Published online Sep 16, 2014. doi: 10.12998/wjcc.v2.i9.409
Revised: June 8, 2014
Accepted: June 27, 2014
Published online: September 16, 2014
Processing time: 167 Days and 20 Hours
Tumoral calcinosis (TC) has long been a controversial clinico-pathological entity. Its pathogenesis and genetic background have been gradually unravelled since its first description in 1943. According to the presence or absence of an underlying calcifying disease process, TC has been divided into primary and secondary varieties. Two subtypes of the primary variety exist; a hyper-phosphatemic type with familial basis represented by mutations in GalNAc transferase 3 gene (GALNT3), KLOTHO or Fibroblast growth factor 23 (FGF23) genes, and a normo-phosphatemic type with growing evidence of underlying familial base represented by mutation in SAMD9 gene. The secondary variety is mainly associated with chronic renal failure and the resulting secondary or tertiary hyperparathyroidism. Diagnosis of TC relies on typical radiographic features (on plain radiographs and computed tomography) and the biochemical profile. Magnetic resonance imaging can be done in difficult cases, and scintigraphy reflects the disease activity. Treatment is mainly surgical for the primary variety; however, a stage-oriented conservative approach using phosphate binders, phosphate restricted diets and acetazolamide should be considered before the surgical approach is pursued due to the high rate of recurrences and complications after surgical intervention. Medical treatment is the mainstay for treatment of the secondary variety, with failure warranting subtotal or total parathyroidectomy. Surgical intervention in these patients should be kept as a last resort.
Core tip: This review of literature on tumoral calcinosis, describes the current understanding of the pathogenesis and classifications of this relatively rare clinico-pathological entity. It discusses the different current diagnostic modalities and treatment options.
- Citation: Fathi I, Sakr M. Review of tumoral calcinosis: A rare clinico-pathological entity. World J Clin Cases 2014; 2(9): 409-414
- URL: https://www.wjgnet.com/2307-8960/full/v2/i9/409.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i9.409
Tumoral calcinosis (TC) is a rare clinical and histopathologic syndrome characterized by calcium salt deposition in different peri-articular soft tissue regions[1,2]. It mainly manifests in childhood or adolescence as painless, firm, tumour-like masses around the joints that may lead to joint function limitations specially when large in size[1,3,4].
Regions most commonly involved by this pathology are soft tissues of peri-articular upper limb (shoulder and elbow) and hip regions. Still; spinal[5-7], temporo-mandibular joint[8,9], metacarpals/metatarsals[10], and popliteal space[11] involvement has also been reported.
The term “tumoral calcinosis” was first stated by Inclan et al[3] in 1943 for a disease characterized by large juxta-articular lobular calcified masses without visceral or skin calcifications in patients showing normal serum calcium and phosphorus levels. The characteristic pathological features of these lesions were the presence of multiple cysts filled with calcified deposits lined by histiocytes, giant cells, and xanthomatous histiocytes. Earlier, Giard[12] and Duret[13] reported a similar condition in the European medical literature in 1898 and 1899, respectively. This disease process was a subject of Teutschlaender[14,15] studies from 1930 to 1950, known as the Teutschlaender disease in the European literature by that time[16].
Since its first description by Inclan et al[3], the term tumoral calcinosis has been widely used in the literature and has been sometimes broadened to include other conditions resulting in similar clinico-pathologic features, or even imprecisely used to describe any massive collection of peri-articular calcifications[17]. In this article, we are aiming at reviewing pathogenesis of the disease and the current diagnostic and treatment options.
Etiology of TC remains uncertain despite the several theories that have been proposed. In 1996, Smack et al[18], retrospectively reviewed 122 cases of TC ending in a proposed pathogenesis-based classification as follows: (1) Primary normo-phosphatemic TC. Normo-calcemia and normo-phoshpatemia are the hallmark of this entity. The majority of patients present before the 2nd decade of life and almost half of them live in tropical or subtropical regions. It is usually characterized by solitary calcifications. Although Smack et al[18], mentioned that there was no evident familial pattern in this entity, recent literature showed growing evidence of familial basis for this type of pathology, involving mutations in the gene encoding for SAMD9 protein[19]; (2) Primary hyper-phosphatemic TC. Normo-calcemia and hyper-phosphatemia are the hallmark of this entity. This type usually presents during the first and second decades[17,20] with predominance in people of African descent (some authors suggested confining the term TC to this variety)[17]. Genetic predisposition is a feature of this type of TC where hyper-phosphatemia arises due to reduced urinary phosphate excretion caused by recessive mutations in GalNAc transferase 3 gene, GALNT3, and KLOTHO, that causes the inactivation of FGF23, a phosphoturic hormone[21-24]; and (3) Secondary TC. Chronic renal failure (CRF) is the most common identifiable condition in this entity.
The histology of the TC lesions in these 3 groups is identical. The reason behind this similarity has not yet been resolved[25]. This classification although bringing clarity to the diagnosis of TC and being widely propagated in the literature is still facing some debates[17] including the dissociation between the lesion and it’s underlying etiology resulting from dealing with the term as a clinico-pathological description rather than a separate disease entity as described by Inclan et al[3].
Although Smack et al[18] described this classification as a pathogenesis based one, they actually classified the condition according to the presence or absence of underlying disease associated with calcification and the biochemical profile of the patients rather than actual pathogenesis of the lesions.
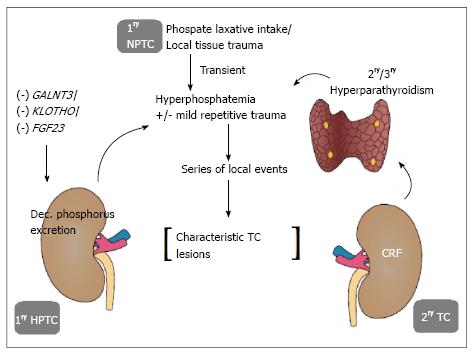
A stepwise approach to the pathogenesis of TC lesions has been proposed[26]. Although this approach has been described for the familial type of TC, it has been later enlarged to contain the other types of this pathology as a common pathway, which eventually results in the formation of the characteristic TC lesions[25] as follows: (1) Minimal repetitive trauma leading to hemorrhages in the peri-articular tissue initiating a foamy histiocytic response. Traumatic Injury preceding the development of TC lesions has been frequently reported specially in the normo-phosphatemic variety[18]. Trauma in the form of chronic pressure has also been accused[27]. The presence of hemosiderin pigment near TC lesions fortifies this theory[28]; and (2) A reparative process is initiated which together with friction forces, lead towards neobursae formation. However, an interplay between multifactorial calcification process and collagenolysis due to proteolytic enzymes produced from disintegrating histiocytes prevents functional bursae and bone formation. This results in the characteristic lesions of TC, representing the active stage of the process[26].
This multifactorial calcification is initiated by elevated calcium phosphorus product with hyper-phosphatemia as the overwhelming component. This hyper-phosphatemia can be explained by genetic mutations in the FGF23, GALNT3 or KLOTHO gene resulting in inactivation of the phosphaturic protein FGF23 in the primary hyper-phosphatemic variety. In the secondary type, this hyper-phosphatemic state is explained by the association with secondary hyperparathyroidism resulting from CRF. On the other hand, in the primary normo-phosphatemic variety, transient hyper-phosphatemia is the proposed mechanism. This transient hyper-phosphatemia is either produced locally due to tissue injury leading to release of phosphate from injured cells into extracellular space specially when injury involves muscles (main phosphate store in soft tissue), or induced by excessive oral or rectal use of a phosphate-saline laxatives[25,29]. This hypothesis still needs to be augmented. Figure 1 shows a schematic illustration of the pathogenesis of the different types of TC.
Finally, calcified debris fills the loculi leading to bone formation with arrest of bursae forming activity and decline in collagenolysis activity, ending in fibrosis that surrounds the TC lesions. Here, the lesions become relatively quiescent[26].
Diagnosis of TC involves differentiating the condition from its mimics and further classifying it into one of the aforementioned categories.
Patients with TC usually present with multiple or solitary swellings related to the joints, discomfort, pain, and joint movement limitation[28,30] most commonly affecting the hip, elbow, shoulder, foot, and wrist[17]. Growth of such lesions is mostly slow and progressive in nature over several years[31]. Sometimes, ulceration of the overlying skin occurs with superadded secondary infection[32,33]. Huge bilateral cases of TC though rare, have been described in the literature[34].
Diagnosis of TC is mainly based on imaging modalities. Plain radiographs show the typical appearance of amorphous, multilobulated and cystic calcifications in a peri-articular location[17]. Computed tomography helps in determining the extent and relations of individual lesions, and as a guide for surgical planning. It usually shows cystic loculi with fluid-fluid levels caused by calcium layering giving rise to “the sedimentation sign”[35]. In other instances, the lesion may appear homogenous denoting decreased activity in the quiescent stage[36,37]. Erosion or osseous destruction by adjacent soft-tissue masses is consistently absent; another hallmark of this pathology[17]. Magnetic resonance imaging shows inhomogeneous high signal intensity on T2-weighted sequences with two patterns frequently observed; diffuse lower-signal-intensity pattern, or nodular pattern with alternating areas of high signal intensity and signal void. The lesions appear inhomogeneous with low-signal intensity on T1-weighted sequences[37].
Scintigraphy using radiolabeled phosphate compounds (technetium-99m methylene diphosphonate) is of great value in detecting multiple lesions, newly-forming lesions, bone marrow affection, and for monitoring therapy reflecting the activity of the lesions. Ultrasonography can also be of value in detecting loculated fluid collections, thus helping in determining the disease activity[17,37,38].
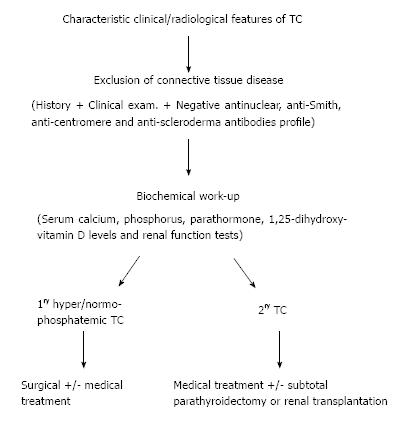
Other conditions including calcinosis universalis, calcinosis circumscripta, calcific tendonitis, synovial osteochondromatosis, synovial sarcoma, osteosarcoma, myositis ossificans, tophaceous gout, and calcific myonecrosis can confuse both the radiologists and the clinicians regarding the nature of these lesions. This can be resolved through combining typical radiological features of TC with the serum biochemical profile (including serum calcium level, serum phosphorus levels, renal function tests , serum parathormone level and 1,25-dihydroxy-vitamin D levels)[17,38]. Detailed family, drug and past history should also be obtained.
It should be emphasized that connective tissue diseases should be excluded before settling the diagnosis as primary TC specially in the setting of normal calcium and phosphorus levels. This can be achieved with a negative antinuclear, anti-Smith, anti-centromere and anti-scleroderma antibodies profile[17].
Although biopsy is better avoided for fear of infection[34]. It may still be done in difficult cases to settle the diagnosis[39]. Histopathological examination of TC lesions after biopsy or surgical excision shows certain characteristic morphologic features differentiating it from other calcifying processes. This includes formation of the characteristic compartments, which contain liquid chalky content together with calcifications. Such compartmentalized configuration frequently remains even in the quiescent stage[25].
Treatment of TC should be tailored according to the type of the lesion, stage of the pathology together with the site, size and relations of the lesion, as well as symptoms of the patient.
Considering the primary variety, primary treatment is early surgical excision[40]. However, the high rate of recurrence warrants repeated excisions[2,33]. During surgery, TC lesions show a cystic nature with white and yellow chalky material formed by calcium hydroxyapatite crystals, calcium carbonate and calcium phosphate[18]. The presence of a hyper-vascular region beyond the periphery of the calcified mass as proven by angiography raises the possibility that a wider surgical resection margin may lead to fewer recurrences. Confirmation of this theory is however, still needed[41]. Slavin et al[26], reported that immobilization after resection may also have a role in decreasing new lesion formation in the adjacent tissues.
Huge lesions may require extensive surgical excision and reconstruction[42]. Relations to important neurovascular structures may be challenging, resulting mostly in partial excision and rapid recurrence. However, partial excision of large symptomatic lesions can be helpful providing significant pain relief[43,44]. Indications for surgical excision also include recurrent infection, ulceration, and functional impairment[34,45].
According to a literature review done by King et al[45], surgical complications of TC excision include postoperative prolonged drainage which can lead to delayed wound healing and sinus tract formation, secondary infections caused by chronic wound problems specially with extensive disease or incomplete resection, and recurrence, which is frequent after incomplete excision and usually has a faster rate of growth.
Medical treatment through phosphate depletion (dietary deprivation of phosphorus and phosphate binding chelating agents such as oral aluminium hydroxide has shown variable success rates in both normo- and hyper-phosphatemic cases[10,33,46]. The combination with acetazolamide to induce phosphaturia may have a valuable synergistic effect in lowering hyper-phosphatemia[47,48]. In-view of the high rate of recurrence after surgical excision, medical treatment in the primary variety could be reasonably considered before the surgical approach, especially in the hyper-phosphatemic entity.
From a pathogenetic point of view, medical treatment during the active stage maybe superior to surgery which is usually doomed with recurrence in this stage. On the other hand, surgical treatment may be more effective in the relatively quiescent stage where encapsulation occurs and hinders the ion exchange process leading to failure of phosphate depletion treatment[26]. Some authors advocate a combination therapy that includes surgical excision and medical treatment as a necessity in some resistant cases[49,50]. Alternative treatment modalities including the administration of steroids, diphosphonates, or calcitonin and radiation therapy have not proven to be effective[2,3,51-53].
On the other hand, treatment of secondary TC (end stage renal disease-related, hemodialysis-related TC) is mainly medical. Surgical excision is associated with more profound complications (infection, fistula formation) aggravated by the patient medical condition, together with the persistence of the etiology[34]. Surgical interventions or biopsies should be kept as a last resort in these patients. Medical treatment includes calcium and phosphorus restricted diets, dialysates, and phosphate binders (except aluminium containing binders). Several other medical treatments including Vinpocetine, Sodium thiosulfate, intravenous Pamidronate, have been used in treatment of the secondary variety of TC with variable success rates[54-58].
Given the underlying secondary or tertiary hyperparathyroidism in most of these patients, subtotal or total parathyroidectomy is the next logical step in the setting of medical treatment failure. This approach has demonstrated significant response[59-61]. Kidney transplantation may also be considered. Figure 2 shows a schematic diagram of the diagnostic and treatment approach for TC.
In view of growing understanding of the pathogenesis of TC and evidence of the familial origin in the normo-phosphatemic, an agreement regarding the clinico-pathological entities to which the term TC should be coined should be sought. Such an agreement may necessitate preserving the term for the familial type of the condition including its two variants after exclusion of underlying disease process, or at least limiting the secondary variant to conditions sharing the same pathogenesis on ultrastructural level. This should be propagated to radiologists, clinicians and pathologists in order to avoid a misleading imprecise diagnosis. The exact diagnosis of TC relies on typical radiologic features and biochemical profile, with the exclusion of connective tissue diseases. Treatment plans should be tailored to individual cases. Generally, conservative treatment is better considered prior to the surgical approach in primary patients, reserving surgical excision to patients with disabling symptoms. In secondary cases, medical treatment in the mainstay. Treatment failure warrants parathyroidectomy, and surgical excision should be the last resort in these cases.
P- Reviewer: Schoenhagen P, Takahashi M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | McClatchie S, Bremner AD. Tumoral calcinosis--an unrecognized disease. Br Med J. 1969;1:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 2. | Lafferty FW, Reynolds ES, Pearson OH. Tumoral calcinosis: a metabolic disease of obscure etiology. Am J Med. 1965;38:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Inclan A, Leon PP, Camejo M. Tumoral calcinosis. J Am Med Ass. 1943;121:490-495. [RCA] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Slavin G, Klenerman L, Darby A, Bansal S. Tumoral calcinosis in England. Br Med J. 1973;1:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Durant DM, Riley LH, Burger PC, McCarthy EF. Tumoral calcinosis of the spine: a study of 21 cases. Spine (Phila Pa 1976). 2001;26:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Kokubun S, Ozawa H, Sakurai M, Tanaka Y. Tumoral calcinosis in the upper cervical spine: a case report. Spine (Phila Pa 1976). 1996;21:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ohashi K, Yamada T, Ishikawa T, Yamaguchi S, Nakajima H, Takagi M. Idiopathic tumoral calcinosis involving the cervical spine. Skeletal Radiol. 1996;25:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Gal G, Metzker A, Garlick J, Gold Y, Calderon S. Head and neck manifestations of tumoral calcinosis. Oral Surg Oral Med Oral Pathol. 1994;77:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Shirasuna K, Sugiyama M, Yasui Y. Tumoral calcinosis around the mandibular condyle. Int J Oral Maxillofac Surg. 1991;20:36-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Mozaffarian G, Lafferty FW, Pearson OH. Treatment of tumoral calcinosis with phosphorus deprivation. Ann Intern Med. 1972;77:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Giardina F, Sudanese A, Bertoni F, Guerra E, Paderni S. Tumoral calcinosis of the popliteal space. Orthopedics. 2004;27:1104-1107. [PubMed] |
| 12. | Giard A. Sur la calcification hibernale. C R Soc Biol. 1898;10:1013-1015. |
| 13. | Duret MH. Tumours multiples et singulieres des bourses sereuses. Bull Mem Soc Anat Paris. 1899;74:725-733. |
| 14. | Teutschlaender O. Die lipoido-calcinosis oder lipoidkalkgicht. Beitr Pathol Ana. 1949;110:402-432. |
| 15. | TEUTSCHLAENDER O. [On progressive lipoidocalcinosis; a differentiation of two types]. Zentralbl Allg Pathol. 1951;87:1-15. [PubMed] |
| 16. | Barrière H, Welin J, Lenne Y, Visset J, Vigier P. [Teutschlaender lipo-calcino-granulomatosis or tumoral calcinosis of Inclan (author’s transl)]. Ann Dermatol Venereol. 1977;104:136-140. [PubMed] |
| 17. | Olsen KM, Chew FS. Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics. 2006;26:871-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Smack D, Norton SA, Fitzpatrick JE. Proposal for a pathogenesis-based classification of tumoral calcinosis. Int J Dermatol. 1996;35:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Hershkovitz D, Gross Y, Nahum S, Yehezkel S, Sarig O, Uitto J, Sprecher E. Functional characterization of SAMD9, a protein deficient in normophosphatemic familial tumoral calcinosis. J Invest Dermatol. 2011;131:662-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Prince MJ, Schaeffer PC, Goldsmith RS, Chausmer AB. Hyperphosphatemic tumoral calcinosis: association with elevation of serum 1,25-dihydroxycholecalciferol concentrations. Ann Intern Med. 1982;96:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 73] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 408] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 361] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 23. | Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7:318-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 25. | Slavin RE, Wen J, Barmada A. Tumoral calcinosis--a pathogenetic overview: a histological and ultrastructural study with a report of two new cases, one in infancy. Int J Surg Pathol. 2012;20:462-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Slavin RE, Wen J, Kumar D, Evans EB. Familial tumoral calcinosis. A clinical, histopathologic, and ultrastructural study with an analysis of its calcifying process and pathogenesis. Am J Surg Pathol. 1993;17:788-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Thomson JG. Calcifying collagenolysis (tumoural calcinosis). Br J Radiol. 1966;39:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Pakasa NM, Kalengayi RM. Tumoral calcinosis: a clinicopathological study of 111 cases with emphasis on the earliest changes. Histopathology. 1997;31:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Markowitz GS, Perazella MA. Acute phosphate nephropathy. Kidney Int. 2009;76:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Asuncion GF, Tzarnas CD. Uremic tumoral calcinosis: acute hand presentations mimicking infection. J Hand Surg Am. 1994;19:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Meltzer CC, Fishman EK, Scott WW. Tumoral calcinosis causing bone erosion in a renal dialysis patient. Clin Imaging. 1992;16:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Geirnaerdt MJ, Kroon HM, van der Heul RO, Herfkens HF. Tumoral calcinosis. Skeletal Radiol. 1995;24:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Kirk TS, Simon MA. Tumoral calcinosis. Report of a case with successful medical management. J Bone Joint Surg Am. 1981;63:1167-1169. [PubMed] |
| 34. | Farzan M, Farhoud AR. Tumoral calcinosis: what is the treatment? Report of two cases of different types and review of the literature. Am J Orthop (Belle Mead NJ). 2011;40:E170-E176. [PubMed] |
| 35. | Hug I, Gunçaga J. Tumoral calcinosis with sedimentation sign. Br J Radiol. 1974;47:734-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Harkess JW, Peters HJ. Tumoral calcinosis. A report of six cases. J Bone Joint Surg Am. 1967;49:721-731. [PubMed] |
| 37. | Martinez S, Vogler JB, Harrelson JM, Lyles KW. Imaging of tumoral calcinosis: new observations. Radiology. 1990;174:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Huang YT, Chen CY, Yang CM, Yao MS, Chan WP. Tumoral calcinosis-like metastatic calcification in a patient on renal dialysis. Clin Imaging. 2006;30:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Petscavage JM, Richardson ML. Tumoral calcinosis mimicking recurrent osteosarcoma. J Radiol Case Rep. 2009;4:336. |
| 40. | Steinbach LS, Johnston JO, Tepper EF, Honda GD, Martel W. Tumoral calcinosis: radiologic-pathologic correlation. Skeletal Radiol. 1995;24:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Neeman Z, Wood BJ. Angiographic findings in tumoral calcinosis. Clin Imaging. 2003;27:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Lykoudis EG, Seretis K, Ristanis S. Huge recurrent tumoral calcinosis needing extensive excision and reconstruction: report of a rare case and brief literature review. Aesthetic Plast Surg. 2012;36:1194-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Tezelman S, Siperstein AE, Duh QY, Clark OH. Tumoral calcinosis. Controversies in the etiology and alternatives in the treatment. Arch Surg. 1993;128:737-744; discussion 744-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Arikawa J, Higaki Y, Mizushima J, Nogita T, Kawashima M. Tumoral calcinosis: a case report with an electron microscopic study. Eur J Dermatol. 2000;10:52-54. [PubMed] |
| 45. | King JJ, Brennan KB, Crawford EA, Fox EJ, Ogilvie CM. Surgical complications associated with extensive tumoral calcinosis. Am J Orthop. 2011;40:247-252. [PubMed] |
| 46. | Davies M, Clements MR, Mawer EB, Freemont AJ. Tumoral calcinosis: clinical and metabolic response to phosphorus deprivation. Q J Med. 1987;63:493-503. [PubMed] |
| 47. | Yamaguchi T, Sugimoto T, Imai Y, Fukase M, Fujita T, Chihara K. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone. 1995;16:247S-250S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Lufkin EG, Wilson DM, Smith LH, Bill NJ, DeLuca HF, Dousa TP, Knox FG. Phosphorus excretion in tumoral calcinosis: response to parathyroid hormone and acetazolamide. J Clin Endocrinol Metab. 1980;50:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Hamada J, Tamai K, Ono W, Saotome K. Uremic tumoral calcinosis in hemodialysis patients: clinicopathological findings and identification of calcific deposits. J Rheumatol. 2006;33:119-126. [PubMed] |
| 50. | Walentynowicz JE, Mahoney MD, Saldana MJ. Tumoral calcinosis. Case report with treatment failure. Orthop Rev. 1989;18:687-690. [PubMed] |
| 51. | Thomson JE, Tanner FH. Tumoral calcinosis. J Bone Joint Surg Am. 1949;31A:132-140. [PubMed] |
| 52. | Gregosiewicz A, Warda E. Tumoral calcinosis: successful medical treatment. A case report. J Bone Joint Surg Am. 1989;71:1244-1249. [PubMed] |
| 53. | Kallmeyer JC, Seimon LP, MacSearraigh ET. The effect of thyrocalcitonin therapy and phosphate deprivation on tumoral calcinosis. S Afr Med J. 1978;54:963-966. [PubMed] |
| 54. | Seyahi A, Atalar AC, Ergin HK. Tumoral calcinosis: Clinical and biochemical aspects of a patient treated with vinpocetine. Eur J Intern Med. 2006;17:436-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Ueyoshi A, Ota K. Clinical appraisal of vinpocetine for the removal of intractable tumoral calcinosis in haemodialysis patients with renal failure. J Int Med Res. 1992;20:435-443. [PubMed] |
| 56. | Minisola S, Romagnoli E, Rosso R. Massive tumoral calcinosis in a patient on long-term hemodialysis. J Bone Miner Res. 2000;15:2056-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Drüeke TB. A clinical approach to the uraemic patient with extraskeletal calcifications. Nephrol Dial Transplant. 1996;11 Suppl 3:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Phanish MK, Kallarackal G, Ravanan R, Lawson TM, Baboolal K. Tumoral calcinosis associated with pyrexia and systemic inflammatory response in a haemodialysis patient: successful treatment using intravenous pamidronate. Nephrol Dial Transplant. 2000;15:1691-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Möckel G, Buttgereit F, Labs K, Perka C. Tumoral calcinosis revisited: pathophysiology and treatment. Rheumatol Int. 2005;25:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Pecovnik-Balon B, Kramberger S. Tumoral calcinosis in patients on hemodialysis. Case report and review of the literature. Am J Nephrol. 1997;17:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Tarrass F, Benjelloun M. Tumoral calcinosis of the elbow in a long-term hemodialysis patient. Saudi J Kidney Dis Transpl. 2008;19:105-106. [PubMed] |