42:3030-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Jover R, Pérez-Serra J, de Vera F, Alamo JM, Muñoz C, Yago C, Martínez-Ramírez R, Vidal JV. Infection by genotype 5a of HCV in a district of southeast Spain. Am J Gastroenterol. 2001;96:3042-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Verbeeck J, Maes P, Lemey P, Pybus OG, Wollants E, Song E, Nevens F, Fevery J, Delport W, Van der Merwe S. Investigating the origin and spread of hepatitis C virus genotype 5a. J Virol. 2006;80:4220-4226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Karatapanis S, Tsoplou P, Papastergiou V, Vasiageorgi A, Stampori M, Saitis I, Tsitsopoulos E, Lisgos P, Skorda L, Ketikoglou I. Hepatitis C virus genotyping in Greece: unexpected high prevalence of genotype 5a in a Greek island. J Med Virol. 2012;84:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Nguyen MH, Keeffe EB. Chronic hepatitis C: genotypes 4 to 9. Clin Liver Dis. 2005;9:411-426, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Oh HB, Kim SO, Cha CH, Hong SP, Folk WR, Kim KM, Suh DJ. Identification of hepatitis C virus genotype 6 in Korean patients by analysis of 5’ untranslated region using a matrix assisted laser desorption/ionization time of flight-based assay, restriction fragment mass polymorphism. J Med Virol. 2008;80:1712-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Peng JS, Wang X, Liu MQ, Zhou DJ, Gong J, Xu HM, Chen JP, Zhu HH, Zhou W, Ho WZ. Genetic variation of hepatitis C virus in a cohort of injection heroin users in Wuhan, China. Virus Res. 2008;135:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Prescott LE, Simmonds P, Lai CL, Chan NK, Pike I, Yap PL, Lin CK. Detection and clinical features of hepatitis C virus type 6 infections in blood donors from Hong Kong. J Med Virol. 1996;50:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Wong DA, Tong LK, Lim W. High prevalence of hepatitis C virus genotype 6 among certain risk groups in Hong Kong. Eur J Epidemiol. 1998;14:421-426. [PubMed] |
| 35. | Zhang YY, Lok AS, Chan DT, Widell A. Greater diversity of hepatitis C virus genotypes found in Hong Kong than in mainland China. J Clin Microbiol. 1995;33:2931-2934. [PubMed] |
| 36. | Kronenberger B, Zeuzem S. New developments in HCV therapy. J Viral Hepat. 2012;19 Suppl 1:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Cha A, Budovich A. Sofosbuvir: a new oral once-daily agent for the treatment of hepatitis C virus infection. P T. 2014;39:345-352. [PubMed] |
| 38. | Kamal SM, Madwar MA, Peters T, Fawzy R, Rasenack J. Interferon therapy in patients with chronic hepatitis C and schistosomiasis. J Hepatol. 2000;32:172-174. [PubMed] |
| 39. | Zylberberg H, Chaix ML, Bréchot C. Infection with hepatitis C virus genotype 4 is associated with a poor response to interferon-alpha. Ann Intern Med. 2000;132:845-846. [PubMed] |
| 40. | Derbala MF, Al Kaabi SR, El Dweik NZ, Pasic F, Butt MT, Yakoob R, Al-Marri A, Amer AM, Morad N, Bener A. Treatment of hepatitis C virus genotype 4 with peginterferon alfa-2a: impact of bilharziasis and fibrosis stage. World J Gastroenterol. 2006;12:5692-5698. [PubMed] |
| 41. | Esmat G, Mohamed MK, Abdel Hamid M, Zalata K, Khattab M, El Batanony M, Abouzied AM, El Raziky M, Shaheen AM, Ismail A. The impact of steatosis on baseline characteristic and end of treatment response for chronic hepatitis (C) genotype 4 patients treated with interferon. J Hepatol. 2003;38:139. |
| 42. | Hasan F, Asker H, Al-Khaldi J, Siddique I, Al-Ajmi M, Owaid S, Varghese R, Al-Nakib B. Peginterferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C genotype 4. Am J Gastroenterol. 2004;99:1733-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Kamal SM, El Tawil AA, Nakano T, He Q, Rasenack J, Hakam SA, Saleh WA, Ismail A, Aziz AA, Madwar MA. Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut. 2005;54:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Shobokshi O, Serebour F, Skakni L, Tantawi T, Dinish M, At Quwaiz M. Efficacy of pegylated (40 KDA) IFN alfa-2a (PEGASYS) plus ribavirin in the treatment of hepatitis C genotype 4 chronic active patients in Saudi Arabia. J Hepatol. 2002;36:129. |
| 45. | Thakeb F, Omar M, Bilharz T, Awady M, Isshak S. Randomized controlled trial of peginterferon alfa- 2a plus ribavirin for chronic hepatitis C virus-genotype 4 among Egyptian patients. Hepatology. 2003;38:278A. |
| 46. | Urquijo JJ, Diago M, Boadas J, Planas R, Solá R, Del Olmo JA, Crespo J, Erdozaín JC, Antón MD, Arocena C. Safety and efficacy of treatment with pegylated interferon alpha-2a with ribavirin in chronic hepatitis C genotype 4. Ann Hepatol. 2013;12:30-35. [PubMed] |
| 47. | Diago M, Hassanein T, Rodés J, Ackrill AM, Sedarati F. Optimized virologic response in hepatitis C virus genotype 4 with peginterferon-alpha2a and ribavirin. Ann Intern Med. 2004;140:72-73. [PubMed] |
| 48. | El-Zayadi AR, Attia M, Barakat EM, Badran HM, Hamdy H, El-Tawil A, El-Nakeeb A, Selim O, Saied A. Response of hepatitis C genotype-4 naïve patients to 24 weeks of Peg-interferon-alpha2b/ribavirin or induction-dose interferon-alpha2b/ribavirin/amantadine: a non-randomized controlled study. Am J Gastroenterol. 2005;100:2447-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Khattab M, Eslam M, Sharwae MA, Shatat M, Ali A, Hamdy L. Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol. 2010;105:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Khuroo MS, Khuroo MS, Dahab ST. Meta-analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Aliment Pharmacol Ther. 2004;20:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Trapero-Marugan M, Moreno-Monteagudo JA, Garcia-Buey L, Borque MJ, Medina J, Garcia-Sanchez A, Moreno-Otero R. Clinical and pathological characteristics and response to combination therapy of genotype 4 chronic hepatitis C patients: experience from a spanish center. J Chemother. 2007;19:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Roulot D, Bourcier V, Grando V, Deny P, Baazia Y, Fontaine H, Bailly F, Castera L, De Ledinghen V, Marcellin P. Epidemiological characteristics and response to peginterferon plus ribavirin treatment of hepatitis C virus genotype 4 infection. J Viral Hepat. 2007;14:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Elefsiniotis IS, Pavlidis C, Dimitroulopoulos D, Vezali E, Mihas C, Mariolis-Sapsakos T, Koutsounas S, Paraskevas E, Saroglou G. Differential viral kinetics in treated genotype 4 chronic hepatitis C patients according to ethnicity. J Viral Hepat. 2009;16:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Moucari R, Ripault MP, Martinot-Peignoux M, Voitot H, Cardoso AC, Stern C, Boyer N, Maylin S, Nicolas-Chanoine MH, Vidaud M. Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut. 2009;58:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Taira S, Hizume M, Note I, Sugimoto J, Okita R. A template for forward planning in prostate cancer treatment: conformal irradiation with segmental intensity-modulation. Igaku Butsuri. 2003;23:59-64. [PubMed] |
| 56. | Thio CL, Thomas DL, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O’Brien SJ, Karacki P, Marti D, Astemborski J. Racial differences in HLA class II associations with hepatitis C virus outcomes. J Infect Dis. 2001;184:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Papastergiou V, Dimitroulopoulos D, Skorda L, Lisgos P, Ketikoglou I, Kostas N, Karatapanis S. Predictors of sustained virological response in Greek and Egyptian patients with hepatitis C genotype 4: does ethnicity matter? J Med Virol. 2012;84:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Asselah T, De Muynck S, Broët P, Masliah-Planchon J, Blanluet M, Bièche I, Lapalus M, Martinot-Peignoux M, Lada O, Estrabaud E. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J Hepatol. 2012;56:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 59. | Derbala M, Rizk NM, Al-Kaabi S, John A, Sharma M, El-dweik N, Yakoob R, Pasic F, Almohanadi M, Alejji K. The predictive value of IL28B rs12979860, rs11881222 and rs8099917 polymorphisms and IP-10 in the therapeutic response of Egyptian genotype 4 patients. Virology. 2013;444:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Gad RR, Males S, El Makhzangy H, Shouman S, Hasan A, Attala M, El Hoseiny M, Zalata K, Abdel-Hamid M, Fontanet A. Predictors of a sustained virological response in patients with genotype 4 chronic hepatitis C. Liver Int. 2008;28:1112-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Martín-Carbonero L, Puoti M, García-Samaniego J, De Luca A, Losada E, Quinzan G, Bruno R, Mariño A, González M, Núñez M. Response to pegylated interferon plus ribavirin in HIV-infected patients with chronic hepatitis C due to genotype 4. J Viral Hepat. 2008;15:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Kamal S, Madwar M, Bianchi L, Tawil AE, Fawzy R, Peters T, Rasenack JW. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver. 2000;20:281-289. [PubMed] |
| 63. | Antaki N, Bibert S, Kebbewar K, Asaad F, Baroudi O, Alideeb S, Hadad M, Abboud D, Sabah H, Bochud PY. IL28B polymorphisms predict response to therapy among chronic hepatitis C patients with HCV genotype 4. J Viral Hepat. 2013;20:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, Hamdy L. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 65. | Hwang SJ, Lee SD. Hepatic steatosis and hepatitis C: Still unhappy bedfellows? J Gastroenterol Hepatol. 2011;26 Suppl 1:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Tsochatzis E, Papatheodoridis GV, Manesis EK, Chrysanthos N, Kafiri G, Petraki K, Hadziyannis E, Pandelidaki H, Zafiropoulou R, Savvas S. Hepatic steatosis in genotype 4 chronic hepatitis C is mainly because of metabolic factors. Am J Gastroenterol. 2007;102:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Boglione L, Cusato J, De Nicolò A, Cariti G, Allegra S, Ghisetti V, Di Perri G, D’Avolio A. Identification of naïve HVC-4 patients who may be treated with pegylated-interferon and ribavirin according to IL28B polymorphisms. Antiviral Res. 2014;106:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 69. | Kamal SM, El Kamary SS, Shardell MD, Hashem M, Ahmed IN, Muhammadi M, Sayed K, Moustafa A, Hakem SA, Ibrahiem A. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: The role of rapid and early virologic response. Hepatology. 2007;46:1732-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Ferenci P, Laferl H, Scherzer TM, Gschwantler M, Maieron A, Brunner H, Stauber R, Bischof M, Bauer B, Datz C. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Ferenci P, Laferl H, Scherzer TM, Maieron A, Hofer H, Stauber R, Gschwantler M, Brunner H, Wenisch C, Bischof M. Peginterferon alfa-2a/ribavirin for 48 or 72 weeks in hepatitis C genotypes 1 and 4 patients with slow virologic response. Gastroenterology. 2010;138:503-512, 512.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 72. | Esmat G, El Kassas M, Hassany M, Gamil M, El Raziky M. Optimizing treatment for HCV genotype 4: PEG-IFN alfa 2a vs. PEG-IFN alfa 2b; the debate continues. Liver Int. 2014;34 Suppl 1:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Bernier L, Willems B, Delage G, Murphy DG. Identification of numerous hepatitis C virus genotypes in Montreal, Canada. J Clin Microbiol. 1996;34:2815-2818. [PubMed] |
| 74. | Delwaide J, Gerard C, Reenaers C, Vaira D, Bastens B, Bataille C, Servais B, Maes B, Belaiche J, Hepatotropes GL. Hepatitis C virus genotype 5 in southern belgium: epidemiological characteristics and response to therapy. Dig Dis Sci. 2005;50:2348-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Legrand-Abravanel F, Sandres-Sauné K, Barange K, Alric L, Moreau J, Desmorat P, Vinel JP, Izopet J. Hepatitis C virus genotype 5: epidemiological characteristics and sensitivity to combination therapy with interferon-alpha plus ribavirin. J Infect Dis. 2004;189:1397-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Antaki N, Bibert S, Kebbewar K, Asaad F, Baroudi O, Alideeb S, Hadad M, Abboud D, Sabah H, Bochud PY. IL28B polymorphisms do not predict response to therapy in chronic hepatitis C with HCV genotype 5. Gut. 2012;61:1640-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Antaki N, Hermes A, Hadad M, Ftayeh M, Antaki F, Abdo N, Kebbewar K. Efficacy of interferon plus ribavirin in the treatment of hepatitis C virus genotype 5. J Viral Hepat. 2008;15:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Bonny C, Fontaine H, Poynard T, Hézode C, Larrey D, Marcellin P, Bourlière M, Bronowicki JP, Merle P, Zarski JP. Effectiveness of interferon plus ribavirin combination in the treatment of naive patients with hepatitis C virus type 5. A French multicentre retrospective study. Aliment Pharmacol Ther. 2006;24:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | D’Heygere F, George C, Van Vlierberghe H, Decaestecker J, Nakad A, Adler M, Delwaide J, Laureys A, Nevens F. Efficacy of interferon-based antiviral therapy in patients with chronic hepatitis C infected with genotype 5: a meta-analysis of two large prospective clinical trials. J Med Virol. 2011;83:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Mauss S, Berger F, Vogel M, Pfeiffer-Vornkahl H, Alshuth U, Rockstroh JK, Niederau C, Hüppe D. Treatment results of chronic hepatitis C genotype 5 and 6 infections in Germany. Z Gastroenterol. 2012;50:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Papastergiou V, Skorda L, Lisgos P, Stampori M, Ntetskas G, Papakonstantinou L, Prodromidou K, Karatapanis S. Hepatitis C virus genotype 5: prospective evaluation of peginterferon/ribavirin treatment efficacy and predictive value of on-treatment virological responses for sustained virological response. J Clin Gastroenterol. 2014;48:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Papastergiou V, Karatapanis S. Letter: Response-guided treatment of hepatitis C virus genotype 5 may be feasible. Aliment Pharmacol Ther. 2014;39:1337-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 83. | Dev AT, McCaw R, Sundararajan V, Bowden S, Sievert W. Southeast Asian patients with chronic hepatitis C: the impact of novel genotypes and race on treatment outcome. Hepatology. 2002;36:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Hui CK, Yuen MF, Sablon E, Chan AO, Wong BC, Lai CL. Interferon and ribavirin therapy for chronic hepatitis C virus genotype 6: a comparison with genotype 1. J Infect Dis. 2003;187:1071-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Fung J, Lai CL, Hung I, Young J, Cheng C, Wong D, Yuen MF. Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin. J Infect Dis. 2008;198:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Nguyen MH, Trinh HN, Garcia R, Nguyen G, Lam KD, Keeffe EB. Higher rate of sustained virologic response in chronic hepatitis C genotype 6 treated with 48 weeks versus 24 weeks of peginterferon plus ribavirin. Am J Gastroenterol. 2008;103:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Chuang WL, Yu ML. Host factors determining the efficacy of hepatitis C treatment. J Gastroenterol. 2013;48:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Bunchorntavakul C, Chavalitdhamrong D, Tanwandee T. Hepatitis C genotype 6: A concise review and response-guided therapy proposal. World J Hepatol. 2013;5:496-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Lam KD, Trinh HN, Do ST, Nguyen TT, Garcia RT, Nguyen T, Phan QQ, Nguyen HA, Nguyen KK, Nguyen LH. Randomized controlled trial of pegylated interferon-alfa 2a and ribavirin in treatment-naive chronic hepatitis C genotype 6. Hepatology. 2010;52:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Tangkijvanich P, Komolmit P, Mahachai V, Poovorawan K, Akkarathamrongsin S, Poovorawan Y. Response-guided therapy for patients with hepatitis C virus genotype 6 infection: a pilot study. J Viral Hepat. 2012;19:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Thu Thuy PT, Bunchorntavakul C, Tan Dat H, Rajender Reddy K. A randomized trial of 48 versus 24 weeks of combination pegylated interferon and ribavirin therapy in genotype 6 chronic hepatitis C. J Hepatol. 2012;56:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Zhou YQ, Wang XH, Hong GH, Zhu Y, Zhang XQ, Hu YJ, Mao Q. Twenty-four weeks of pegylated interferon plus ribavirin effectively treat patients with HCV genotype 6a. J Viral Hepat. 2011;18:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | De Meyer S, Ghys A, Dierynck I, Beumont M, Luo D, Picchio G. Virologic characterization of genotype 4 hepatitis C virus variants in patients treated with telaprevir. Virol J. 2014;11:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Hézode C. Boceprevir and telaprevir for the treatment of chronic hepatitis C: safety management in clinical practice. Liver Int. 2012;32 Suppl 1:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Gaetano JN. Benefit-risk assessment of new and emerging treatments for hepatitis C: focus on simeprevir and sofosbuvir. Drug Healthc Patient Saf. 2014;6:37-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Moreno C, Hezode C, Marcellin P, Bourgeois S, Francque S, Samuel D, Zoulim F, Grange JD, Lenz O, Ouwerkerk-Mahadevan S. Once-daily simeprevir (TMC435) with peginterferon/ribavirin in treatment-naive or treatment-experienced chroniv HCV genotype 4-infected patients: final results of a phase III trial. J Hepatol. 2014;60:S535. |
| 97. | Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, Brown A, Peeters M, Lenz O. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669-1679.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 98. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 99. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 100. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 101. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 102. | Ruane PJ, Ain D, Meshrekey R, Riad J, Soliman M, Mikhail S, Wolfe PR, Kersey K, Doehle B, Jiang D. Sofosbuvir plus ribavirin, an interferon-free regimen, in the treatment of treatment-naive and treatment-experienced patients with chronic genotype 4 HCV infection. J Hepatol. 2014;60:S503-S504. |
| 103. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 104. | Stepanova M, Nader F, Cure S, Bourhis F, Hunt S, Younossi ZM. Patients’ preferences and health utility assessment with SF-6D and EQ-5D in patients with chronic hepatitis C treated with sofosbuvir regimens. Aliment Pharmacol Ther. 2014;40:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 105. | Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, Nelson D, Nader F, Hunt S. Minimal impact of sofosbuvir and ribavirin on health related quality of life in Chronic Hepatitis C (CH-C). J Hepatol. 2014;60:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 106. | Herbst DA, Reddy KR. NS5A inhibitor, daclatasvir, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013;22:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Hezode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, Shafran SD, Thuluvath PJ, Tatum HA, Waked I, Esmat G. Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut. 2014;Jul 30; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 108. | Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran-Jaramillo T, Andreone P, Bruno S, Ferenci P, Zeuzem S. DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha-2a/ribavirin in HCV genotypes 1 or 4. Liver Int. 2015;35:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Bronowicki JP, Ratziu V, Gadano A, Thuluvath PJ, Bessone F, Martorell CT, Pol S, Terg R, Younes Z, He B. Randomized trial of asunaprevir plus peginterferon alfa and ribavirin for previously untreated genotype 1 or 4 chronic hepatitis C. J Hepatol. 2014;61:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 110. | Pockros PJ, Jensen D, Tsai N, Taylor R, Ramji A, Cooper C, Dickson R, Tice A, Kulkarni R, Vierling JM. JUMP-C: a randomized trial of mericitabine plus pegylated interferon alpha-2a/ribavirin for 24 weeks in treatment-naïve HCV genotype 1/4 patients. Hepatology. 2013;58:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Wedemeyer H, Jensen D, Herring R, Ferenci P, Ma MM, Zeuzem S, Rodriguez-Torres M, Bzowej N, Pockros P, Vierling J. PROPEL: a randomized trial of mericitabine plus peginterferon alpha-2a/ribavirin therapy in treatment-naïve HCV genotype 1/4 patients. Hepatology. 2013;58:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |