Published online Jun 16, 2014. doi: 10.12998/wjcc.v2.i6.224
Revised: February 14, 2014
Accepted: April 17, 2014
Published online: June 16, 2014
Processing time: 171 Days and 15.8 Hours
We report our first simultaneous bilateral robot assisted partial nephrectomy (RAPN) in order to show and critically discuss the feasibility of this procedure. Materials and methods A 69-year-old male patient visited our department due to incidental finding of bilateral mesorenal small masses (2.5 cm on the right and 3.5 cm on the left) suspicious for malignancy. We started from the right side with patient in flank position. Port placement: 12-mm periumbilical camera port, two 8-mm robotic ports in wide ‘‘V’’configuration, additional 12 mm assistant port on the midline between the umbilicus and symphysis pubis. A right unclamping RAPN with sliding clip renorrhaphy was performed. The trocars were removed and the robot undocked. Without interrupting the anesthesiological procedures, the patient was reported in supine position and, after 180 degrees rotation of the surgical bed, was newly placed in contralateral flank position. Using both the previous periumbilical and midline ports, two other 8-mm robotic trocars were placed. The robot was then redocked and RAPN was also performed on the left side using the same previously reported technique. Results Total time: 285 min. Estimated blood losses: 150 cc. Postoperative period: uneventful. Pathological examination: bilateral renal cell carcinoma, negative surgical margins. Conclusions Our experience was encouraging and confirmed the feasibility and safety of this procedure. The planning of our technique was time and cost effective with cosmetic benefit for the patient. However, we think that an appropriate selection of the patients and a skill in robotic renal surgery are advisable before approaching this type of surgery.
Core tip: Very few papers have been reported concerning simultaneous bilateral robot assisted partial nephrectomy. We think that our technique was noteworthy for some important aspects: the number of the ports was minimized, the disposition of the operatory room allows the quick rotation of the patient’s bed and the redocking of the robot, the operative time was acceptable, the unclamping technique decreased the risk of renal insufficiency, the cost for two nephrectomies was decreased. In conclusion, our technique was safe, feasible, time and cost effective with a cosmetic benefit for the patient.
- Citation: Giberti C, Gallo F, Schenone M, Cortese P. Simultaneous bilateral robotic partial nephrectomy: Case report and critical evaluation of the technique. World J Clin Cases 2014; 2(6): 224-227
- URL: https://www.wjgnet.com/2307-8960/full/v2/i6/224.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i6.224
In the last few years, robot-assisted partial nephrectomy (RAPN) has become a promising procedure able to bridge the technical difficulties of laparoscopic partial nephrectomy (LPN), permitting a broader diffusion of laparoscopic treatment of renal masses[1-3].
In fact, the 3D vision, the optical magnification and the robotic instruments allow surgeons to realize very precise tumor resection and to simplify the reconstructive steps of the procedure, minimizing the potential risks due to the ischemia time.
More recently, the expanded role of robot-assisted surgery has also included the simultaneous treatment of bilateral renal tumors[4,5].
This type of procedure, which is certainly fascinating, still needs to be well defined regarding the indications and the technique. We report our first case of simultaneous bilateral robotic partial nephrectomy in order to show the feasibility of our technique and critically discuss both the advantages and the disadvantages of this procedure.
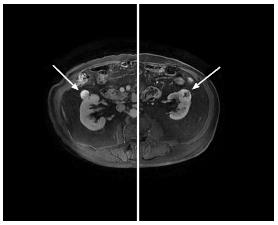
A 69-year-old male patient visited our department due to the incidental finding of bilateral small renal masses. Magnetic resonance scans showed a 2.5 cm mass in the middle portion of the right kidney and a 3.5 cm mass in the middle portion of the left kidney with no involvement of the collecting systems. The two masses were suspicious for malignancy (Figure 1). The differential diagnosis was made with benign tumors and complicated cysts. There was no past surgical history. General physical examination and the preoperative exams were normal. The body mass index (BMI) was 23.51.
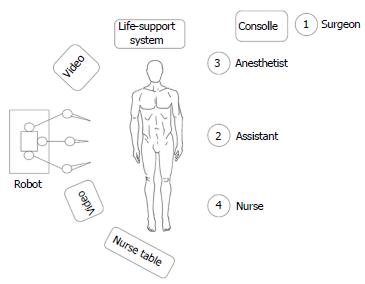
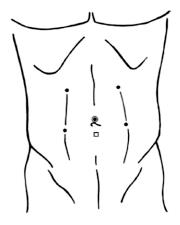
The operating theatre was set up as shown in Figure 2. The procedure was performed using a three-arm Da Vinci Robot, standard version, starting from the right side. The patient was secured in a flank position with the table slightly bent. Regarding the port placement, a 12-mm periumbilical port was placed for the camera. Two 8-mm robotic instrument ports were placed approximately 8 cm from the camera in a wide ‘‘V’’ configuration centered on the renal tumor. An additional 12 mm assistant trocar was placed on the midline between the umbilicus and symphysis pubis (Figure 3). A 30° angle lens was used. The robotic instruments included bipolar fenestrated forceps, monopolar cautery scissors, and two needle drivers. The peritoneum was incised sharply along the line of Toldt and the bowel was mobilized medially exposing the Gerota’s fascia. The renal artery and vein were isolated and vessel loops were placed around them. The Gerota’s fascia was dissected off the surface of the kidney and the kidney was extensively mobilized until easy access to the tumor was achieved from all sides. The RAPN was then performed without hilar clamping. The renal specimen was retrieved using an endobag. The inner defect was closed with a running outside-in Monocryl 4-0 suture preloaded with a Hem-o-lok clip, taking care to include retracted vessels or calyces into the suture. The borders of the defect were closed with another running outside to inside Monocryl 2-0 suture including a haemostatic agent and secured with Hem-o-lok clips at each bite. Through the sliding clip technique, the right tension was brought on these sutures[6].
The Gerota’s fascia and the peritoneum were closed. A wound drain was introduced through the inferior 8-mm port. All the trocars were removed and the robot was undocked.
Without interrupting the anesthesia, the patient was repositioned in the supine position and, after a 180 degree rotation of the surgical bed, he was placed in the contralateral flank position. Using both the previous periumbilical and midline ports for the camera and the additional 12-mm assistant trocar, respectively, the other 8-mm robotic trocars were placed in a wide ‘‘V’’configuration centered on the left renal tumor (Figure 3). The robot was then redocked without changing any disposition of the instruments, the furniture or the staff inside the operating theatre.
A second RAPN without hilar clamping was also performed on the left side following the previously reported technique. Intraoperative ultrasonography was used on this side in order to score the margins of the lesion.
The total operation time was 285 min and total console time was 240 min. The estimated blood loss was 150 cc. The postoperative period was uneventful. The patient was mobilized on day 2. The urethral catheter was removed on day 2. The right and left drains were removed on days 2 and 3, respectively. The patient was discharged on day 4. The pathological examination reported bilateral renal cell carcinoma, Fuhrman grade 1, with negative surgical margins. Six months after surgery, computed tomography scan did not show tumor local recurrences.
The robotic approach for conservative renal surgery is becoming increasingly common due to the reported encouraging outcomes in terms of safety, feasibility and efficacy of this procedure[1-3,7]. Very few papers have been reported in literature concerning simultaneous bilateral RAPNs probably due to the low incidence of bilateral renal tumors but also due to the difficulties of this type of surgery[4-5]. The potential benefits of simultaneous bilateral surgery could be related to the advantages of a unique surgical procedure with single anesthesia, shorter overall hospitalization, faster overall recovery and lower costs than two separate procedures. Furthermore, a cosmetic benefit due to the reuse of some ports for both sides could be considered. However, these advantages could be balanced by the risks of longer total anesthesia time, higher blood loss and postoperative renal insufficiency. In this setting, the procedure should be planned appropriately in order to maximize the benefits and minimize the risks. We think that our technique was noteworthy for some aspects.
The positioning of the ports was planned accurately in order to minimize the number of the abdominal incisions. In particular, the camera and the assistant ports were positioned on the xifopubic line and used for both sides. In the end, the bilateral RAPN was performed using only six ports.
The disposition of the operating theatre was studied in order to allow the rotation of the patient’s bed without changing the positions of the robot, the instruments and the operators. This detail allowed us to undock and redock the robot very quickly between the two nephrectomies, avoiding the waste of precious minutes.
Overall, the entire operation lasted less than 5 h including anesthesiological procedures, patient positioning and trocar placement. We think that this is an acceptable anesthesia time for a bilateral procedure as confirmed by the regular observations made in the postoperative period.
The surgical technique with no arterial clamping decreased the risk of postoperative renal insufficiency which can be more frequent after a bilateral procedure, especially when bilateral clamping is performed, as already reported in literature[4].
This procedure was really cost effective. In fact, the two nephrectomies were performed without shutting the robot down and using the same surgical instruments. These aspects helped strongly to decrease the costs of a robotic operation.
Some limitations of our technique should be mentioned. In fact, an appropriate selection of the patients (mainly regarding the size, the location of the tumors or the preexisting condition of chronic renal insufficiency) and a very good skill in renal robotic surgery are really advisable before approaching this type of surgery.
In conclusion, our experience was encouraging and confirmed the feasibility and the safety of this procedure. Furthermore, the planning of our technique was time and cost effective with a cosmetic benefit for the patient. However, we think that an appropriate selection of the patients and skill in robotic renal surgery are really advisable before approaching this type of surgery.
We thank Dr. Jennifer McDermott for the language revision.
A 69-year-old male patient visited our department due to the incidental finding of bilateral small renal masses.
The general physical examination was normal, the renal masses were not palpable. The body mass index was 23.51.
Benign tumors, complicated cysts.
The preoperative exams were normal.
Magnetic resonance scans showed a 2.5 cm mass in the middle portion of the right kidney and a 3.5 cm mass in the middle portion of the left kidney with no involvement of the collecting systems.
The pathological examination reported bilateral renal cell carcinoma, Fuhrman grade 1, with negative surgical margins.
A simultaneous bilateral robotic partial nephrectomy was performed.
The surgical treatment of small renal masses is well known using different approaches (mainly open surgery, laparoscopy and cryotherapy). However, very few papers have been reported in literature concerning simultaneous bilateral robot assisted partial nephrectomy probably due to the low incidence of bilateral renal tumors but also due to the difficulties of this type of surgery.
This case report showed the feasibility, the safety, the time and cost effectiveness of this procedure with a cosmetic benefit for the patient.
This is a well written and interesting case.
P- Reviewers: Gbolade BA, Sanju G S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Leslie S, Goh AC, Gill IS. Partial nephrectomy--contemporary indications, techniques and outcomes. Nat Rev Urol. 2013;10:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Mottrie A, Borghesi M, Ficarra V. Is traditional laparoscopy the real competitor of robot-assisted partial nephrectomy? Eur Urol. 2012;62:1034-1036; discussion 1038-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Aboumarzouk OM, Stein RJ, Eyraud R, Haber GP, Chlosta PL, Somani BK, Kaouk JH. Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2012;62:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Jung JH, Arkoncel FR, Lee JW, Oh CK, Yusoff NA, Kim KJ, Rha KH. Initial clinical experience of simultaneous robot-assisted bilateral partial nephrectomy and radical prostatectomy. Yonsei Med J. 2012;53:236-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Seo IY, Rim JS. Bilateral robotic single-site partial nephrectomy. J Laparoendosc Adv Surg Tech A. 2011;21:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009;55:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Haber GP, White WM, Crouzet S, White MA, Forest S, Autorino R, Kaouk JH. Robotic versus laparoscopic partial nephrectomy: single-surgeon matched cohort study of 150 patients. Urology. 2010;76:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |