Published online Dec 16, 2014. doi: 10.12998/wjcc.v2.i12.893
Revised: October 28, 2014
Accepted: October 31, 2014
Published online: December 16, 2014
Processing time: 266 Days and 22.9 Hours
AIM: To conduct a detailed systematic review of the current evidence on the administration and efficacy of tranexamic acid in patients with menorrhagia due to uterine fibroids.
METHODS: We conducted an electronic search on the following databases PubMed and Medline (1950-2013); (1980-2013); Cochrane library (1993-2013).
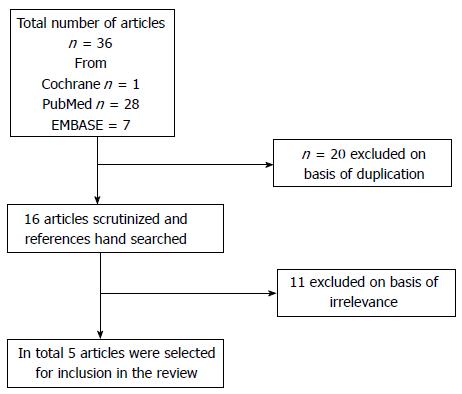
RESULTS: A total of 36 articles were retrieved after the initial electronic search. Careful assessment of the retrieved studies led to the final selection of 5 articles for inclusion in the review.
CONCLUSION: Tranexamic acid may reduce blood loss perioperatively in myomectomies. It may reduce the menorrhagia in patients with fibroids, however a stratification of fibroids by size and location is required to define the responses. It is safe in general, with mild adverse effects observed in some cases. More studies with a double-blind randomized design and larger numbers of participants are necessary to reach more precise and safe conclusions.
Core tip: Uterine fibroid tumors are the most common gynecologic causes for menorrhagia. Tranexamic acid is a safe non-hormonal medication that significantly reduces abnormal menstrual bleeding. We conducted a systematic review of the contemporary evidence on the administration and efficacy of tranexamic acid in patients with menorrhagia associated with fibroid tumors of the uterus. Antifibrinolytic treatment may reduce blood loss perioperatively in myomectomies, and reduce menorrhagia in patients with fibroids. More double randomized studies with larger numbers of participants are necessary to reach more precise and safe conclusions.
- Citation: Peitsidis P, Koukoulomati A. Tranexamic acid for the management of uterine fibroid tumors: A systematic review of the current evidence. World J Clin Cases 2014; 2(12): 893-898
- URL: https://www.wjgnet.com/2307-8960/full/v2/i12/893.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i12.893
Worldwide, approximately 235 million women are affected by uterine fibroids and about 20%-40% of women will be diagnosed with leiomyomas at some point in their life, though only a fraction of those will cause problems or require treatment[1]. Uterine fibroid tumors or leiomyomas very often lead to abnormal menstrual bleeding or menorrhagia[2]. Menorrhagia is abnormal extensive menstrual bleeding in cases where the quantity of the overall blood loss exceeds 80 mL in every menses[3].
The treatment of uterine leiomyoma may be surgical or conservative. Surgical management consists of total or subtotal hysterectomy and myomectomy, but in some cases less invasive procedures, such as uterine artery embolization are successful[3].
Data show the presence of extensive fibrinolysis in the menstrual blood of women suffering from menorrhagia, and this has triggered the use of antifibrinolytic drugs as a therapeutic option[4]. Tranexamic acid (cyklokapron) is a non-hormonal medication that decreases menstrual hemorrhage and it is an excellent therapeutic option in patients with menorrhagia who opt for nonhormonal management[5]. Tranexamic acid achieves hemostasis and elicits its antifibrinolytic action by reversible block of the locus that connects with lysine on plasminogen molecules. It inactivates the plasminogen activator of the endometrium and thus stops fibrinolysis and degradation of the clotting complexes[6]. Tranexamic acid has been administered on a daily basis to reduce excessive hemorrhaging and the need for transfusion during and after major cardiac or orthopedic surgeries[7].
In the international literature, several randomized clinical trials have been published which have evaluated and reviewed the efficacy of tranexamic acid in the management of abnormal gynecological hemorrhagic conditions. It is not certain how efficient tranexamic acid is in treating women with normal reproductive function and diagnosed with abnormal bleeding caused be uterine fibroids[8].
The aim of the study was to conduct a systematic review of the current evidence on the administration and efficacy of tranexamic acid in patients with menorrhagia caused by uterine myomas. The administration of tranexamic acid during the preoperative and postoperative period as a method of reducing blood loss is also reviewed. No previous systematic review of the use of tranexamic acid in women with fibroids has been reported.
We conducted an electronic search on the following databases PubMed and Medline (1950-2014); EMBASE (1980-2013); Cochrane library (1993-2014). The Medical Subject Headings which were utilized were as follows: “tranexamic acid” and “fibroids” and “myomas” and “leiomyomas” and “myomectomy”.
Manuscripts written in English or French languages were selected for inclusion in the study. The retrieved studies were scrutinized and their references were examined carefully in order to reveal any relevant studies not identified initially by the electronic search. The included studies were reviewed independently by two authors (PP and AK). In cases of discrepancy and lack of evidence, the corresponding authors of the studies were contacted to provide further information and clarification. Studies from conferences and scientific meetings were also searched.
From each study, we gathered the following clinical data: author and year of publication; country of origin of study; type of study; number of participants in the study; aim of the study; dosage, type and length of administration of tranexamic acid; data about adverse effects and the conclusion of study results. The clinical data were collected and presented in a table according to chronological order of publication.
All the studies reported the administration of tranexamic acid for treatment of hemorrhage in women of reproductive age with symptomatic fibroids. In addition, studies that reported the administration of tranexamic acid preoperatively or postoperatively to myomectomy procedures were selected for inclusion.
Studies that reported the use of tranexamic acid in pregnant women and in women diagnosed with malignant gynecological disease were excluded. Also studies in women with non-symptomatic fibroids, postmenopausal women and women with hemorrhage related to reasons other than fibroids (dysfunctional uterine bleeding; hematological disorders) were not included in the review.
The quality assessment of studies was performed according to the guidelines reported from The Scottish Intercollegiate Guidelines Network (SIGN)[9].
A total of 36 articles were retrieved after the initial electronic search. These 36 articles were scrutinized for duplicate results. The flowchart diagram of the selection process is shown in Figure 1. Eleven articles were excluded because they were not concerned with management of menorrhagia in women with fibroid tumors. Careful assessment of the retrieved studies led to the final selection of 5 articles to be included in the study. A summary of the selected articles is presented in Table 1[10-14]. The reviewed studies originated from Europe[10,11]; Аsia[12] and America[13,14]. All articles were written in English. The studies were published between 1998 and 2013. The total study population from the 5 studies was 349 women; 206 patients were treated with tranexamic acid and 101 patients were allocated to the placebo groups. All patients were premenopausal with a mean age of 37.65 ± 3.2 years. All patients in the study groups presented with menorrhagia and pelvic pain due to uterine fibroids. Three studies had a double-blind randomized design[11,13,14], one had an observational design[12] and one had a prospective longitudinal design[10]. Tranexamic acid was administered orally in four studies[10,12-14], and intravenously in one study[11]. Tranexamic acid reduced the blood loss perioperatively in women undergoing myomectomy in comparison to women not receiving tranexamic acid according to the authors[11]. Management of excessive bleeding during the menstrual cycle with tranexamic acid decreased hemorrhage despite the existence of myomas[13].
| Ref. | Country | Type of study | Meanage | Symptom-atology | Participants | Aim of thestudy | Regimenadministration | Results | Adverse effects | Comment |
| Lakhani et al[10] | United Kingdom | Longitudinal Prospective | 42.8 | Menorrhagia Pelvic pain | n = 12 | Ultrasound assesment of PI and RI of UA in women with TA administration | Tranexamic acid P.O. 1 g x 3 for 2 cycles | No significant changes in blood loss or PI and RI | Not reported | No changes in UA-PI resistance in women with fibroids |
| Caglar et al[11] | Turkey | Prospective randomized double-blind placebo | 34.2 | Menorrhagia Pelvic pain | n = 50 (TA) n = 50 (Placebo) | To compare the perioperative blood loss in patients undergoing myomectomy and taking TA with patients not taking TA | Tranexamic acid 10 mg/kg iv (max 1 g) 15 min before incision | Significant statistical differences in two groups postoperative, total blood loss and duration of surgery (P < 0.01) in favor of TA | Not reported | TA does not reduce perioperative blood loss nor Hb levels. It reduces postoperative and total blood loss and surgery time in correlation with myoma size. However further investigations required |
| Ip et al[12] | Hong Kong | Observational | 43.8 ± 25 | Menorrhagia Pelvic pain | n = 22 | Pathology assesment of fibroid specimen in women receiving TA | Tranexamic acid Per os dosage not reported | Necrosis and infarcts in resected fibroids. Larger daimeter fibroids more prone to necrosis changes. Size is an independent factor | Not reported | Authors emphasize the necrosis and thrombosis in fibroids but suggest precaution for complications |
| Lukes et al[14] | United States | Randomized double-blind placebo | 36.5 | Menorrhagia Pelvic pain | n = 42 (TA) n = 26 (Placebo) | To assess the efficacy and safety of TA for heavy menstrual bleeding | Tranexamic acid Per os 1.3 g daily for 5 d up to 6 cycles | Reduction in menstrual blood loss in women receiving TA compared to placebo. No statistically significant changes in blood loss in patients with fibroids | Mild adverse effects Menstrual cramps Gastrointestinal allergies | TA was effective in the treatment of heavy menstrual bleeding regardless of the presence or absence of fibroids |
| Eder et al[13] | United States | Randomized double-blind placebo | 38 | Menorrhagia Pelvic pain | n = 96 (TA) n = 51 (Placebo) | To compare the menstrual blood loss in women with fibroids and TA and women with fibroids not taking TA. consisting placebo group | Tranexamic acid Per os 3.9 g/d for 5 d up to 6 menstrual cycles | Menstrual blood loss reduced in women receiving TA (P < 0.001) | 3 patients in TA group and 3 in placebo group reported headache | TA was well tolerated and reduced menstrual blood loss |
Furthermore, tranexamic acid reduced the quantity of bleeding in patients with menorrhagia in a pivotal phase III randomized double blind study[14]. The authors reported that they estimated the quantity of blood via a validated alkaline hematin method in patients with sonographically confirmed fibroids[14]. Tranexamic acid did not alter the pulsatility index during ultrasound assessment of women with fibroids[10]. Significant pathologic changes were noted in specimens from women who received tranexamic acid and underwent myomectomy. Very mild complications of treatment were seen in 2 studies[13,14], and 3 women in a single study reported headache[14].
The quality assessment of the selected studies according to SIGN criteria is shown in Table 2. Three studies were graded 2++ (high quality studies)[11,13,14] and 2 studies were graded 2+ (well conducted studies)[10,12]. All studies were conducted in University teaching hospitals[10-14], 3 were conducted in a single setting[10-12], and 2 were conducted in multicenter settings[13,14]. Three studies received financial support from pharmaceutical companies[10,13,14].
| Ref. | Setting | Sign grade | Interpretation |
| Lakhani et al[10] 1998 | University teaching hospital | 2+ | Well conducted study |
| Caglar et al[11] 2007 | University teaching hospital | 2++ | High quality study |
| Ip et al[12] 2007 | University teaching hospital | 2+ | Well conducted study |
| Lukes et al[14] 2010 | University teaching hospital | 2++ | High quality study |
| Eder et al[13] 2013 | Private research institution and University teaching hospital | 2++ | High quality study |
In practice, tranexamic acid has been administered in many clinical situations in which the inhibition of fibrinolysis has shown beneficial effects in managing hemorrhage. The use of tranexamic acid in Obstetrics and Gynecology as a conservative method for reducing blood loss has been extensive[15]. TA provides a non-hormonal, treatment for patients with excessive hemorrhage during the menstrual period[13]. How tranexamic acid manages menorrhagia provoked by leiomyoma is still unclear and unknown due to the limited data.
In the current review, according to the reported studies, tranexamic acid is a safe treatment and may reduce menorrhagia in women with fibroids. It reduces the blood loss perioperatively with no adverse effects in women undergoing laparotomy and myomectomy. Tranexamic acid causes necrosis in myomas but does not alter pulsatility indices in ultrasound assessment. However, the current review has some limitations because of the quantity and quality of studies published in the literature and the presence of bias related to the size and location of fibroids.
Despite the fact that tranexamic acid administration has shown a risk for complications like thrombosis and embolism due to its antifibrinolytic effect, thromboembolic events were not been reported in the selected studies. Only mild headaches, allergies and discomfort were reported in a small population of patients[13,14]. In the study by Lukes et al[14], the authors did not specify the exact type and number of adverse effects in patients with fibroids, and stated that the most common adverse effect was menstrual discomfort.
Tranexamic acid has been administered widely in Scandinavian and European countries in general as a first-line management option for menorrhagia since the 1970s, and data have shown no increase in the frequency of adverse clotting disorders[16,17]. However, the optimal dose and duration of treatment with tranexamic acid has not been established[18].
The efficacy and safety of tranexamic acid when given intravenously for peri- and postoperative hemorrhage has been investigated more in orthopedic and cardiovascular surgical interventions[19]. In the study by Caglar et al[11], the authors reported that tranexamic acid succeeded in decreasing perioperative blood loss during excision of myomas; however, they emphasized the importance of various parameters such as type of surgery, surgical skills, and duration of surgery for perioperative blood loss. In the same study, the location and type of myoma (subserous, intramural, submucous) were highlighted and also the number and size of fibroid tumors. Multiple fibroid tumors may increase the duration of surgery in contrast to a single large myoma > 6 cm[12].
Ip et al[12] concluded that tranexamic acid induced necrosis of fibroids. Larger fibroids were more prone to necrosis. The authors emphasized the significance of tranexamic acid in conservative management of fibroids, thus sparing unnecessary surgical interventions. However possible complications such as pelvic pain and low grade fever maybe present in these patients[12].
It has been reported in clinical studies that the levels of plasminogen activator are elevated 30 min after the initiation of surgery, and this mechanism may elicit a reduction in bleeding in surgical patients[11,19].
One randomized study investigated whether tranexamic acid was effective in comparison with placebo for the management of menorrhagia in patients with no pathological findings in the pelvis[13]. The factual limitations of this study were that in women diagnosed with fibroid tumors, myomas were not found in large numbers and their size was not significant to justify surgical removal. Although the goal of this trial was not to assess the effect of tranexamic acid on abnormal vaginal bleeding caused by myomas, outcomes showed that tranexamic acid was effective in treating heavy menorrhagia, and this was not related to the presence of absence of myomas. However, based on the design of the study, it is hard to postulate that treatment with tranexamic acid is influenced by the size and type of the fibroids[18]. In the study by Lakhani et al[10], women with fibroids were found to have no significant changes in various sonographic parameters. However, these findings may exhibit bias and limitations because the women were not divided into different groups with different sizes and types of myomas[18].
The Food and Drug Administration approved tranexamic acid 650 mg (Lysteda-Ferring) in November 2009. Treatment with tranexamic acid while using hormonal contraceptives may increase the risk of developing thrombosis, cardiac complications, and stroke[20].
Tranexamic acid has been used extensively in patients with heavy menstrual bleeding with good results, and enough evidence is available to support its use. Tranexamic acid may reduce blood loss perioperatively in myomectomies, and may reduce menorrhagia in patients with fibroids, but stratification of fibroids by size and location is required to define the responses to tranexamic acid. Physicians should be aware that tranexamic acid may cause drug-induced necrosis of fibroids and surgical management can be avoided, but complications such as pelvic pain and low grade fever can be present in these patients. It is safe in general, and mild adverse effects are observed in some cases. More studies of a double-blind randomized design and larger numbers of participants are required to reach clearer conclusions about the use of tranexamic acid in patients with fibroids.
Menorrhagia due to fibroid tumors of the uterus is one the leading causes of abnormal menstrual bleeding. Tranexamic acid is non-hormonal and has been used previously for the treatment of dysfunctional uterine bleeding. The role of tranexamic acid in the treatment of abnormal menstrual bleeding due to uterine fibroid tumors in unclear. The authors have reviewed the literature and demonstrated that tranexamic acid may reduce bleeding in myomectomies and also may reduce the amount of bleeding in patients with menorrhagia caused by uterine fibroids.
Tranexamic acid may reduce blood loss in patients with menorrhagia due to fibroids. It may cause drug-induced necrosis of fibroids and surgical management can be avoided, but complications such as pelvic pain and low grade fever can be present in these patients.
The study showed that tranexamic acid reduced blood loss in patients undergoing myomectomy. It may cause necrosis in fibroids and may reduce the menorrhagia due to fibroids. Tranexamic acid has shown mild adverse effects during its administration. It may be used in patients who do not want hormonal treatment.
To ensure that tranexamic acid can be used in patients with menorrhagia caused be uterine fibroids, further double-blind randomized studies are required in order to ensure that the regimen is safe, efficient and does not cause severe effects.
Tranexamic acid is a hemostatic agent that elicits its antifibrinolytic action by reversibly blocking the lysine-binding sites on plasminogen molecules. It inactivates the plasminogen activator in endometrial cells and thus stops fibrinolysis and degradation of the clotting complexes. A number of studies have reported the use of tranexamic in reducing blood loss in cardiac and orthopedic operations. Tranexamic acid has been used to decrease blood loss in patients with menorrhagia. Menorrhagia is abnormal extensive menstrual bleeding where the quantity of overall blood loss exceeds 80 mL in every menses.
The authors here performed a systematic review of the current evidence on the administration and efficacy of Tranexamic acid for these patients.
P- Reviewer: Mais V, Sonoda K, Wang PH S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393-406. [PubMed] |
| 2. | Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstet Gynecol Scand. 2003;82:405-422. [PubMed] |
| 3. | Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404. [PubMed] |
| 4. | Dockeray CJ, Sheppard BL, Daly L, Bonnar J. The fibrinolytic enzyme system in normal menstruation and excessive uterine bleeding and the effect of tranexamic acid. Eur J Obstet Gynecol Reprod Biol. 1987;24:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K, Mayerhofer K. Estrogen and progesterone receptor expression in patients with uterine smooth muscle tumors. Fertil Steril. 2004;81:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Callender ST, Warner GT, Cope E. Treatment of menorrhagia with tranexamic acid. A double-blind trial. Br Med J. 1970;4:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ido K, Neo M, Asada Y, Kondo K, Morita T, Sakamoto T, Hayashi R, Kuriyama S. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120:518-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Stein K, Ascher-Walsh C. A comprehensive approach to the treatment of uterine leiomyomata. Mt Sinai J Med. 2009;76:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developers’ handbook. Edinburgh: SIGN 2001; . |
| 10. | Lakhani KP, Marsh MS, Purcell W, Hardiman P. Uterine artery blood flow parameters in women with dysfunctional uterine bleeding and uterine fibroids: the effects of tranexamic acid. Ultrasound Obstet Gynecol. 1998;11:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Caglar GS, Tasci Y, Kayikcioglu F, Haberal A. Intravenous tranexamic acid use in myomectomy: a prospective randomized double-blind placebo controlled study. Eur J Obstet Gynecol Reprod Biol. 2008;137:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Ip PP, Lam KW, Cheung CL, Yeung MC, Pun TC, Chan QK, Cheung AN. Tranexamic acid-associated necrosis and intralesional thrombosis of uterine leiomyomas: a clinicopathologic study of 147 cases emphasizing the importance of drug-induced necrosis and early infarcts in leiomyomas. Am J Surg Pathol. 2007;31:1215-1224. [PubMed] |
| 13. | Eder S, Baker J, Gersten J, Mabey RG, Adomako TL. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Womens Health (Lond Engl). 2013;9:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Lukes AS, Moore KA, Muse KN, Gersten JK, Hecht BR, Edlund M, Richter HE, Eder SE, Attia GR, Patrick DL. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opin Pharmacother. 2011;12:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Berntorp E, Follrud C, Lethagen S. No increased risk of venous thrombosis in women taking tranexamic acid. Thromb Haemost. 2001;86:714-715. [PubMed] |
| 17. | Rybo G. Tranexamic acid therapy: effective treatment in heavy menstrual bleeding: clinical update on safety. Therapeutic Advances. 1991;4:1-8. |
| 18. | Naoulou B, Tsai MC. Efficacy of tranexamic acid in the treatment of idiopathic and non-functional heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2012;91:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 728] [Article Influence: 28.0] [Reference Citation Analysis (0)] |