Published online May 16, 2014. doi: 10.12998/wjcc.v2.i5.167
Revised: March 20, 2014
Accepted: April 16, 2014
Published online: May 16, 2014
Processing time: 149 Days and 14.7 Hours
Pancreatic tuberculosis (TB) is a rare condition, even in immunocompetent hosts. A case is presented of pancreatic TB that mimicked pancreatic head carcinoma in a 40-year-old immunocompetent male patient. The patient was admitted to our hospital after suffering for nine days from epigastralgia and obstructive jaundice. Computed tomography revealed a pancreatic mass that mimicked a pancreatic head carcinoma. The patient had undergone an operation four months prior for thoracic TB and was undergoing anti-TB therapy. A previous abdominal ultrasound was unremarkable with the exception of gallbladder steroid deposits. The patient underwent surgery due to the progressive discomfort of the upper abdomen and a mass that resembled a pancreatic malignancy. A biopsy of the pancreas and lymph nodes was performed, revealing TB infection. The patient received a cholecystostomy tube and recovered after being administered standard anti-TB therapy for 15 mo. This case is reported to emphasize the rare contribution of pancreatic TB to pancreatic masses and obstructive jaundice.
Core tip: Pancreatic tuberculosis (TB) is rare in immunocompetent or immunosuppressed hosts. We report a case of a 40-year-old immunocompetent host with pancreatic TB that mimicked pancreatic head carcinoma with obstructive jaundice. The patient had previously been operated on for thoracic TB and was receiving anti-TB therapy. We report this case to emphasize rare causes of pancreatic masses and obstructive jaundice, and to discuss alternate treatments for pancreatic TB.
- Citation: Yang YJ, Li YX, Liu XQ, Yang M, Liu K. Pancreatic tuberculosis mimicking pancreatic carcinoma during anti-tuberculosis therapy: A case report. World J Clin Cases 2014; 2(5): 167-169
- URL: https://www.wjgnet.com/2307-8960/full/v2/i5/167.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i5.167
Pancreatic tuberculosis (TB) is a rare occurrence in either immunocompetent or immunosuppressed hosts; human immunodeficiency virus (HIV)-infected patients only have a 0.46% incidence[1]. Isolated pancreatic TB is predominantly observed in areas of widespread TB dissemination such as a military setting. Currently, there are few reports detailing pancreatic TB cases, and none of the patients were receiving anti-TB therapies at the time of diagnosis. Here, a case of pancreatic TB is presented in an immunocompetent host that was receiving anti-TB treatment. This article highlights the importance of understanding this rare disease as a cause of pancreatic masses and obstructive jaundice.
In July 2012, a 40-year-old male patient was admitted to our hospital after suffering from epigastralgia and jaundice for nine days. Upon eating, the patient complained of nausea and a worsening pain that radiated to his back. An abdominal examination revealed sensitivity in the upper abdomen without hepatosplenomegaly or ascites. The patient’s medical history revealed that he had been previously diagnosed with thoracic TB and had undergone an operation for a thoracic abscess four months prior. Since his diagnosis, the patient was treated with a multi-drug anti-TB regimen that included 300 mg/d isoniazid, 600 mg/d rifampicin, and 750 mg/d ethambutol.
The patient did not present with signs of any immunosuppressive diseases and a serological test for HIV was negative. Laboratory tests revealed the following: 9.75 × 109 white blood cells (WBC)/L [reference range (RR): 4.0 - 10.0 × 109 WBC/L], 30.5 μmol/L total bilirubin (RR: 5.0 - 28.0 μmol/L), 24.5 μmol/L direct bilirubin (RR: < 8.8 μmol/L), 135 U/L alanine aminotransferase (RR: < 55 U/L), 64 U/L aspartate aminotransferase (RR: < 46 U/L), 715 U/L gamma glutamyl transpeptidase (RR: 6 - 46 U/L), 335 U/L alkaline phosphatase (RR: 47 - 138 U/L), and 66.84 U/mL carbohydrate antigen 199 (RR: < 22 U/mL).
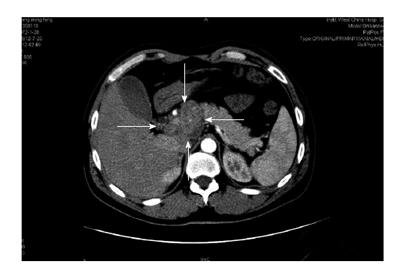
Computed tomography (CT) revealed a soft tissue shadow in the head of the pancreas. The lesion, which was necrotic and calcified, measured 5.6 cm × 4.2 cm and mimicked a pancreatic head carcinoma. CT images also revealed upstream biliary dilatation and multiple lymphadenopathies surrounding the head of pancreas, the vena cava and the liver artery. The celiac trunk and descending part of duodenum could not be clearly identified in the lesion, suggesting that the above areas were involved. A pancreatic neoplasm with multiple lymph node metastases was suspected after CT (Figure 1). A chest CT revealed small nodes in the superior lobe of the left lung and in the lower lobes of both lungs. As the patient had a medical history of TB, the abdominal ultrasound taken four months prior was reexamined, but found to be unremarkable with the exception of steroid deposits in the gallbladder. Progressive discomfort in the patient’s upper abdomen and the identification of a potential malignancy via CT led to the decision to operate and perform a biopsy.
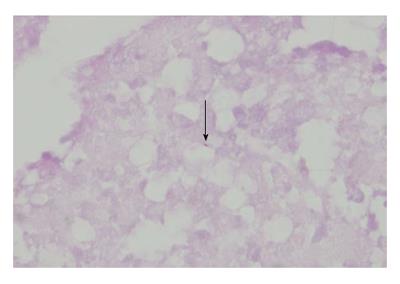
Caseous necrosis and lymphadenectasis was observed during the biopsy procedure. Subsequently, a cholecystostomy tube was placed. Samples from the lymph nodes and biopsies of the mass were obtained for histopathologic examination. Ziehl-Neelsen staining revealed caseous granulomatous inflammation and necrosis with acid-fast bacilli (Figure 2). The presence of Mycobacterium tuberculosis DNA was confirmed within the biopsy samples by polymerase chain reaction (PCR). The patient was therefore administered the standard anti-TB therapy of 300 mg/d isoniazid, 600 mg/d rifampicin, 1.5 g/d pyrazinamide, and 750 mg/d ethambutol for nine months followed by six months of 300 mg/d isoniazid, 600 mg/d rifampicin, and 1.5 g/d pyrazinamide. The patient recovered quickly and was without abdominal pain during the 15 mo of treatment.
Pancreatic TB is extremely rare, even in immunocompromised hosts, and this rarity is attributed to the resistance provided by the pancreatic enzymes[1,2]. Despite its rarity, pancreatic TB can occur even when patients are undergoing the standard anti-TB drug (ATD) regimen, as was demonstrated by the case reported here. A diverse spectrum of symptoms can arise during pancreatic TB, ranging from abdominal discomfort, obstructive jaundice, fever, loss of appetite or nausea, to night sweats and weight loss[3,4]. Imaging of the pancreas by ultrasound or CT has demonstrated that pancreatic TB can mimic a pancreatic neoplasm[4-6]. When this is the case, pancreatic head carcinoma must also be considered during the operation. We report the present case to emphasize alternate, though more rare, causes of pancreatic masses and obstructive jaundice.
According to the literature, endoscopic ultrasound-guided fine needle aspiration biopsy (FNAB) and a PCR-based approach are recommended for pancreatic TB diagnosis[7-9]. However, FNAB is not always employed due to the increased risk for pancreatitis and tumor dissemination. Thus, an operation with an incisional biopsy represents a more specific diagnostic modality and an effective therapy for pancreatic abscesses. Current literature also promotes the use of standard ATD therapy for six to twelve months as an effective strategy to treat pancreatic TB in a majority of cases[1,2,7,10]. As pancreatic TB can occur during treatment, however, ATD may be an insufficient therapy for patients. In the present case, we feel that an operation accompanied by cholecystostomy tube placement aided in treating the patient.
In conclusion, the case report emphasizes a rare cause of pancreatic mass and obstructive jaundice, and shows that an operation and tube cholecystostomy in combination with ATD therapy are an effective treatment for pancreatic TB.
A 40-year-old male patient with a history of thoracic tuberculosis (TB) presented with epigastralgia and jaundice while receiving anti-TB therapy.
An abdominal examination revealed sensitivity in the upper abdomen without hepatosplenomegaly or ascites.
Pancreatic carcinoma and pancreatitis.
The following laboratory results were obtained: 9.75 × 109 white blood cells/L; 30.5 μmol/L total bilirubin; 24.5 μmol/L direct bilirubin; 135 U/L alanine aminotransferase; 64 U/L aspartate aminotransferase; 715 U/L gamma glutamyl transpeptidase; 335U/L alkaline phosphatase; 66.84 U/mL carbohydrate antigen 199.
Computed tomography revealed a necrotic and calcified cystic lesion in the head of the pancreas that measured 5.6 cm × 4.2 cm and resembled a pancreatic malignancy.
Biopsies of the lymph nodes and mass revealed caseous granulomatous inflammation and necrosis with acid-fast bacilli; a polymerase chain reaction-based approach confirmed the presence of Mycobacterium tuberculosis DNA.
After surgery, the patient was administered the standard anti-TB regimen of isoniazid, rifampicin, pyrazinamide, and ethambutol for nine months followed by a regimen of isoniazid, rifampicin, and pyrazinamide for an additional six months.
TB is most often observed as necrotic granulomas within the lungs. Extrapulmonary TB, however, accounts for approximately 10%-30% of all cases and more than 5% of patients have abdominal involvement. In the abdominal cavity, TB predominantly affects the peritoneum, gastrointestinal tract (especially the ileum and cecum), liver, spleen, and lymph nodes. Pancreatic TB is extremely rare, likely due to resistance provided by pancreatic enzymes, and may occur as a result of bacterial dissemination from lymph nodes in the area. Pancreatic TB is often confused with pancreatic malignancy in clinical and radiological examinations.
TB is a disease caused by Mycobacterium tuberculosis bacteria. While the bacteria predominantly affect the lungs, they can also damage other parts of the body. Pancreatic TB occurs when these bacteria affect the pancreas. Pancreatic TB is extremely rare and can mimic a carcinoma, lymphoma or cystic neoplasia.
This case report highlights pancreatic TB as a rare cause of pancreatic masses and obstructive jaundice that can mimic pancreatic head carcinoma and be treated via tube cholecystostomy in combination with anti-TB therapy.
This article presented with the case report of a pancreatic TB which mimicking pancreatic head carcinoma in an immunocompetent host. After anti-tuberculin therapy, the patient recovered soon. This report is interesting and informative that provides useful information of rare disease as a cause of pancreatic masses and obstructive jaundice.
P- Reviewers: Kawa S, Seleem MI, Zippi M S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Meesiri S. Pancreatic tuberculosis with acquired immunodeficiency syndrome: a case report and systematic review. World J Gastroenterol. 2012;18:720-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Raghavan P, Rajan D. Isolated pancreatic tuberculosis mimicking malignancy in an immunocompetent host. Case Rep Med. 2012;2012:501246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hellara O, Noomène F, Toumi O. A pseudotumoral presentation of pancreatic tuberculosis. J Visc Surg. 2012;149:e282-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ozkan F, Bulbuloglu E, Inci MF, Sayar H, Kahraman H, Yuksel M. Isolated pancreatic tuberculosis mimicking malignancy and causing obstructive jaundice. J Gastrointest Cancer. 2013;44:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Arora A, Mukund A, Garg H. Isolated pancreatic tuberculosis: a rare occurrence. Am J Trop Med Hyg. 2012;87:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Lee WK, Van Tonder F, Tartaglia CJ, Dagia C, Cazzato RL, Duddalwar VA, Chang SD. CT appearances of abdominal tuberculosis. Clin Radiol. 2012;67:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Cheng J, Tadi K, Halpern M, Feurdean M, McNelis J, Brensilver J. Pancreatic tuberculosis in a human immunodeficiency virus positive patient: a case report. World J Gastroenterol. 2008;14:939-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Arroyo Martínez Q, DeCruz P, Murugananthan A, Desmond P, Chen R. Endoscopic ultrasound-guided fine needle aspirate: a useful method in the diagnosis of pancreatic tuberculosis. ANZ J Surg. 2012;82:282-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Chatterjee S, Schmid ML, Anderson K, Oppong KW. Tuberculosis and the pancreas: a diagnostic challenge solved by endoscopic ultrasound. A case series. J Gastrointestin Liver Dis. 2012;21:105-107. [PubMed] |
| 10. | Yavuz A, Buluş H, Aydin A, Coşkun A. Pancreatic tuberculosis mimicking inoperable pancreatic cancer. Turk J Gastroenterol. 2012;23:95-97. [PubMed] |