Published online May 16, 2014. doi: 10.12998/wjcc.v2.i5.126
Revised: March 15, 2014
Accepted: April 17, 2014
Published online: May 16, 2014
Processing time: 146 Days and 6.5 Hours
In this mini-review several commonly used animal models of atherosclerosis have been discussed. Among them, emphasis has been made on mice, rabbits, pigs and non-human primates. Although these animal models have played a significant role in our understanding of induction of atherosclerotic lesions, we still lack a reliable animal model for regression of the disease. Researchers have reported several genetically modified and transgenic animal models that replicate human atherosclerosis, however each of current animal models have some limitations. Among these animal models, the apolipoprotein (apo) E-knockout (KO) mice have been used extensively because they develop spontaneous atherosclerosis. Furthermore, atherosclerotic lesions developed in this model depending on experimental design may resemble humans’ stable and unstable atherosclerotic lesions. This mouse model of hypercholesterolemia and atherosclerosis has been also used to investigate the impact of oxidative stress and inflammation on atherogenesis. Low density lipoprotein (LDL)-r-KO mice are a model of human familial hypercholesterolemia. However, unlike apo E-KO mice, the LDL-r-KO mice do not develop spontaneous atherosclerosis. Both apo E-KO and LDL-r-KO mice have been employed to generate other relevant mouse models of cardiovascular disease through breeding strategies. In addition to mice, rabbits have been used extensively particularly to understand the mechanisms of cholesterol-induced atherosclerosis. The present review paper details the characteristics of animal models that are used in atherosclerosis research.
Core tip: This mini-review provides the essential information obtained from a number of animal models in the field of cardiovascular research. Such information can help researchers design their studies for understanding the pathophysiology of atherosclerosis.
- Citation: Kapourchali FR, Surendiran G, Chen L, Uitz E, Bahadori B, Moghadasian MH. Animal models of atherosclerosis. World J Clin Cases 2014; 2(5): 126-132
- URL: https://www.wjgnet.com/2307-8960/full/v2/i5/126.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i5.126
Atherosclerosis is still a leading cause of mortality and morbidity worldwide[1]. Several modifiable and non-modifiable risk factors have been identified for the disease. Many clinical and experimental attempts have been made to understand the pathophysiology of the disease. Among them, a number of animal models have been used for understanding the mechanisms involved in both induction and regression of atherosclerotic lesions. It was first in 1908 that Ignatowski reported atherogenesis in a rabbit model[2]. Subsequent studies documented a significant relationship between elevated levels of serum cholesterol and development of atherosclerotic lesions in experimental animals[3-6]. That was the basis of cholesterol-feeding trials in experimental models of atherosclerosis. However, later a naturally defective rabbit model namely Watanabe Heritable Hyperlipidemic (WHHL) rabbits was discovered and used in most of experimental settings[7-13]. Recent technology has allowed generation of a number of genetically-modified animal models in this field of research.
Overall, one may think that an ideal animal model for studying human disease should possess several features including availability, affordability and close resemblance to human conditions. In particular, an optimal animal model of atherosclerosis should develop various stages of the disease including fatty streaks, accumulation of foam cells, vulnerable and stable plaques as well as relevant complications such as calcification, ulceration, hemorrhage, plaque rapture, thrombosis and stenosis and the formation of aneurysms. Efforts are being made to develop animal models that replicate human atherosclerosis, however each of current animal models have some limitations. In this paper, attempts have been made to summarize the important features of the common animal models of atherosclerosis.
The mouse has been used in medical research for decades. Well-known genetic background, easy to breed and low cost of maintenance are among advantages of this model. However, small size and some physiological characteristics may be considered as limiting factors. For example, the plasma lipoprotein profile in mice is very different from that in humans. The circulating cholesterol is mainly in high density lipoprotein (HDL) particles in the mouse, while it is in low density lipoprotein (LDL) particles in humans. This is probably the main reason that wild-type mice do not develop atherosclerosis, but humans do. One reason for this lipoprotein profile differences is the absence of cholesterol-ester transfer protein (CETP) in the mouse[14]. Another difference between the mouse and the man is their response to dietary cholesterol. The mouse does not absorb dietary cholesterol significantly[15], while the man absorbs approximately 50% of dietary cholesterol. This may also be seen as a limiting factor for cholesterol-induced atherogensis in wild-type mice (C57BL/6J).
To overcome these limitations, researchers have used DNA technology to generate a number of genetically modified mouse models. It was in 1992 that the very first line of such genetically modified mouse model was introduced to our research community. Zhang et al[3] reported a successful deletion of mouse apolipoprotein (apo) E gene. Further research led to generation of other genetically modified mouse models suitable for studying human dyslipidemia and atherosclerosis. Among them, LDL receptor-knockout (KO)[16], hepatic lipase-KO[17], human apo B100 expression[18] and human CETP expression[19] can be named.
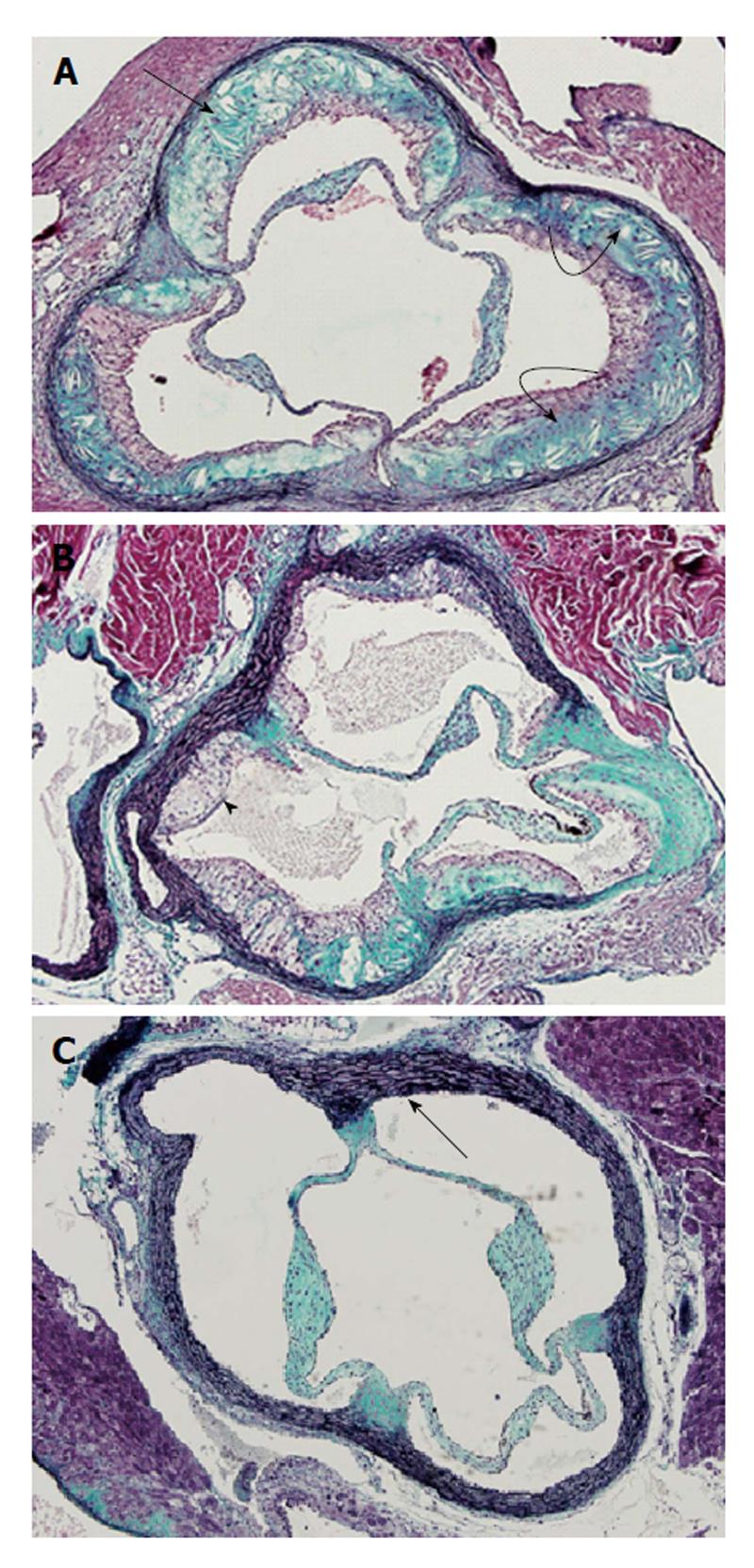
Of these genetically modified mouse models, apo E-KO mice develop spontaneous atherosclerosis. This is associated with elevated levels of circulating cholesterol-rich very LDL (VLDL) particles. This feature makes this animal model to be very robust for both induction and prevention of the disease. Several studies have reported that hypercholesterolemia and atherosclerosis can be prevented by dietary plant sterols in this animal model[20-22]. Unlike apo E-KO mice, LDL-r-KO mice need dietary cholesterol to develop hypercholesterolemia and atherosclerosis[16,23]. Morphological features of atherosclerotic lesions in apo E-KO and LDLr-KO mice are illustrated on Figure 1. Another mouse model which develops hypercholesterolemia and atherosclerosis on high fat/high cholesterol diets is apo E*3-Leiden transgenic mice[24-26]. Furthermore, a number of breeding experiments have been carried out to generate additional mouse models of human dyslipidemia and atherosclerosis. For example, cross breeding of human apo B100 transgenic mice with LDL receptor deficient mice produced a highly susceptible strain (HuBTg+/+Ldlr-/-) with severe hypercholesterolemia and atherosclerosis[16]. Furthermore, Föger et al[19] reported that when human lecithin cholesterol acyl transferase (LCAT) transgenic mice were cross-bred with CETP transgenic mice they produce offspring with low total cholesterol levels and reduced atherosclerosis burden. Similarly, Lweis et al[27] generated apoE/GPx1 double KO (ApoE-/- GPx1-/-), by cross breeding GPx1-deficient mice with apo E-deficient mice. This model features combined hyperlipidemia and hyperglycemia with increased oxidative stress. Chen et al[28] reported a mouse model that develops unstable/ruptured atherosclerotic plaques. They used surgical procedures to introduce a tandem stenosis in the carotid artery of apo E-/- mice fed a high fat diet, in order to develop unstable plaques in these mice. Atherosclerosis is known to be an inflammatory disease. We know that there are major differences in immune system between mice and humans[29]. This is another reason for questioning the mouse model for studying human atherosclerosis. Several inflammatory markers have been detected in both atherosclerotic plaques and in circulation of subjects or animals with atherosclerosis. Several strategies have been implemented to understand the role of inflammatory pathways in the progression of the disease and its complications. One of such experimental strategies was application of bone marrow transplant. Ishibashi and colleagues used bone marrow transplant procedures to produce apo E-KO mice with and without deficiency of chemoattractant protein-1 receptor, CCR2[30]. Results from their studies suggest that CCR2 plays a crucial role in vascular inflammation and atherosclerosis. In another study, bone marrow transplant procedures were used to investigate the effects of macrophage-derived apo E on atherogenesis in apo E-KO mice[31]. Transplantation of bone marrow from wild-type mice to apo E-KO mice resulted in significant reductions in the formation of atherosclerotic lesions in apo E-KO mice.
Although we have significantly advanced our knowledge in understanding the process of induction of atherosclerotic lesions using various mouse model, our knowledge on regression of these lesions is still very limited. In an attempt to regress atherosclerotic lesions we used apo E-Ko mice. Over a 42-wk of low-fat diet and diets enriched phytosterols, we were unable to regress atherosclerotic lesions in this animal model[32]. Similarly, literature search did not show any significant evidence for a successful regression of advanced atherosclerotic lesions.
The rabbit has been used in many research facilities as an animal model of diet induced atherosclerosis. This species shares several aspects of lipoprotein metabolism with humans. These include composition of apo B containing lipoproteins[33], production of apo B100 containing VLDL by the liver[34] plasma CETP activity[35], and high absorption rate of dietary cholesterol[35]. However, the lack of hepatic lipase makes the rabbit to be different from man[36]. Dietary approach is a common method to induce atherogenesis in rabbits. Under these conditions, the animals develop atherosclerotic lesions in the aortic arch and thoracic aorta rather than abdominal aorta which is almost always affected in humans[36].
Two strains of rabbits, namely WHHL and St. Thomas’ Hospital (STH) rabbits are naturally defective and relevant models for human hyperlipidemia[7-13,37-39]. The WHHL rabbits are deficient in LDL receptors and therefore resemble human familial hypercholesterolemia[7-13,37], while STH rabbits are used as a model for human hypertriglyceridemia and combined hyperlipidemia[38,39]. Recent advances in gene technology have allowed generation of transgenic rabbits. For example, New Zealand White rabbits have been used to produce human apo B100 transgenic rabbits; these animals manifest combined hyperlipidemia with reduced HDL-cholesterol concentrations[33]. On the other hand, over expression of human apo AI or human LCAT in rabbits was associated with elevated HDL-cholesterol levels and reduced atherosclerosis[34,35].
Pigs have been used for induction of coronary atherosclerosis by several different laboratories[40-42]. However, induction of advanced atherosclerotic lesions required high levels of dietary cholesterol (up to 4% w/w)[41,43,44]. A strain of pigs has been discovered with three lipoprotein-associated mutations (designated Lpb5, Lpr1, and Lpu1) developing hypercholesterolemia and atherosclerosis without dietary cholesterol[43,44]. In addition to coronary arteries, iliac and femoral arties also develop atherosclerotic lesions which become complicated by 2 years of age[44].
Spontaneous atherosclerosis has been reported in squirrel monkeys, baboons, wooly and spider monkeys[45]. Similar to humans, monkeys can be divided into “hyper-responders” and “hypo-responders”[46,47]. Anatomical locations of atherosclerotic lesions vary among different strains of monkeys. For example, majority of atherosclerotic lesions are found in the anterior descending and circumflex branches of the left coronary artery in rhesus monkeys, while cebus monkeys develop such lesions in their carotid bifurcation and coronary arteries[45,48]. The cynomolgus monkeys and African green monkeys also vary from each other in the location of atherosclerotic lesions being developed in coronary arteries and abdominal aorta, respectively[49].
Dogs[50,51], hamsters[52,53], guinea pigs[54], and birds[55,56] have been also used in experimental atherosclerosis. However, these animals have shown a significant amount of limitations that has not allowed popularity for the use of such animals extensively.
This mini-review aims to summarize the features of the most commonly used animal models of atherosclerosis. Despite many advances in medical research, we still do not have specific animal models for specific human conditions. Every animal model has its own advantages and disadvantages. For example, while non-human-primates are the closest animals to humans, variability in lesion development, high cost, availability, possible hazard, ethical issues and handling matters are among major limitations in the use of these animals. Mice and rabbits also vary from humans in regard to lipoprotein metabolism and development of atherosclerotic lesions. However, these species have been the most common animal models used so far. Among these species, either naturally defective animals such as WHHL and STH rabbits[7-13,37-39,57-59], or genetically modified mice have been used extensively in atherosclerosis research. In particular, LDL-r-KO mice[23,60], apo E-KO mice[61-63] , Cystathionine γ-lyase-KO mice[64] have contributed to our understanding of the disease pathophysiology. Furthermore, surgical procedures and infectious agents have been also used in a number of animal models to study the postoperative injuries such as neointimal hyperplasia[65], atherosclerotic plaque instability[28] or the role of Chlamydophila pneumoniae[66,67] in disease development. The use of these animal models has certainly advanced our knowledge of the induction of atherosclerotic lesions. However, there is no reliable animal model for regression of atherosclerotic lesions. Further research and development is needed to generate such animal models. The list of animal model and their characteristic are summarized in Table 1.
| Model | Features | Ref. |
| Mice | ||
| Apo E-/- mice | Develops spontaneous atherosclerosis, associated with elevated levels of circulating cholesterol-rich VLDL particles | Zhang et al[3] |
| LDL receptor deficient KO mice | This model needs dietary cholesterol to develop hypercholesterolemia and atherosclerosis- associated with elevated levels of circulating cholesterol-rich LDL and VLDL particles | Sanan et al[16] |
| Apo E*3-Leiden transgenic mice | This model needs dietary cholesterol to develop hypercholesterolemia and atherosclerosis-associated with elevated levels of circulating cholesterol | Groot et al[24] van Vlijmen et al[25] |
| Hepatic lipase-KO mice | This model lacks hepatic lipase and develops elevated levels of plasma cholesterol, phospholipids, and HDL cholesterol and can be used for the study of HDL metabolism. | Homanics et al[17] |
| Human apo B100 transgenic mice | This mice model, associated with substantial increased level of LDL cholesterol level and useful for studying various aspects of lipoprotein metabolism and for further delineating the role of LDL in atherogenesis. | Greeve et al[18] |
| Human CETP transgenic mice | This model has reported to have decreased HDL cholesterol levels with variable degree of atherosclerosis | Föger et al[19] |
| Cross breeding of human apo B100 transgenic mice with LDL receptor deficient mice | This model develops severe hypercholesterolemia and atherosclerosis | Sanan et al[16] |
| Cross breeding of human LCAT transgenic mice with CETP transgenic mice. | A mouse model with low total cholesterol levels and reduced atherosclerosis burden. | Föger et al[19] |
| Apo E/GPx1 double knockout (apo E-/- GPx1-/-) | This model features combined hyperlipidemia and hyperglycemia with increased oxidative stress | Lewis et al[27] |
| Surgical model of apo E-/- mice | A mouse model for studying unstable/ruptured atherosclerotic plaques | Chen et al[28] |
| Animal model developed using bone marrow technique | Apo E-KO mice model with and without deficiency of CCR2 | Ishibashi et al[30] |
| Rabbits | ||
| WHHL | Naturally deficient in LDL receptors resembling human familial hypercholesterolemia | Watanabe[10] |
| STH | Rabbit model for human hypertriglyceridemia and combined hyperlipidemia | Beaty et al[38] |
| NZW-human apo B100 transgenic rabbits | Transgenic animal model manifesting combined hyperlipidemia with reduced HDL-cholesterol concentrations | Fan et al[33] |
| NZW-human apo AI or human LCAT transgenic rabbits | Rabbit model with elevated HDL-cholesterol levels and reduced atherosclerosis | Duverger et al[34] |
| Pigs | ||
| Lipoprotein-associated mutations (designated Lpb5, Lpr1, and Lpu1) | This pig model develops hypercholesterolemia and atherosclerosis without dietary cholesterol. In addition to coronary arteries, iliac and femoral arties also develop atherosclerotic lesions which become complicated by 2 year of age. | Prescott et al[44] |
| Non-human primates | ||
| Rhesus monkeys | Develops spontaneous atherosclerosis. This animal model develops majority of atherosclerotic lesions in the anterior descending and circumflex branches of the left coronary artery | Carey[45] |
| Cebus monkeys | Develops spontaneous atherosclerosis. This animal model develops atherosclerotic lesions in their carotid bifurcation and coronary arteries | Carey[45] |
| Cynomolgus monkeys and African green monkeys | These monkeys develop spontaneous atherosclerosis. Atherosclerotic lesions being developed in coronary arteries and abdominal aorta, respectively | Hollander et al[49] |
| Others | ||
| Dogs Hamsters Guinea pigs Birds | These animal models have significant amount of limitations that have not extensively used | Geer et al[50] Nistor et al[52] Fernandez et al[54] Wagner et al[56] |
Dr. Moghadasian’s research program currently is supported by Natural Sciences and Engineering Research Council of Canada. SG is a recipient of Manitoba Health Research Council post-doctoral fellowship.
P- Reviewers: Carlos F, Prosser HC, Ramji DP, Tzortzis N S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | World Health Organization. Cardiovascular diseases (CVDs). 2013; Available from: http: //www.who.int/mediacentre/factsheets/fs317/en/. |
| 2. | Ignatowski AC. Influence of animal food on the organism of rabbits. S Peterb Izviest Imp Voyenno-Med Akad. 1908;16:154-173. |
| 3. | Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1628] [Cited by in RCA: 1707] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 4. | Véniant MM, Zlot CH, Walzem RL, Pierotti V, Driscoll R, Dichek D, Herz J, Young SG. Lipoprotein clearance mechanisms in LDL receptor-deficient “Apo-B48-only” and “Apo-B100-only” mice. J Clin Invest. 1998;102:1559-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Mezdour H, Jones R, Dengremont C, Castro G, Maeda N. Hepatic lipase deficiency increases plasma cholesterol but reduces susceptibility to atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 1997;272:13570-13575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Purcell-Huynh DA, Farese RV, Johnson DF, Flynn LM, Pierotti V, Newland DL, Linton MF, Sanan DA, Young SG. Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J Clin Invest. 1995;95:2246-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Kondo T, Watanabe Y. A heritable hyperlipemic rabbit. Jikken Dobutsu. 1975;24:89-94. [PubMed] |
| 8. | Watanabe Y, Ito T, Kondo T. Breeding of a rabbit strain of hyperlipidemia and characteristics of the strain. Exp Anim. 1977;26:35-42. |
| 9. | Watanabe Y. Studies on characteristics of spontaneously hyperlipemic rabbits and development of the strain with such property. Bull Azabu Vet Coll. 1977;2:99-124. |
| 10. | Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis. 1980;36:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 434] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Tanzawa K, Shimada Y, Kuroda M, Tsujita Y, Arai M, Watanabe H. WHHL-rabbit: a low density lipoprotein receptor-deficient animal model for familial hypercholesterolemia. FEBS Lett. 1980;118:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Raymond TL, Reynolds SA, Swanson JA, Patnode CA, Bell FP. The effect of oral l-carnitine on lipoprotein composition in the Watanabe Heritable Hyperlipidemic Rabbit (Oryctolagus cuniculus). Comp Biochem Physiol A Comp Physiol. 1987;88:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Szeto A, Rossetti MA, Mendez AJ, Noller CM, Herderick EE, Gonzales JA, Schneiderman N, McCabe PM. Oxytocin administration attenuates atherosclerosis and inflammation in Watanabe Heritable Hyperlipidemic rabbits. Psychoneuroendocrinology. 2013;38:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Barter PJ, Brewer HB, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 636] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 15. | Carter CP, Howles PN, Hui DY. Genetic variation in cholesterol absorption efficiency among inbred strains of mice. J Nutr. 1997;127:1344-1348. [PubMed] |
| 16. | Sanan DA, Newland DL, Tao R, Marcovina S, Wang J, Mooser V, Hammer RE, Hobbs HH. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc Natl Acad Sci USA. 1998;95:4544-4549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Homanics GE, de Silva HV, Osada J, Zhang SH, Wong H, Borensztajn J, Maeda N. Mild dyslipidemia in mice following targeted inactivation of the hepatic lipase gene. J Biol Chem. 1995;270:2974-2980. [PubMed] |
| 18. | Greeve J, Altkemper I, Dieterich JH, Greten H, Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993;34:1367-1383. [PubMed] |
| 19. | Föger B, Chase M, Amar MJ, Vaisman BL, Shamburek RD, Paigen B, Fruchart-Najib J, Paiz JA, Koch CA, Hoyt RF. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J Biol Chem. 1999;274:36912-36920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Moghadasian MH, McManus BM, Pritchard PH, Frohlich JJ. “Tall oil”-derived phytosterols reduce atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Moghadasian MH, McManus BM, Godin DV, Rodrigues B, Frohlich JJ. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Circulation. 1999;99:1733-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Xu Z, Le K, Moghadasian MH. Long-term phytosterol treatment alters gene expression in the liver of apo E-deficient mice. J Nutr Biochem. 2008;19:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Surendiran G, Goh C, Le K, Zhao Z, Askarian F, Othman R, Nicholson T, Moghadasian P, Wang YJ, Aliani M. Wild rice (Zizania palustris L.) prevents atherogenesis in LDL receptor knockout mice. Atherosclerosis. 2013;230:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Groot PH, van Vlijmen BJ, Benson GM, Hofker MH, Schiffelers R, Vidgeon-Hart M, Havekes LM. Quantitative assessment of aortic atherosclerosis in APOE*3 Leiden transgenic mice and its relationship to serum cholesterol exposure. Arterioscler Thromb Vasc Biol. 1996;16:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | van Vlijmen BJ, Pearce NJ, Bergö M, Staels B, Yates JW, Gribble AD, Bond BC, Hofker MH, Havekes LM, Groot PH. Apolipoprotein E*3-Leiden transgenic mice as a test model for hypolipidaemic drugs. Arzneimittelforschung. 1998;48:396-402. [PubMed] |
| 26. | Lutgens E, Daemen M, Kockx M, Doevendans P, Hofker M, Havekes L, Wellens H, de Muinck ED. Atherosclerosis in APOE*3-Leiden transgenic mice: from proliferative to atheromatous stage. Circulation. 1999;99:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Chen YC, Bui AV, Diesch J, Manasseh R, Hausding C, Rivera J, Haviv I, Agrotis A, Htun NM, Jowett J. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ Res. 2013;113:252-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731-2738. [PubMed] |
| 30. | Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, Charo IF, Kura S, Tsuzuki T, Takeshita A. Bone marrow-derived monocyte chemoattractant protein-1 receptor CCR2 is critical in angiotensin II-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:e174-e178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Boisvert WA, Spangenberg J, Curtiss LK. Treatment of severe hypercholesterolemia in apolipoprotein E-deficient mice by bone marrow transplantation. J Clin Invest. 1995;96:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Moghadasian MH, Godin DV, McManus BM, Frohlich JJ. Lack of regression of atherosclerotic lesions in phytosterol-treated apo E-deficient mice. Life Sci. 1999;64:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Fan J, McCormick SP, Krauss RM, Taylor S, Quan R, Taylor JM, Young SG. Overexpression of human apolipoprotein B-100 in transgenic rabbits results in increased levels of LDL and decreased levels of HDL. Arterioscler Thromb Vasc Biol. 1995;15:1889-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation. 1996;94:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Hoeg JM, Santamarina-Fojo S, Bérard AM, Cornhill JF, Herderick EE, Feldman SH, Haudenschild CC, Vaisman BL, Hoyt RF, Demosky SJ. Overexpression of lecithin: cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc Natl Acad Sci USA. 1996;93:11448-11453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Warren RJ, Ebert DL, Mitchell A, Barter PJ. Rabbit hepatic lipase cDNA sequence: low activity is associated with low messenger RNA levels. J Lipid Res. 1991;32:1333-1339. [PubMed] |
| 37. | Aliev G, Burnstock G. Watanabe rabbits with heritable hypercholesterolaemia: a model of atherosclerosis. Histol Histopathol. 1998;13:797-817. [PubMed] |
| 38. | Beaty TH, Prenger VL, Virgil DG, Lewis B, Kwiterovich PO, Bachorik PS. A genetic model for control of hypertriglyceridemia and apolipoprotein B levels in the Johns Hopkins colony of St. Thomas Hospital rabbits. Genetics. 1992;132:1095-1104. [PubMed] |
| 39. | Nordestgaard BG, Lewis B. Intermediate density lipoprotein levels are strong predictors of the extent of aortic atherosclerosis in the St. Thomas’s Hospital rabbit strain. Atherosclerosis. 1991;87:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Stout LC. Pathogenesis of diffuse intimal thickening (DIT) in aortas and coronary arteries of 2 1/2-year-old miniature pigs. Exp Mol Pathol. 1982;37:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Griggs TR, Bauman RW, Reddick RL, Read MS, Koch GG, Lamb MA. Development of coronary atherosclerosis in swine with severe hypercholesterolemia. Lack of influence of von Willebrand factor or acute intimal injury. Arteriosclerosis. 1986;6:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Holvoet P, Theilmeier G, Shivalkar B, Flameng W, Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arterioscler Thromb Vasc Biol. 1998;18:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Rapacz J, Hasler-Rapacz J, Taylor KM, Checovich WJ, Attie AD. Lipoprotein mutations in pigs are associated with elevated plasma cholesterol and atherosclerosis. Science. 1986;234:1573-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Prescott MF, McBride CH, Hasler-Rapacz J, Von Linden J, Rapacz J. Development of complex atherosclerotic lesions in pigs with inherited hyper-LDL cholesterolemia bearing mutant alleles for apolipoprotein B. Am J Pathol. 1991;139:139-147. [PubMed] |
| 45. | Carey KD. Non-human primate models of atherosclerosis. Atherosclerosis: Its Pediatric Aspects. Orlando Grune and Stratton 1978; 41-83. |
| 46. | Bullock BC, Lehner ND, Clarkson TB, Feldner MA, Wagner WD, Lofland HB. Comparative primate atherosclerosis. I. Tissue cholesterol concentration and pathologic anatomy. Exp Mol Pathol. 1975;22:151-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Clarkson TB, Lofland HB, Bullock BC, Goodman HO. Genetic control of plasma cholesterol. Studies on squirrel monkeys. Arch Pathol. 1971;92:37-45. [PubMed] |
| 48. | Bullock BC, Clarkson TB, Lehner ND, Lofland HB, St Clair RW. Atherosclerosis in Cebus albifrons monkeys. [. Clinical and pathologic studies. Exp Mol Pathol. 1969;10:39-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Hollander W, Kirkpatrick B, Paddock J, Colombo M, Nagraj S, Prusty S. Studies on the progression and regression of coronary and peripheral atherosclerosis in the cynomolgus monkey. I. Effects of dipyridamole and aspirin. Exp Mol Pathol. 1979;30:55-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Geer JC, Guitry MA. Experimental canine atherosclerosis. In: Roberts JC, Straus R, eds. Comparative atherosclerosis. Hoeber, Harper and Row, New York 1965; 170-185. |
| 51. | Mahley RW, Weisgraber KH, Innerarity T. Canine lipoproteins and atherosclerosis. II. Characterization of the plasma lipoproteins associated with atherogenic and nonatherogenic hyperlipidemia. Circ Res. 1974;35:722-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Nistor A, Bulla A, Filip DA, Radu A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis. 1987;68:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Dillard A, Matthan NR, Lichtenstein AH. Use of hamster as a model to study diet-induced atherosclerosis. Nutr Metab (Lond). 2010;7:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Fernandez ML, Volek JS. Guinea pigs: a suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr Metab (Lond). 2006;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Horlick L, Katz LN. Retrogression of atherosclerotic lesions on cessation of cholesterol feeding in the chick. J Lab Clin Med. 1949;34:1427-1442. [PubMed] |
| 56. | Wagner WD, Clarkson TB, Feldner MA, Prichard RW. The development of pigeon strains with selected atherosclerosis characteristics. Exp Mol Pathol. 1973;19:304-319. [PubMed] |
| 57. | Brousseau ME, Hoeg JM. Transgenic rabbits as models for atherosclerosis research. J Lipid Res. 1999;40:365-375. [PubMed] |
| 58. | Moghadasian MH, Frohlich JJ, McManus BM. Advances in experimental dyslipidemia and atherosclerosis. Lab Invest. 2001;81:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Konya A. Animal models for atherosclerosis, restenosis, and endovascular aneurysm repair. In: Conn PM (Ed). Sourcebook of models for biomedical research. Springer-Verlag New York, LLC 2008; 369-384. |
| 60. | Bombo RP, Afonso MS, Machado RM, Lavrador MS, Nunes VS, Quintão ER, Koike M, Catanozi S, Lin CJ, Nakandakare ER. Dietary phytosterol does not accumulate in the arterial wall and prevents atherosclerosis of LDLr-KO mice. Atherosclerosis. 2013;231:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Zhao R, Moghadasian MH, Shen GX. Involvement of NADPH oxidase in up-regulation of plasminogen activator inhibitor-1 and heat shock factor-1 in mouse embryo fibroblasts induced by oxidized LDL and in apolipoprotein E-deficient mice. Free Radic Res. 2011;45:1013-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Tan Z, Le K, Moghadasian M, Shahidi F. Enzymatic synthesis of phytosteryl docosahexaneates and evaluation of their anti-atherogenic effects in apo-E deficient mice. Food Chem. 2012;134:2097-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Cleverley K, Du X, Premecz S, Le K, Zeglinski M, Nicholson T, Goh CY, Lu Y, Anderson HD, Moghadasian MH. The effects of fish oil consumption on cardiovascular remodeling in ApoE deficient mice. Can J Physiol Pharmacol. 2013;91:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 65. | Reis ED, Roque M, Dansky H, Fallon JT, Badimon JJ, Cordon-Cardo C, Shiff SJ, Fisher EA. Sulindac inhibits neointimal formation after arterial injury in wild-type and apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 2000;97:12764-12769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Mäkelä PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983-986. [PubMed] |
| 67. | Deniset JF, Cheung PK, Dibrov E, Lee K, Steigerwald S, Pierce GN. Chlamydophila pneumoniae infection leads to smooth muscle cell proliferation and thickening in the coronary artery without contributions from a host immune response. Am J Pathol. 2010;176:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |