Published online Dec 16, 2014. doi: 10.12998/wjcc.v2.i12.769
Revised: July 12, 2014
Accepted: September 23, 2014
Published online: December 16, 2014
Processing time: 202 Days and 13.8 Hours
Breast cancer is the most frequent female malignancy worldwide. Current strategies in breast cancer therapy, including classical chemotherapy, hormone therapy, and targeted therapies, are usually associated with chemoresistance and serious adverse effects. Advances in our understanding of changes affecting the interactome in advanced and chemoresistant breast tumors have provided novel therapeutic targets, including, cyclin dependent kinases, mammalian target of rapamycin, Notch, Wnt and Shh. Inhibitors of these molecules recently entered clinical trials in mono- and combination therapy in metastatic and chemo-resistant breast cancers. Anticancer epigenetic drugs, mainly histone deacetylase inhibitors and DNA methyltransferase inhibitors, also entered clinical trials. Because of the complexity and heterogeneity of breast cancer, the future in therapy lies in the application of individualized tailored regimens. Emerging therapeutic targets and the implications for personalized-based therapy development in breast cancer are herein discussed.
Core tip: Emerging therapeutic targets may overcome chemoresistance in breast cancer.
- Citation: Kamdje AHN, Etet PFS, Vecchio L, Tagne RS, Amvene JM, Muller JM, Krampera M, Lukong KE. New targeted therapies for breast cancer: A focus on tumor microenvironmental signals and chemoresistant breast cancers. World J Clin Cases 2014; 2(12): 769-786
- URL: https://www.wjgnet.com/2307-8960/full/v2/i12/769.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i12.769
The incidence of breast cancer, the most common cancer in women and the second cause of cancer death in women worldwide[1,2], is currently growing[3,4]. Cancers are diseases characterized by aberrant microenvironment and intrinsic signaling causing a continuous proliferation of affected cells (“cancer cells”). Clinical features and prognosis of cancers vary tremendously according to the tissue and organs they originate from and affect. Breast cancer may start in milk ducts, and can be invasive [invasive ductal carcinoma (IDC)] or not (ductal carcinoma in situ). IDC would represent up to 80% of cases[5,6]. Breast cancer may also start in the lobules, with invasive features (invasive lobular carcinoma) or not (lobular carcinoma in situ ). In metastatic breast cancer malignant cells originating from breast primary tumors invade other tissues and organs of the body, resulting in a systemic disease. As disease early detection is associated with better prognosis, screening campaigns involving healthy female subjects are performed worldwide. Notably, mammography, which requires the use of low-dose X-rays to capture images inside the breast, is the current goal standard screening for detection of breast cancer asymptomatic cases[7,8]. However, although the technique requires X-rays, the benefits of the earlier detection of breast cancer outweigh the risk of radiation exposure, which can be associated with the development of breast cancer in previously healthy women is present[9,10]. New approaches for early detection have been proposed, and may also contribute to reducing breast cancer mortality (for review see[11,12]).
Three major therapeutic approaches are used today to treat or control breast cancer: surgical removal of primary tumors, irradiation of cancer cells to stop their growth, and anticancer drugs, which kill cancer cells or inhibit their proliferation. Notably, oncoplastic surgery, a technique combining classical lumpectomy (or partial mastectomy) and plastic surgery techniques have revolutionized breast-conserving surgery for removal of lumps and malignant masses. However, surgery or radiotherapy still requires chemotherapy to eradicate remaining malignant cells and impede relapses. Anticancer drugs are based on three therapeutic approaches: (1) the classical chemotherapy, where cancer cell proliferation is stopped by the indiscriminate targeting of rapid cell divisions in the body; (2) hormone therapy, devised to stop cancer cell growth by targeting the receptors and downstream signaling molecules of hormones pivotal for the proliferation of these cells; and (3) and the emerging and promising targeted therapy, where signaling pathways deregulated in primary breast tumors are specifically targeted. Breast cancer treatment is still challenging, as drugs in use are expensive, have serious undesired effects[13-15], and drug resistance is common, particularly in metastatic cases[16,17], underlying the need for new targeted therapies. Interestingly, recent advances in the understanding of breast cancer biology have highlighted the tumor microenvironment as a major player in breast carcinogenesis and have provided new avenues for targeted therapy.
The present review summarizes and discusses the current understanding of changes affecting breast microenvironment during breast tumorigenesis, with a particular emphasis on signaling pathways currently targeted for therapy and emerging therapeutic targets. Personalized-based targeting implementation is also discussed.
Numerous stromal cell types are found in the extracellular matrix of the breast stroma, including endothelial cells, fibroblasts, adipocytes, and resident immune cells[18]. In addition to these cell types, cancer-affected microenvironment contains malignant cells termed as cancer-associated fibroblasts (CAFs), which are the most numerous cell type, and infiltrating macrophages termed as tumor-associated macrophages (TAMs).
CAFs were reported to play key roles in malignant cell proliferation and tumor maintenance[18,19]. An in vivo study involving xenograft of MDA-MB-231 breast cells in SCID mice revealed that CAFs induce p53-dependent antimitogenic responses in normal stromal fibroblast[20], at least partly through Notch-dependent mechanisms[21]. In another study, CAFs expressed vascular endothelial growth factor in presence of hypoxia inducible factor 1 α/G-protein estrogen receptor (HIF-1α/GPER) signaling, suggesting a role for these cells in hypoxia-dependent tumor angiogenesis[22]. Under the same conditions, CAFs were shown to express Notch molecules[23], which promotes cancer cell survival, proliferation[24,25], as well as angiogenesis[26]. In addition, Luga et al[27] showed that CAFs release exosomes, which stimulate invasiveness and malignant cell metastasis via a Wnt11-dependent mechanism. On the same hand, CAFs induced phenotypical changes in adipocytes resulting in the generation of fibroblast-like cells [adipocyte-derived fibroblasts (ADF)], which in turn increased migratory abilities of metastatic cells by releasing high levels of collagen I and fibronectin[28]. Notably, CAF-induced ADF phenotype generation was mediated by reactivation of the oncogenic Wnt/β-catenin pathway in the latter cells in response to Wnt3a produced by the cancer cells, suggesting CAFs and ADFs as potential therapeutic targets in metastatic breast cancer. Furthermore, CAFs may promote breast cancer initiation and progression to metastasis via tumor-α9β1 integrin signaling[29] and fibroblast growth factor signaling[30], as well as malignancy orchestration and tumor stroma reprogramming through activation of heat shock factor 1[31], a transcriptional regulator.
Interestingly, Capparelli et al[32,33] have hypothesized that senescent fibroblasts may promote tumor growth through an autophagy-dependent mechanism termed as “autophagy-senescence transition”. In order to test such hypothesis, these authors introduced autophagy genes such as bnip3, ctsb or ATG16L1 in immortalized human fibroblasts that resulted in the induction of a constitutive autophagic phenotype (characterized by mitophagy, aerobic glycolysis, L-lactate and ketone body production) with senescence features associated with increased β-galactosidase activity, increased level of cyclin dependent kinase inhibitor (CDKI) p21, and cellular hypertrophy. Interestingly, “autophagic-senescent” fibroblasts were able to induce tumor growth and metastasis independently of angiogenesis, with stronger effects (up to 11-fold) in autophagic fibroblasts producing large amounts of ketone bodies. These observations were confirmed in vivo, as the lysosomal enzyme and biomarker of senescence, β-galactosidase, was also found in human breast cancer stroma. A recent in vivo study revealed the ability of CAF autophagy and senescence to promote tumor growth and metastasis increasing the rate of glycolysis and enhancing the generation of mitochondrial fuels including bodies[33] in a compartment-specific fashion, thus supporting the role of CAFs to metabolically regulate tumorigenesis. In this study, the injection of the antidiabetic molecule along with peroxisome proliferator-activated receptor gamma (PPARγ), known to stimulate glycolysis and pro-autophagy, into stromal cells enhanced the growth of co-injected breast cancer cells by 60%, whereas PPARγ injection in cancer cells reduced the growth of breast cancer cells by 40%[34].
TAMs infiltration into neoplastic tissues is an important negative prognostic factor[35,36], and a hallmark of triple negative breast cancer[37], a chemoresistant subtype of breast cancer[38,39]. Overall, emerging evidence suggests that TAMs are major player in anticancer drug resistance in breast cancer. For instance, Yamashina et al[40] recently reported that cancer stem-like cells originating from chemoresistant tumor promote macrophage colony-stimulating factor production via an interferon regulatory factor 5 -dependent mechanism, and transform recruited CD14(+) monocytes in tumorigenic M2-macrophages (immunoregulatory), probably through CXCR3 downregulation[41]. Interestingly, the differentiation inducer dimethyl sulfoxide exerted antitumor effects in a mouse breast cancer model (4T1) possibly by inducing M1-phenotype in TAMs[42].
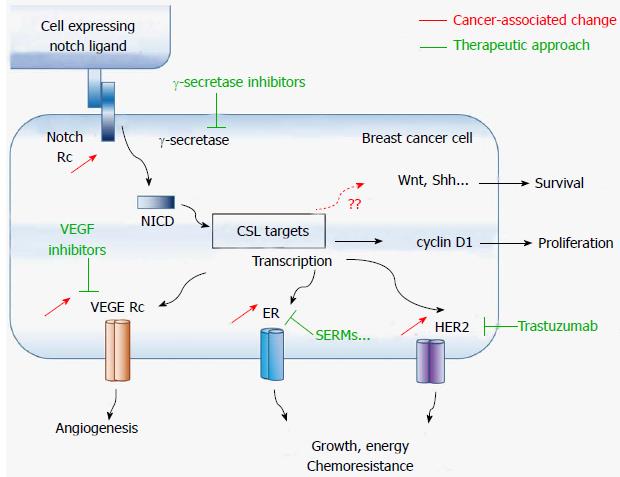
Furthermore, TAMs may promote carcinogenesis and metastasis via Wnt signaling, which mediates the angiogenic switch and metastatic processes in breast cancer[43,44]. Notably, TAMs release high levels of the Wnt family ligand Wnt7b[45], and cancer stem-like cells may trigger the metastatic effect of TAMs through enhancement of the β-catenin pathway via vitamin D receptor suppression by tumor necrosis factor alpha[46]. In addition, in vivo and in vitro studies supported a pivotal role for Wnt 5a signaling in TAMs-induced metastasis[47,48], and a strong correlation was found between Wnt5a expression in malignant cells and the number of CD163(+) M2-macrophages[49]. In a relatively recent study investigating the potential of the phosphodiesterase type 5 inhibitor (vasodilator) drug dipyridamole in xenograft mice, anticancer effects were mediated at least partly by decreasing β-catenin cytosolic levels[50]. Altogether, these findings implicated TAMs as a key links between chemoresistance and tumorigenic activities of cancer stem-like cells, and thus, positioning TAMs as potential therapeutic targets for breast cancer. Figure 1 shows the main signaling pathways currently in use for targeted breast cancer therapy, as well as some possible new targets.
The Notch family of membrane bound receptors and ligands regulate several cell processes including cell invasion, survival and apoptosis, via the Notch signaling pathway. The pathway comprises four receptors (Notch1 through Notch4) and five Notch ligands (Delta-like 1, 3, and 4, and Jagged1 and 2). Notch ligands include an extracellular domain containing multiple epidermal growth factor (EGF)-like repeats and an extracellular DSL where ligand binding occurs, and an intracellular domain with a PDZ-binding motif at C-terminal domain[51,52]. Notch receptors are also made of an extracellular and an intracellular domain covalently linked. Notch receptor extracellular domain also contains EGF-like repeats (26-29 depending on the Notch receptor), whereas Notch intracellular domain (NICD) presents with LIN12/Notch-related repeats preventing ligand-independent signaling, cysteine residues, and a C-terminal transactivation domain containing a PEST sequence with proteolytic activity.
Notch ligands are expressed on the plasma membrane of one cell and interact with Notch receptors on the plasma membrane of a neighboring cell, initiating the cleavage of the receptor by proteases [ADAM (a disintegrin and metalloprotease) and γ-secretase] that culminates in the release of the NICD[53]. Released NICD translocate to the nucleus and forms a transcriptional activator complex with C-promoter binding factor 1/Suppressor of Hairless and Lag-1 (CSL) transcription factor. Together with cofactors like mastermind-like protein, NICD-CSL complex induces the transcription of cell fate key target genes such as vegfr3 and, notch1 that regulate angiogenesis and apoptosis, p21 that regulates the cell cycle, as well as transcription factor genes such as the basic helix-loop-helix and hairy/enhancer of split/-related (hes and hey) [54,55] (Figure 1).
As already mentioned (section 2), Notch signaling is used by CAFs to promote cancer cell survival and proliferation. Early reports revealed that upregulation of Notch signaling suffices to transform normal breast epithelial cells in malignant cells in vitro, and that high levels of NICD are present in breast primary tumors[56-59]. Notch carcinogenic effects are mediated via the silencing pro-apoptotic signaling pathways and growth-inhibitory molecules like TGF-β[58]. Notch-induced TGF-β silencing also promotes bone metastasis[60,61]. In addition, Notch signaling, which is required for physiological angiogenesis, may also be a key player in neoangiogenesis[62]. A Notch 3 addiction of the lymphovascular embolus was reported in a xenograft model of inflammatory breast carcinoma, a subtype of breast cancer whose hallmark is lymphovascular invasion[63].
In vitro studies in estrogen receptor (ER)-negative breast cancer cells (MDA-MB-231) performed by Lee et al[64] revealed that Notch signaling up-regulates the transcription of the apoptosis inhibitor survivin. In another study, these authors showed that Notch-1-survivin functional gene signature is common in basal breast cancer[65]. In addition, crosstalk between Notch and signaling pathways involved in cell growth were reported as well, including the estrogen receptor[66], human epidermal growth factor receptor 2 (HER2)[67], and the metabolic signaling pathways phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt)/mammalian target of rapamycin (mTOR)[68,69] and MAP kinase/ERK[70,71]. Interestingly combined targeting of Notch and EGFR signaling suppressed chemoresistance in a basal-like breast cancer in vivo model[72], suggesting that co-targeting of Notch and associated pathways may represent a new avenue for overcoming chemoresistance (Figure 1).
Tumor initiating cells of tumors overexpressing HER2/neu also express high levels of Notch molecules, whose signaling is known to enhance HER2 expression[73]. Chemoresistance to HER2+ breast cancers to trastuzumab, a monoclonal antibody against HER2, is associated with the overexpression of Notch-1 and its ligand Jagged-1[74,75]. Similarly, cancer stem-like cells also achieve resistance against chemotherapy and radiotherapy via Notch signaling[76], and targeting of this signaling pathway reduces the stem-like population[77]. The γ-secretase inhibitor MRK-003 induced long-term recurrence-free survival in a transgenic mouse model of HER2+ breast cancer[78]. Similarly, co-targeting of Notch and HER2 signaling pathways prevented breast tumor recurrence in orthotopic breast tumor xenograft using trastuzumab-resistant BT474 cells[79].
Platelet-derived growth factor-D, another marker of breast cancer poor prognosis, may increase breast tumor aggressiveness by activating Notch and NF-κB signaling pathways[80]. Furthermore, Notch-1 and Notch-4, established bio-markers of the chemoresistant breast cancer subtype[81], were reported as novel transcriptional targets in triple negative breast cancer[82,83]. Jagged1/Notch4 signaling was shown to induce epithelial-to-mesenchymal transition[84]. Notch signaling was also reported as a mechanism of resistance to PI3K inhibitors[85] and hormone therapy[86].
Notch signaling inhibitors have a promising clinical efficacy as they abrogate HER2-Notch axis of chemoresistance. Notch silencing by ɣ-secretase inhibitors (GSIs) inhibited the proliferation of breast cancer cells partly by causing cell cycle arrest and apoptosis[76], and by sensitizing chemoresistant breast cancer cells to the BH3 mimetic ABT-737[87]. Notably, GSIs induce toxicity to breast cancer both in vitro and in vivo models, however mechanisms of such cytotoxicity are complex and may involve proteasome inhibition and downregulation of Bax and Bcl-2[88,89].
Following encouraging pre-clinical studies[83,90,91], the oral gamma secretase inhibitor R04929097 recently entered phase-I trial in patients with advanced solid tumors. Early reports of combination therapies with the kinase inhibitor temsirolimus[92], the antimetabolites of the pyrimidine analog family gemcitabine (PHL-078/CTEP 8575)[93] or cediranib (PJC-004/NCI 8503) revealed that the combinations were safe and promising in breast, tracheal, and pancreas cancer patients. However, anemia, diarrhea, fatigue, hypertension, neutropenia, and nausea were observed, among other side effects. GSI reported side effects seem to be mediated primarily through proteasome inhibition[88,94]. Thus, CSL inhibition, which was reported to mediate a more effective inhibition of Notch-dependent carcinogenic processes than GSIs[95], may represent a less toxic approach for Notch signaling targeting.
Another GSI, PF-03084014, also presented promising results in breast xenograft models[96], with gastrointestinal toxicity easily abrogated by glucocorticoids[97]. Other promising pre-clinical observations included a synergistic effect with the antimitotic drug docetaxel in breast cancer[98], colorectal cancer[99], and metastatic pancreatic cancer[100] models. Antiangiogenic effects where also reported in combinations with the tyrosine kinase inhibitor sunitinib in solid tumors[101], whereas in chronic lymphocytic leukemia cells combinations with the nucleoside metabolic inhibitor fludarabine inhibited angiogenesis as well as migration and invasion of Notch 1-mutated cancer cells[102,103]. PF-03084014 therefore appears as an appealing GSI for both solid and blood cancers and may be a good targeted-therapy drug in breast cancer.
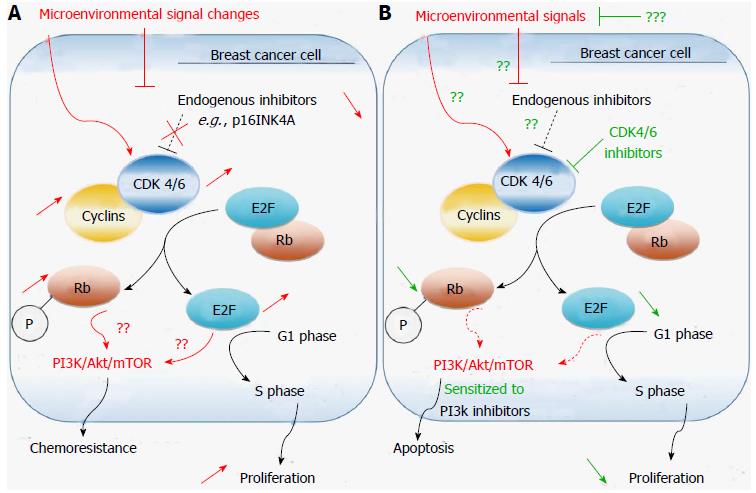
Cyclins, CDK inhibitors (CDKIs, e.g., p16INK4, p15INK4B, p18INK4C. p21WAF1/CIP1[104,105]) and CDKs are the three key classes of regulatory molecules that determine cell cycle progression through the G0-G1-S-G2 and M phases[106,107]. Numerous CDKs are found in eukaryotic cells, of which some are pivotal cell cycle regulators, such as CDK1/2/4/6 (Figure 2). CDKs (catalytic subunits, heterodimeric serine/threonine kinase class) associate with cyclins (regulatory subunits) to form an active catalytic complex favoring G1/S cell-cycle progression in mitosis. For instance, CDK1/A2 or CDK1/B1 complexes trigger mitosis in mammalian cells by phosphorylating downstream cell cycle regulatory proteins[108]. Other CDKs are involved in the regulation of cellular transcription, such as CDK7-11[107,109]. A recent proteomic analysis of the CDK family in human cells has identified a CDK5 complex as a key regulator of non-neural cell growth and migration factor[110].
Early and emerging evidence suggests that cyclin D1 promotes breast tumorigenesis[111,112]. CDK1 activity was recently reported as a powerful predictor of taxane chemosensitivity, indicating a role for CDK1 in breast tumorigenesis[111]. Notably, taxanes are the drug class most used for breast cancer pre-operative chemotherapy; they induce apoptosis in malignant cells by stopping their replication[113,114]. Moreover, studies investigating genes that are synthetically lethal in Myc-dependent cancer identified numerous CDKs as Myc synthetic-lethal genes[115-117]. Interestingly, in one of such studies CDK1, but not CDK2 or CDK4/6 was selectively lethal to Myc-dependent breast cancer cells[117]. This observation indicates that targeting CDK1 may induce apoptosis in Myc-dependent cancers, where Myc drives cancer cell growth and cycle progression[118]. Increases in activities and levels of other CDK complexes were also reported in breast cancer primary tumors and experimental models, including CDK4/6 and cyclin E/CDK2 complexes[119-121]. The occurrence of cyclin E/CDK2 proteolytic cleavage products associates with poor clinical outcome in breast cancer patients and increases tumorigenicity in experimental models at least partly by promoting stem-like properties of tumor cells[120]. Transcriptional regulator CDK8 targeting was also recently reported to inhibit both the proliferation and the migration of breast cancer cells[122]. In addition, BRCA2 gene, whose aberrant activating mutations associate with familial breast cancer[123,124], was reported to induce genomic stability in malignant cells through a CDK-dependent mechanism[125].
A link between the cell cycle and steroid hormone metabolism involving CDK4/6 was recently uncovered in breast cancer primary tumor cells[126]. In this study, malignant cells appeared to control the activity of steroid metabolic enzymes, i.e., the expression of steroid hormone receptors (including ER), by alteration of CDK4/6-levels (overexpression of CDK4 and decrease of its homolog CDK6). Such mechanism may play a pivotal role in the carcinogenesis and chemoresistance of steroid hormone-dependent cancers. In another recent study the newly synthesized compound KU004 that had a potent anticancer effect by targeting HER2 induced a decrease in CDK4 expression[127]. On the same hand, CDK 4/6 inhibitors sensitized PIK3CA mutant breast cancer to PI3K inhibitors in a xenograft study[128] (Figure 2), further suggesting a role for CDK4/6 imbalance in breast tumorigenesis.
CDK4/6 inhibitors are more efficient and less toxic antineoplastic agents than molecules targeting other CDKs[129]. The selective cyclin D kinase 4/6 inhibitor palbociclib (PD-0332991) is currently entering phase III trial for ER+ breast cancer patients, following encouraging results in progression free survival in phase II trials[130]. Using the bioluminescence imaging technology, an early study in xenograft models displaying metastatic progression revealed powerful antimetastatic effects, comparable to avastin, and docetaxel effects[131]. In addition, palbociclib, preferentially inhibited the proliferation of luminal ER+ breast cancer cell lines in vitro[132], suppressed malignant cell proliferation in approximately 85% of cases irrespective of ER+/- or HER2+/- statuses[133]. Furthermore, palbociclib induced growth arrest in hormone-resistant MCF-7 breast cancer cells by a mechanism consistent with cellular senescence[134]. This observation is not surprising considering the functional link between tumor microenvironment carcinogenic activity, ageing, and autophagy discussed above (section 2.1), and indicate that the drug may also affect metabolic processes in CAFs and stem-like tumor cells[33,34].
Chemoresistance to CDK4/6 inhibitors has been reported[133,135]. Analyses of primary tumor cells of cases resistant to CDK4/6 inhibitors showed that these cells lack the tumor suppressor retinoblastoma protein (RB)[133], which is necessary for CDK4/6 control of the cell cycle restriction point[135]. Interestingly, RB-deficient chemoresistant breast cancers, such as RB-deficient triple negative breast cancers, are more sensitive to the metabolic inhibitor of the folate analog family methotrexate and to the anthracycline topoisomerase inhibitor doxorubicin compared to RB+ cell lines[136], indicating that combination therapy may improve CDK4/6 inhibitor response in resistant cases. However, a report by Roberts and colleagues cautioned against the use of these agents in combination with DNA-damaging drugs (e.g., doxorubicin, carboplatin), considering the potential genotoxic side effects[129]. The dangers that may result from such combination also emerged in other pre-clinical studies[137,138].
The CDKI dinaciclib (MK-7965), which selectively binds to the ATP site of CDKs and acts as a protein-protein inhibitor of bromodomains[139,140], also displayed encouraging anticancer properties in pre-clinical studies in human cancer models[141,142]. The drug recently entered phase III in leukemia[139] and phase II trial in solid cancers. The drug is well tolerated in monotherapy, but revealed an antitumor activity whose efficacy was not superior to the nucleoside metabolic inhibitor capecitabine in a phase II trial in advanced breast cancer patients[143]. Comparable observations were reported in non-small cell lung cancer where the drug was compared with the protein kinase inhibitor erlotinib[144]. Similar combination therapy studies in progress for breast cancer[143,144] may provide alternative strategies for breast cancer therapy.
A number of reports have suggested that Wnt signaling pathway, which is normally involved in embryonic induction and cell fate[145,146], is aberrantly activated in blood cancers[147-149] and solid cancers, such as head and neck, lung, gastrointestinal, and breast cancer[27,150-155]. Wnt5a and Wnt11 are major players in macrophage-induced malignant invasion in metastatic breast cancer[27,151], and several breast tumors constitutively release-inducible Wnt ligands[156]. In addition, the naturally occurring pentacyclic triterpenoid ursolic acid, which is known to exert antitumor activity in various solid cancers including breast cancer, may act through inhibition of canonical (Wnt/β-catenin) signaling[150]. Similarly, the natural plant polyphenol rottlerin was reported to inhibit Wnt/β-catenin signaling in cancer cells by promoting the degradation of Wnt co-receptor LRP6 (low density lipoprotein receptor-related protein 6)[157]. Such inhibition resulted in cell death in various cancer cell lines, including MDA-MB-231 and T-47D breast cancer cells. Salinomycin, another novel LRP6 inhibitor, induced comparable effects in breast and prostate cancer cell lines, by inhibiting both Wnt/β-catenin and PI3K/Akt/mTOR signaling[158].
The development of specific Wnt inhibitors is in progress. Recently, a specific inhibitor of Porcupine (PORCN, an O-acyltransferase required for the secretion of Wnt ligands[159]) termed as LGK974 was developed. LGK974 displayed potent anticancer properties in in vitro and in vivo models of breast cancer and pancreatic adenocarcinoma mediated by reduction of the transcriptional expression of Wnt target genes[147,160]. However, another recent report revealed that Wnt signaling molecules are differentially expressed in breast cancer clinical subtypes and in cancer stem-like cells, indicating that the development of more specific Wnt-targeted therapies in breast cancer may be necessary[161]. Wnt signaling was also reported a major role in malignant cell acquired resistance to classical chemotherapy, including resistance to tamoxifen[162], and in chemoresistant cells from triple negative breast cancer patients[163], suggesting the potential of Wnt inhibitor combination therapies.
Early studies have suggested that Sonic Hedgehog (Shh) overexpression, mediated by both NF-κB up-regulation and shh promoter hypomethylation in breast cancer[164], is a critical event in the development of various solid cancers[165-167]. For instance, Shh signaling was reported to promote the survival of cancer epithelial cells, but not their normal counterparts[168]. Targeting of Shh transcription activator Gli1 enhanced apoptosis and attenuated migration in inflammatory breast cancer cells[169]. In addition, Shh non-classical activation was reported as a multidrug resistance enhancer, including resistance to Smo inhibitors[170], suggesting that targeting these pathways specifically may abrogate the associated chemoresistance.
Smo inhibitor anticancer drug cyclopamine, which inhibits Shh signaling by antagonizing its downstream target Smo, is metabolically stable and is currently investigated for the treatment of various cancers[171-173]. The chemotherapy drug paclitaxel used in combination with cyclopamine was shown to antagonize chemoresistant breast cancer cells both in vivo and in vitro[174], suggesting Shh signaling as a candidate for targeted therapy in chemoresistant cancer cells. Similarly, cyclopamine also sensitized chemoresistant tumor cells to taxane drugs in ovarian cancer[175], another hormone-related cancer. Not surprisingly, Shh targeting was reported as a therapeutic option in endocrine-resistant breast cancer due to its ability to sensitize PI3K/AKT signaling-induced tamoxifen chemoresistant malignant cells[176].
Notably, ER-α physiologically regulates non-canonical Shh signaling in the mammary gland, and is essential for mammary gland morphogenesis at puberty[177,178]. However, Gli1 expression also enhances migration and invasion of malignant cells in ERα-negative and triple negative breast cancers, where it represents a predictor of poor prognosis[179]. These observations indicate that Shh signaling involvement in breast cancer cells is complex and therefore targeting Shh in chemoresistant cancer therapy can also compromise its normal physiological function.
The major challenges in breast cancer treatment include resistance to chemotherapy, hormone therapy and even targeted therapy (Table 1), which underline the need for developing novel targeted therapies. Although the main molecular events driving cancer involve the activation of proto-oncogenes or the inactivation of tumor suppressors, deregulation of various signaling intermediates and metabolic factors have been well documented[72,77,82,83,149,161]. The events triggering cancer development affect proto-oncogenes such as Notch, Wnt, and Shh, which are the developmental genes driving embryonic induction and organogenesis during fetal life. These genes, whose expression is normally transcriptionally reduced or silenced in most adult tissues (except stem-like cells) by regulator molecules, are aberrantly overexpressed in cancer cells, conferring them stem-like properties[72,77,82,83,149,161].
| Drug | Trade name | Class | Anticancer mechanism |
| Classical chemotherapy | |||
| Methotrexate | Abitrexate®, Mexate®, Folex® | Antimetabolites, folate analogs | Folate receptor competitive antagonist[218] |
| 5-fluorouracil | Adrucil®, Efudex®, Fluoroplex®, prodrug capecitabine/Xeloda® | Antimetabolite, pyrimidine analogs | Inhibition of the phosphatase and tensin homolog thymidylate synthase[219] |
| Gemcitabine hydrochloride | Gemzar® | ||
| Doxorubicin hydrochloride | Adriamycin® | Anthracycline | Deoxyribonuclease inhibitor[220] |
| Epirubicin hydrochloride | Ellence® | ||
| Pamidronate disodium | Aredia® | Nitrogen-containing bisphosphonate | Inhibition of farnesyl pyrophosphate synthase activity[221] |
| Cyclophosphamide | Clafen®, Cytoxan®, Neosar® | Nitrogen mustard alkylating agent | Inhibition of DNA replication by interacting with the alkyl group of DNA guanine base[222] |
| Paclitaxel | Abraxane® Taxol® | Taxanes | Microtubule Inhibitors[223,224] |
| Docetaxel | Docecad®, Taxotere® | ||
| Ixabepilone | Ixempra® | Epothilone B analog | |
| Targeted therapy | |||
| Everolimus | Afinitor® | mTOR inhibitor | Silencing of PI3K/Akt/mTOR signaling[225] |
| Trastuzumab | Herceptin® | HER2 inhibitor | Anti-HER2 monoclonal antibodies[226,227] |
| Pertuzumab | Perjeta® | ||
| Ado-Trastuzumab Emtansine | Kadcyla® | Antibody-drug conjugate | HER2 inhibitor and cytotoxic agent[228] |
| Lapatinib ditosylate | Tykerb® | Dual tyrosine kinase inhibitor | EGFR/HER2 inhibitor[229] |
| Hormone therapy | |||
| Toremifene | Fareston® | Selective ER modulator | Silence ER signaling[230,231] |
| Fulvestrant | Faslodex® | ER antagonists | |
| Tamoxifen citrate | Nolvadex® | ||
| Anastrozole | Arimidex® | Aromatase inhibitors | Inhibit estrogen synthesis[232-234] |
| Exemestane | Aromasin® | ||
| Letrozole | Femara® | ||
| Goserelin acetate | Zoladex® | GnRH agonist | |
| Megestrol acetate | Megace® | Progesterone derivative | Progestational and antigonadotropic effects[235] |
Concomitantly, neoplastic tissue growth is fuelled by the upregulation and overexpression of receptors such as HER2, ER and, IGF-1R[70,71,180], the upregulation and/or activation of signaling molecules associated with cell proliferation[111,112], cell migration[181,182], oxidative stress, hypoxia and neoangiogenesis[22,26], all which are characteristic of tumor microenvironment. Thus, the complete characterization of all these tumor promoting events will pave the way for the development more efficient and less toxic anticancer drugs. Computational causal network models aimed at improving the current understanding of signaling molecule interactions in breast cancer, which will allow the determination of specific subsets of patients susceptible to a given therapeutic approach, are currently in development[156,183]. Although the complexity of such networks makes this effort challenging, nonetheless, the development of such tool would allow implementation of a highly efficient personalized-based therapy in breast cancer.
Epigenetics describes heritable alterations in gene expression patterns that do not alter the primary DNA sequence, but play critical roles in normal differentiation and development. Epigenetic alterations include modifications such as DNA methylation, histone modifications and nucleosome remodeling. The plasticity and reversibility of epigenetic events enable a better control of the dynamism of cellular processes. However, deregulation of the normal epigenetic patterns can lead to aberrant expression of cell growth regulatory genes that can culminate in cancer. Epigenetic factors affect gene expression both pre- and post-transcriptionally and probably account for the high inter-individual variability in clinical course and treatment outcome of both blood and solid cancers[184,185]. There is ample evidence linking the etiology of breast to abnormal genetic and epigenetic events[180,186,187]. Cancer-specific DNA methylation changes and well as dysregulation of histone modification have been characterized as contributors to breast cancer development. Progress in our understanding of epigenetics mechanisms in breast cancer have led to the identification of novel therapeutic targets. Recent therapeutic strategies involving the use of epigenetic agents alone or in combination with chemotherapy and/or endocrine therapy are showing promising results in breast cancer patients including chemoresistant cases[186,188].
The technological breakthrough of “omics era” has allowed the development of high-throughput sequencing technology allowing both global and comprehensive investigations of the interactome, the epigenome, and the transcriptome (i.e., active signaling pathways, cascades of pre- and post-translational changes affecting specific genes, and changes in gene expression)[189-191] at individual level. Epigenetic alterations in cancer constitute appealing therapeutic targets due to their pivotal roles in disease initiation, progression, and chemoresistance, and to their reversibility. For instance, chemoresistance to the ER antagonist fulvestrant is mediated by epigenetic modulation (more specifically hSWI/SNF-mediated chromatin remodeling) of GPER and CDK6 expression[192], suggesting that adjuvant therapy targeting SWI/SNF activity may induce apoptosis in resistant cancer cells. SWI/SNF tumor-dependency has also been reported in other solid cancers and in leukemias[193,194].
Epigenetic targets in breast cancer: histone deacetylation and DNA hypermethylation
Studies have shown that the transcriptional expression of various signaling molecules associated with breast cancer and other cancers may result from selective epigenetic silencing of regulator genes mediated by histone deacetylation and gene promoter (DNA) hypermethylation[195-197], among other potential epigenetic mechanisms[186,198]. For instance, the reduction in ER expression observed in various chemoresistant breast tumors may be mediated by epigenetic silencing (e.g., erβ1 silencing)[199]; and some histone deacetylases (HDACs) such as HDAC3/8 were reported to play pivotal regulatory roles in the proliferation of normal and MDA-MB-231 cells[200].
Data from numerous pre-clinical in vivo and in vitro studies support the potential of DNA methylation status targeting in breast cancer. Both the HDAC inhibitor (HDACI) trichostatin A and the DNA methyltransferase (DNMT) inhibitor (DNMTI) deoxycytidine (5-aza-2’-deoxycytidine) induced apoptosis in various breast cancer cell lines[201-205]. The HDACI Romidepsin (FK-288) eliminated both primary and metastatic tumors in combination with Paclitaxel in the Mary-X pre-clinical model of inflammatory breast cancer[206]. The green tea-derived anticancer molecule epigallocatechin-3-gallate suppressed invasiveness in MDA-MB-231 and MCF-7 breast cancer cells by silencing matrix metalloproteinase 2 (MMP2) and MMP-9 and inducing TIMP-3 through increased activities of the enhancer of zeste homolog 2 and HDACs[207]. Suberoylanilide hydroxamic acid, another naturally occurring HDACI, restored radiosensitivity and suppressed breast cancer lung metastasis in vitro and in vivo[208].
The HDACI Vorinostat sensitized mesenchymal-like triple-negative breast cancer cell lines to hormone therapy by reactivating ERα[209] and PI3K/Akt/mTOR signaling sensitivity[210], corroborating the role of epigenetic alterations in chemoresistance development in breast tumors. Furthermore, the HDACI abexinostat induced cancer-like stem cells differentiation in 16 breast cancer cell lines[211]. Because of these interesting observations, the HDACIs belinostat, panobinostat, and vorinostat, previously used only in blood cancers, have entered phase I and II clinical trials in solid tumors, such as lung, prostate, gastrointestinal, ovarian and breast cancer, where they are showing encouraging results (for review see[212]). Various DNMTI are also showing encouraging responses in metastatic and chemoresistant breast cancers in monotherapy and in combination therapies in phase I and II trials[213-217].
Targeted therapies are associated with reduced adverse effects and better outcome. Tumor microenvironment cells such as cancer-associated fibroblasts and tumor-associated macrophages undergo aberrant genetic and epigenetic changes that trigger the overexpression of signaling molecules promoting neoplasia and neoplastic tissue survival. Many therapeutic targets have emerged. They include Notch, CDKs, mTOR, Wnt, and Shh, whose inhibitors are showing promising results in ongoing clinical trials, both in monotherapy and in combination therapy. Similarly, epigenetic drugs are also showing encouraging results in breast cancer, particularly in advanced and chemoresistant cases. New technological advances will enable the identification of precise alterations affecting the interactome, transcriptome, and the epigenome, leading to the design of more specific tailored therapies. Such therapeutic approach may also be beneficial in the treatment of chemoresistant breast cancers.
P- Reviewer: Peng Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Azim HA, Ibrahim AS. Breast cancer in Egypt, China and Chinese: statistics and beyond. J Thorac Dis. 2014;6:864-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 2. | Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11:101-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 186] [Reference Citation Analysis (0)] |
| 3. | Villarreal-Garza C, Aguila C, Magallanes-Hoyos MC, Mohar A, Bargalló E, Meneses A, Cazap E, Gomez H, López-Carrillo L, Chávarri-Guerra Y. Breast cancer in young women in Latin America: an unmet, growing burden. Oncologist. 2013;18:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | de Azambuja E, Ameye L, Paesmans M, Zielinski CC, Piccart-Gebhart M, Preusser M. The landscape of medical oncology in Europe by 2020. Ann Oncol. 2014;25:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Zengel B, Yararbas U, Duran A, Uslu A, Elıyatkın N, Demırkıran MA, Cengiz F, Simşek C, Postacı H, Vardar E. Comparison of the clinicopathological features of invasive ductal, invasive lobular, and mixed (invasive ductal + invasive lobular) carcinoma of the breast. Breast Cancer. 2013;Aug 8; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Arps DP, Jorns JM, Zhao L, Bensenhaver J, Kleer CG, Pang JC. Re-Excision Rates of Invasive Ductal Carcinoma with Lobular Features Compared with Invasive Ductal Carcinomas and Invasive Lobular Carcinomas of the Breast. Ann Surg Oncol. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Al-Foheidi M, Al-Mansour MM, Ibrahim EM. Breast cancer screening: review of benefits and harms, and recommendations for developing and low-income countries. Med Oncol. 2013;30:471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Onega T, Weaver D, Geller B, Oster N, Tosteson AN, Carney PA, Nelson H, Allison KH, O’Malley FP, Schnitt SJ. Digitized whole slides for breast pathology interpretation: current practices and perceptions. J Digit Imaging. 2014;27:642-648. [PubMed] |
| 9. | Suzuki A, Ishida T, Ohuchi N. Controversies in breast cancer screening for women aged 40-49 years. Jpn J Clin Oncol. 2014;44:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Paci E, Broeders M, Hofvind S, Puliti D, Duffy SW. European breast cancer service screening outcomes: a first balance sheet of the benefits and harms. Cancer Epidemiol Biomarkers Prev. 2014;23:1159-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Plescia M, White MC. The National Prevention Strategy and breast cancer screening: scientific evidence for public health action. Am J Public Health. 2013;103:1545-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Nishikawa RM, Gur D. CADe for early detection of breast cancer-current status and why we need to continue to explore new approaches. Acad Radiol. 2014;21:1320-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS, Nowak AK. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29:4498-4504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Dadla A, Tannenbaum S, Yates B, Holle L. Delayed hypersensitivity reaction related to the use of pegfilgrastim. J Oncol Pharm Pract. 2014;Jul 3; Epub ahead of print. [PubMed] |
| 15. | Karczmarek-Borowska B, Drzymała M, Golon K. Hepatotoxicity of acetaminophen in a patient treated with capecitabine due to breast cancer. Pol Merkur Lekarski. 2014;36:348-351. [PubMed] |
| 16. | Hurvitz S, Guerin A, Brammer M, Guardino E, Zhou ZY, Latremouille Viau D, Wu EQ, Lalla D. Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist. 2014;19:901-908. [PubMed] |
| 17. | Hansen RN, Ramsey SD, Lalla D, Masaquel A, Kamath T, Brammer M, Hurvitz SA, Sullivan SD. Identification and cost of adverse events in metastatic breast cancer in taxane and capecitabine based regimens. Springerplus. 2014;3:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 528] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 19. | Vivacqua A, Romeo E, De Marco P, De Francesco EM, Abonante S, Maggiolini M. GPER mediates the Egr-1 expression induced by 17β-estradiol and 4-hydroxitamoxifen in breast and endometrial cancer cells. Breast Cancer Res Treat. 2012;133:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Farmaki E, Chatzistamou I, Bourlis P, Santoukou E, Trimis G, Papavassiliou AG, Kiaris H. Selection of p53-Deficient Stromal Cells in the Tumor Microenvironment. Genes Cancer. 2012;3:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Tao L, Roberts AL, Dunphy KA, Bigelow C, Yan H, Jerry DJ. Repression of mammary stem/progenitor cells by p53 is mediated by Notch and separable from apoptotic activity. Stem Cells. 2011;29:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs). Breast Cancer Res. 2013;15:R64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 23. | Pupo M, Pisano A, Abonante S, Maggiolini M, Musti AM. GPER activates Notch signaling in breast cancer cells and cancer-associated fibroblasts (CAFs). Int J Biochem Cell Biol. 2014;46:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Nwabo Kamdje AH, Mosna F, Bifari F, Lisi V, Bassi G, Malpeli G, Ricciardi M, Perbellini O, Scupoli MT, Pizzolo G. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood. 2011;118:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Nwabo Kamdje AH, Bassi G, Pacelli L, Malpeli G, Amati E, Nichele I, Pizzolo G, Krampera M. Role of stromal cell-mediated Notch signaling in CLL resistance to chemotherapy. Blood Cancer J. 2012;2:e73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. [PubMed] |
| 27. | Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73:6843-6847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657-5668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 29. | Ota D, Kanayama M, Matsui Y, Ito K, Maeda N, Kutomi G, Hirata K, Torigoe T, Sato N, Takaoka A. Tumor-α9β1 integrin-mediated signaling induces breast cancer growth and lymphatic metastasis via the recruitment of cancer-associated fibroblasts. J Mol Med (Berl). 2014;Aug 8; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 30. | Ishikawa M, Inoue T, Shirai T, Takamatsu K, Kunihiro S, Ishii H, Nishikata T. Simultaneous expression of cancer stem cell-like properties and cancer-associated fibroblast-like properties in a primary culture of breast cancer cells. Cancers (Basel). 2014;6:1570-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158:564-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 32. | Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG, Goldberg AF, Pestell RG, Howell A, Sneddon S. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle. 2012;11:2285-2302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Capparelli C, Chiavarina B, Whitaker-Menezes D, Pestell TG, Pestell RG, Hulit J, Andò S, Howell A, Martinez-Outschoorn UE, Sotgia F. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle. 2012;11:3599-3610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Avena P, Anselmo W, Whitaker-Menezes D, Wang C, Pestell RG, Lamb RS, Hulit J, Casaburi I, Andò S, Martinez-Outschoorn UE. Compartment-specific activation of PPARγ governs breast cancer tumor growth, via metabolic reprogramming and symbiosis. Cell Cycle. 2013;12:1360-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Tymoszuk P, Evens H, Marzola V, Wachowicz K, Wasmer MH, Datta S, Müller-Holzner E, Fiegl H, Böck G, van Rooijen N. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. Eur J Immunol. 2014;44:2247-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Xuan QJ, Wang JX, Nanding A, Wang ZP, Liu H, Lian X, Zhang QY. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol Oncol Res. 2014;20:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci USA. 2014;111:E2120-E2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 38. | Liu H, Wang Y, Li X, Zhang YJ, Li J, Zheng YQ, Liu M, Song X, Li XR. Expression and regulatory function of miRNA-182 in triple-negative breast cancer cells through its targeting of profilin 1. Tumour Biol. 2013;34:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, Chang G, Li X, Li Q, Wang S. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS One. 2014;9:e96228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Yamashina T, Baghdadi M, Yoneda A, Kinoshita I, Suzu S, Dosaka-Akita H, Jinushi M. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res. 2014;74:2698-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, Ganju RK, Satoskar AR. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014;143:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Deng R, Wang SM, Yin T, Ye TH, Shen GB, Li L, Zhao JY, Sang YX, Duan XG, Wei YQ. Dimethyl Sulfoxide Suppresses Mouse 4T1 Breast Cancer Growth by Modulating Tumor-Associated Macrophage Differentiation. J Breast Cancer. 2014;17:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Milovanovic T, Planutis K, Nguyen A, Marsh JL, Lin F, Hope C, Holcombe RF. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol. 2004;25:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Yeo EJ, Cassetta L, Qian BZ, Lewkowich I, Li JF, Stefater JA, Smith AN, Wiechmann LS, Wang Y, Pollard JW. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74:2962-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 46. | Zhang Y, Guo Q, Zhang Z, Bai N, Liu Z, Xiong M, Wei Y, Xiang R, Tan X. VDR status arbitrates the prometastatic effects of tumor-associated macrophages. Mol Cancer Res. 2014;12:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trümper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA. 2006;103:5454-5459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 48. | Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702-712. [PubMed] |
| 49. | Bergenfelz C, Medrek C, Ekström E, Jirström K, Janols H, Wullt M, Bredberg A, Leandersson K. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol. 2012;188:5448-5458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 50. | Spano D, Marshall JC, Marino N, De Martino D, Romano A, Scoppettuolo MN, Bello AM, Di Dato V, Navas L, De Vita G. Dipyridamole prevents triple-negative breast-cancer progression. Clin Exp Metastasis. 2013;30:47-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 797] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 52. | Pintar A, De Biasio A, Popovic M, Ivanova N, Pongor S. The intracellular region of Notch ligands: does the tail make the difference? Biol Direct. 2007;2:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Zhang P, Ostrander JH, Faivre EJ, Olsen A, Fitzsimmons D, Lange CA. Regulated association of protein kinase B/Akt with breast tumor kinase. J Biol Chem. 2005;280:1982-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Wu L, Sun T, Kobayashi K, Gao P, Griffin JD. Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol Cell Biol. 2002;22:7688-7700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 688] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 56. | Sun Y, Lowther W, Kato K, Bianco C, Kenney N, Strizzi L, Raafat D, Hirota M, Khan NI, Bargo S. Notch4 intracellular domain binding to Smad3 and inhibition of the TGF-beta signaling. Oncogene. 2005;24:5365-5374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 441] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 58. | Liu Z, Teng L, Bailey SK, Frost AR, Bland KI, LoBuglio AF, Ruppert JM, Lobo-Ruppert SM. Epithelial transformation by KLF4 requires Notch1 but not canonical Notch1 signaling. Cancer Biol Ther. 2009;8:1840-1851. [PubMed] |
| 59. | Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 60. | Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 61. | Xing F, Okuda H, Watabe M, Kobayashi A, Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30:4075-4086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 62. | Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Xiao Y, Ye Y, Zou X, Jones S, Yearsley K, Shetuni B, Tellez J, Barsky SH. The lymphovascular embolus of inflammatory breast cancer exhibits a Notch 3 addiction. Oncogene. 2011;30:287-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Lee CW, Raskett CM, Prudovsky I, Altieri DC. Molecular dependence of estrogen receptor-negative breast cancer on a notch-survivin signaling axis. Cancer Res. 2008;68:5273-5281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, Hsieh CC, Altieri DC. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10:R97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226-5235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 67. | Dai J, Ma D, Zang S, Guo D, Qu X, Ye J, Ji C. Cross-talk between Notch and EGFR signaling in human breast cancer cells. Cancer Invest. 2009;27:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Mungamuri SK, Yang X, Thor AD, Somasundaram K. Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Cancer Res. 2006;66:4715-4724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 69. | Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res. 2010;70:2476-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 70. | Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 71. | Mittal S, Subramanyam D, Dey D, Kumar RV, Rangarajan A. Cooperation of Notch and Ras/MAPK signaling pathways in human breast carcinogenesis. Mol Cancer. 2009;8:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Dong Y, Li A, Wang J, Weber JD, Michel LS. Synthetic lethality through combined Notch-epidermal growth factor receptor pathway inhibition in basal-like breast cancer. Cancer Res. 2010;70:5465-5474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 74. | Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, Reedijk M. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007;20:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 75. | Osipo C, Patel P, Rizzo P, Clementz AG, Hao L, Golde TE, Miele L. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene. 2008;27:5019-5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Zang S, Ji Ch, Qu X, Dong X, Ma D, Ye J, Ma R, Dai J, Guo D. A study on Notch signaling in human breast cancer. Neoplasma. 2007;54:304-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Grudzien P, Lo S, Albain KS, Robinson P, Rajan P, Strack PR, Golde TE, Miele L, Foreman KE. Inhibition of Notch signaling reduces the stem-like population of breast cancer cells and prevents mammosphere formation. Anticancer Res. 2010;30:3853-3867. [PubMed] |
| 78. | Kondratyev M, Kreso A, Hallett RM, Girgis-Gabardo A, Barcelon ME, Ilieva D, Ware C, Majumder PK, Hassell JA. Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene. 2012;31:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | Pandya K, Meeke K, Clementz AG, Rogowski A, Roberts J, Miele L, Albain KS, Osipo C. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer. 2011;105:796-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Ahmad A, Wang Z, Kong D, Ali R, Ali S, Banerjee S, Sarkar FH. Platelet-derived growth factor-D contributes to aggressiveness of breast cancer cells by up-regulating Notch and NF-κB signaling pathways. Breast Cancer Res Treat. 2011;126:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Speiser J, Foreman K, Drinka E, Godellas C, Perez C, Salhadar A, Erşahin Ç, Rajan P. Notch-1 and Notch-4 biomarker expression in triple-negative breast cancer. Int J Surg Pathol. 2012;20:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Clementz AG, Rogowski A, Pandya K, Miele L, Osipo C. NOTCH-1 and NOTCH-4 are novel gene targets of PEA3 in breast cancer: novel therapeutic implications. Breast Cancer Res. 2011;13:R63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Azzam DJ, Zhao D, Sun J, Minn AJ, Ranganathan P, Drews-Elger K, Han X, Picon-Ruiz M, Gilbert CA, Wander SA. Triple negative breast cancer initiating cell subsets differ in functional and molecular characteristics and in γ-secretase inhibitor drug responses. EMBO Mol Med. 2013;5:1502-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 84. | Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935-2948. [PubMed] |
| 85. | Muellner MK, Uras IZ, Gapp BV, Kerzendorfer C, Smida M, Lechtermann H, Craig-Mueller N, Colinge J, Duernberger G, Nijman SM. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol. 2011;7:787-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 86. | Haughian JM, Pinto MP, Harrell JC, Bliesner BS, Joensuu KM, Dye WW, Sartorius CA, Tan AC, Heikkilä P, Perou CM. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc Natl Acad Sci USA. 2012;109:2742-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 87. | Séveno C, Loussouarn D, Bréchet S, Campone M, Juin P, Barillé-Nion S. γ-Secretase inhibition promotes cell death, Noxa upregulation, and sensitization to BH3 mimetic ABT-737 in human breast cancer cells. Breast Cancer Res. 2012;14:R96. [PubMed] |
| 88. | Han J, Ma I, Hendzel MJ, Allalunis-Turner J. The cytotoxicity of gamma-secretase inhibitor I to breast cancer cells is mediated by proteasome inhibition, not by gamma-secretase inhibition. Breast Cancer Res. 2009;11:R57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 89. | Rasul S, Balasubramanian R, Filipović A, Slade MJ, Yagüe E, Coombes RC. Inhibition of gamma-secretase induces G2/M arrest and triggers apoptosis in breast cancer cells. Br J Cancer. 2009;100:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 90. | Luistro L, He W, Smith M, Packman K, Vilenchik M, Carvajal D, Roberts J, Cai J, Berkofsky-Fessler W, Hilton H. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res. 2009;69:7672-7680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 91. | Debeb BG, Cohen EN, Boley K, Freiter EM, Li L, Robertson FM, Reuben JM, Cristofanilli M, Buchholz TA, Woodward WA. Pre-clinical studies of Notch signaling inhibitor RO4929097 in inflammatory breast cancer cells. Breast Cancer Res Treat. 2012;134:495-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Diaz-Padilla I, Hirte H, Oza AM, Clarke BA, Cohen B, Reedjik M, Zhang T, Kamel-Reid S, Ivy SP, Hotte SJ. A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest New Drugs. 2013;31:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Richter S, Bedard PL, Chen EX, Clarke BA, Tran B, Hotte SJ, Stathis A, Hirte HW, Razak AR, Reedijk M. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Invest New Drugs. 2014;32:243-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 94. | Han J, Shen Q. Targeting γ-secretase in breast cancer. Breast Cancer (Dove Med Press). 2012;4:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Yong T, Sun A, Henry MD, Meyers S, Davis JN. Down regulation of CSL activity inhibits cell proliferation in prostate and breast cancer cells. J Cell Biochem. 2011;112:2340-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Zhang CC, Pavlicek A, Zhang Q, Lira ME, Painter CL, Yan Z, Zheng X, Lee NV, Ozeck M, Qiu M. Biomarker and pharmacologic evaluation of the γ-secretase inhibitor PF-03084014 in breast cancer models. Clin Cancer Res. 2012;18:5008-5019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, Tsaparikos K, Jani JP, Hosea N, Sands M. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther. 2010;9:1618-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 98. | Zhang CC, Yan Z, Zong Q, Fang DD, Painter C, Zhang Q, Chen E, Lira ME, John-Baptiste A, Christensen JG. Synergistic effect of the γ-secretase inhibitor PF-03084014 and docetaxel in breast cancer models. Stem Cells Transl Med. 2013;2:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 99. | Arcaroli JJ, Quackenbush KS, Purkey A, Powell RW, Pitts TM, Bagby S, Tan AC, Cross B, McPhillips K, Song EK. Tumours with elevated levels of the Notch and Wnt pathways exhibit efficacy to PF-03084014, a γ-secretase inhibitor, in a preclinical colorectal explant model. Br J Cancer. 2013;109:667-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 101. | Zhang CC, Yan Z, Giddabasappa A, Lappin PB, Painter CL, Zhang Q, Li G, Goodman J, Simmons B, Pascual B. Comparison of dynamic contrast-enhanced MR, ultrasound and optical imaging modalities to evaluate the antiangiogenic effect of PF-03084014 and sunitinib. Cancer Med. 2014;3:462-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | López-Guerra M, Xargay-Torrent S, Rosich L, Montraveta A, Roldán J, Matas-Céspedes A, Villamor N, Aymerich M, López-Otín C, Pérez-Galán P. The γ-secretase inhibitor PF-03084014 combined with fludarabine antagonizes migration, invasion and angiogenesis in NOTCH1-mutated CLL cells. Leukemia. 2014;Apr 30; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Carol H, Maris JM, Kang MH, Reynolds CP, Kolb EA, Gorlick R, Keir ST, Wu J, Kurmasheva RT, Houghton PJ. Initial testing (stage 1) of the notch inhibitor PF-03084014, by the pediatric preclinical testing program. Pediatr Blood Cancer. 2014;61:1493-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Musgrove EA, Lilischkis R, Cornish AL, Lee CS, Setlur V, Seshadri R, Sutherland RL. Expression of the cyclin-dependent kinase inhibitors p16INK4, p15INK4B and p21WAF1/CIP1 in human breast cancer. Int J Cancer. 1995;63:584-591. [PubMed] |
| 105. | Zariwala M, Liu E, Xiong Y. Mutational analysis of the p16 family cyclin-dependent kinase inhibitors p15INK4b and p18INK4c in tumor-derived cell lines and primary tumors. Oncogene. 1996;12:451-455. [PubMed] |
| 106. | Nurse PM. Nobel Lecture. Cyclin dependent kinases and cell cycle control. Biosci Rep. 2002;22:487-499. [PubMed] |
| 107. | Malumbres M. Cyclins and related kinases in cancer cells. J BUON. 2007;12 Suppl 1:S45-S52. [PubMed] |
| 108. | Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 109. | Kuwajima M, Kumano G, Nishida H. Regulation of the number of cell division rounds by tissue-specific transcription factors and Cdk inhibitor during ascidian embryogenesis. PLoS One. 2014;9:e90188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Xu S, Li X, Gong Z, Wang W, Li Y, Nair BC, Piao H, Yang K, Wu G, Chen J. Proteomic Analysis of the Human Cyclin-dependent Kinase Family Reveals a Novel CDK5 Complex Involved in Cell Growth and Migration. Mol Cell Proteomics. 2014;13:2986-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Torikoshi Y, Gohda K, Davis ML, Symmans WF, Pusztai L, Kazansky A, Nakayama S, Yoshida T, Matsushima T, Hortobagyi GN. Novel functional assay for spindle-assembly checkpoint by cyclin-dependent kinase activity to predict taxane chemosensitivity in breast tumor patient. J Cancer. 2013;4:697-702. [PubMed] |
| 112. | Casimiro MC, Velasco-Velázquez M, Aguirre-Alvarado C, Pestell RG. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present. Expert Opin Investig Drugs. 2014;23:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 113. | Jinno H, Matsuda S, Hayashida T, Takahashi M, Hirose S, Ikeda T, Kitagawa Y. Differential pathological response to preoperative chemotherapy across breast cancer intrinsic subtypes. Chemotherapy. 2012;58:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Yamaguchi T, Mukai H. Ki-67 index guided selection of preoperative chemotherapy for HER2-positive breast cancer: a randomized phase II trial. Jpn J Clin Oncol. 2012;42:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 115. | Toyoshima M, Howie HL, Imakura M, Walsh RM, Annis JE, Chang AN, Frazier J, Chau BN, Loboda A, Linsley PS. Functional genomics identifies therapeutic targets for MYC-driven cancer. Proc Natl Acad Sci USA. 2012;109:9545-9550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 116. | Kessler JD, Kahle KT, Sun T, Meerbrey KL, Schlabach MR, Schmitt EM, Skinner SO, Xu Q, Li MZ, Hartman ZC. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science. 2012;335:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 117. | Kang J, Sergio CM, Sutherland RL, Musgrove EA. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer. 2014;14:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 118. | Cunningham JT, Pourdehnad M, Stumpf CR, Ruggero D. Investigating Myc-dependent translational regulation in normal and cancer cells. Methods Mol Biol. 2013;1012:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 119. | Said TK, Medina D. Cell cyclins and cyclin-dependent kinase activities in mouse mammary tumor development. Carcinogenesis. 1995;16:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Duong MT, Akli S, Macalou S, Biernacka A, Debeb BG, Yi M, Hunt KK, Keyomarsi K. Hbo1 is a cyclin E/CDK2 substrate that enriches breast cancer stem-like cells. Cancer Res. 2013;73:5556-5568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Rath SL, Senapati S. Why are the truncated cyclin Es more effective CDK2 activators than the full-length isoforms? Biochemistry. 2014;53:4612-4624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 122. | Li XY, Luo QF, Wei CK, Li DF, Fang L. siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells. Int J Clin Exp Pathol. 2014;7:92-100. [PubMed] |
| 123. | Pisanò M, Mezzolla V, Galante MM, Alemanno G, Manca C, Lorusso V, Malvasi A, Tinelli A. A new mutation of BRCA2 gene in an Italian healthy woman with familial breast cancer history. Fam Cancer. 2011;10:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Pruss D, Morris B, Hughes E, Eggington JM, Esterling L, Robinson BS, van Kan A, Fernandes PH, Roa BB, Gutin A. Development and validation of a new algorithm for the reclassification of genetic variants identified in the BRCA1 and BRCA2 genes. Breast Cancer Res Treat. 2014;147:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Yata K, Bleuyard JY, Nakato R, Ralf C, Katou Y, Schwab RA, Niedzwiedz W, Shirahige K, Esashi F. BRCA2 coordinates the activities of cell-cycle kinases to promote genome stability. Cell Rep. 2014;7:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 126. | Jia Y, Domenico J, Swasey C, Wang M, Gelfand EW, Lucas JJ. Modulated expression of genes encoding estrogen metabolizing enzymes by G1-phase cyclin-dependent kinases 6 and 4 in human breast cancer cells. PLoS One. 2014;9:e97448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 127. | Fu J, Tian C, Xing M, Wang X, Guo H, Sun L, Sun L, Jiang Z, Zhang L. KU004 induces G1 cell cycle arrest in human breast cancer SKBR-3 cells by modulating PI3K/Akt pathway. Biomed Pharmacother. 2014;68:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 128. | Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, Lockerman EL, Pollack SF, Liu M, Li X. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 129. | Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, Zamboni WC, Wong KK, Perou CM, Sharpless NE. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 130. | Akin S, Babacan T, Sarici F, Altundag K. A novel targeted therapy in breast cancer: cyclin dependent kinase inhibitors. J BUON. 2014;19:42-46. [PubMed] |
| 131. | Zhang C, Yan Z, Arango ME, Painter CL, Anderes K. Advancing bioluminescence imaging technology for the evaluation of anticancer agents in the MDA-MB-435-HAL-Luc mammary fat pad and subrenal capsule tumor models. Clin Cancer Res. 2009;15:238-246. [PubMed] |
| 132. | Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Dean JL, McClendon AK, Hickey TE, Butler LM, Tilley WD, Witkiewicz AK, Knudsen ES. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle. 2012;11:2756-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 134. | Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr Relat Cancer. 2011;18:C19-C24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 135. | Rocca A, Farolfi A, Bravaccini S, Schirone A, Amadori D. Palbociclib (PD 0332991) : targeting the cell cycle machinery in breast cancer. Expert Opin Pharmacother. 2014;15:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 136. | Robinson TJ, Liu JC, Vizeacoumar F, Sun T, Maclean N, Egan SE, Schimmer AD, Datti A, Zacksenhaus E. RB1 status in triple negative breast cancer cells dictates response to radiation treatment and selective therapeutic drugs. PLoS One. 2013;8:e78641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 137. | McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, Farber JL, Force T, Koch WJ, Knudsen ES. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11:2747-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 138. | DiRocco DP, Bisi J, Roberts P, Strum J, Wong KK, Sharpless N, Humphreys BD. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2014;306:F379-F388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 139. | Martin MP, Olesen SH, Georg GI, Schönbrunn E. Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. ACS Chem Biol. 2013;8:2360-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 140. | Nguyen TK, Grant S. Dinaciclib (SCH727965) inhibits the unfolded protein response through a CDK1- and 5-dependent mechanism. Mol Cancer Ther. 2014;13:662-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Paruch K, Dwyer MP, Alvarez C, Brown C, Chan TY, Doll RJ, Keertikar K, Knutson C, McKittrick B, Rivera J. Discovery of Dinaciclib (SCH 727965): A Potent and Selective Inhibitor of Cyclin-Dependent Kinases. ACS Med Chem Lett. 2010;1:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 142. | Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, Seghezzi W, Paruch K, Dwyer MP, Doll R. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 435] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 143. | Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, Statkevich P, Zhu Y, Yao SL, Small K. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer. 2014;14:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 144. | Stephenson JJ, Nemunaitis J, Joy AA, Martin JC, Jou YM, Zhang D, Statkevich P, Yao SL, Zhu Y, Zhou H. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer. 2014;83:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 145. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [PubMed] |
| 146. | Asai N, Ohkawara B, Ito M, Masuda A, Ishiguro N, Ohno K. LRP4 induces extracellular matrix productions and facilitates chondrocyte differentiation. Biochem Biophys Res Commun. 2014;451:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 147. | Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. 2013;110:20224-20229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 660] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 148. | Lento W, Congdon K, Voermans C, Kritzik M, Reya T. Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harb Perspect Biol. 2013;5:pii: a008011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 149. | Seke Etet PF, Vecchio L, Bogne Kamga P, Nchiwan Nukenine E, Krampera M, Nwabo Kamdje AH. Normal hematopoiesis and hematologic malignancies: role of canonical Wnt signaling pathway and stromal microenvironment. Biochim Biophys Acta. 2013;1835:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 150. | Park JH, Kwon HY, Sohn EJ, Kim KA, Kim B, Jeong SJ, Song JH, Koo JS, Kim SH. Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacol Rep. 2013;65:1366-1374. [PubMed] |
| 151. | Menck K, Klemm F, Gross JC, Pukrop T, Wenzel D, Binder C. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4:2057-2066. [PubMed] |
| 152. | Tumova L, Pombinho AR, Vojtechova M, Stancikova J, Gradl D, Krausova M, Sloncova E, Horazna M, Kriz V, Machonova O. Monensin inhibits canonical Wnt signaling in human colorectal cancer cells and suppresses tumor growth in multiple intestinal neoplasia mice. Mol Cancer Ther. 2014;13:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Kim JT, Liu C, Zaytseva YY, Weiss HL, Townsend CM Jr, Evers BM. Neurotensin, a novel target of Wnt/β-catenin pathway, promotes growth of neuroendocrine tumor cells. Int J Cancer. 2014;Aug 5; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 154. | Yu DH, Zhang X, Wang H, Zhang L, Chen H, Hu M, Dong Z, Zhu G, Qian Z, Fan J. The essential role of TNIK gene amplification in gastric cancer growth. Oncogenesis. 2014;2:e89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 155. | Many AM, Brown AM. Both canonical and non-canonical Wnt signaling independently promote stem cell growth in mammospheres. PLoS One. 2014;9:e101800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 156. | Klinke DJ. Induction of Wnt-inducible signaling protein-1 correlates with invasive breast cancer oncogenesis and reduced type 1 cell-mediated cytotoxic immunity: a retrospective study. PLoS Comput Biol. 2014;10:e1003409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 157. | Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/β-catenin and mTORC1 signaling in breast and prostate cancer cells. J Cell Biochem. 2014;115:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 158. | Lu W, Lin C, Li Y. Rottlerin induces Wnt co-receptor LRP6 degradation and suppresses both Wnt/β-catenin and mTORC1 signaling in prostate and breast cancer cells. Cell Signal. 2014;26:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 159. | Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 160. | Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA. 2013;110:12649-12654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 161. | Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One. 2013;8:e67811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 162. | Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer. 2013;13:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 163. | Yin S, Xu L, Bonfil RD, Banerjee S, Sarkar FH, Sethi S, Reddy KB. Tumor-initiating cells and FZD8 play a major role in drug resistance in triple-negative breast cancer. Mol Cancer Ther. 2013;12:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 164. | Cui W, Wang LH, Wen YY, Song M, Li BL, Chen XL, Xu M, An SX, Zhao J, Lu YY. Expression and regulation mechanisms of Sonic Hedgehog in breast cancer. Cancer Sci. 2010;101:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 165. | Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 166. | Wang TP, Hsu SH, Feng HC, Huang RF. Folate deprivation enhances invasiveness of human colon cancer cells mediated by activation of sonic hedgehog signaling through promoter hypomethylation and cross action with transcription nuclear factor-kappa B pathway. Carcinogenesis. 2012;33:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 167. | Singh AP, Arora S, Bhardwaj A, Srivastava SK, Kadakia MP, Wang B, Grizzle WE, Owen LB, Singh S. CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor κB: implications for bidirectional tumor-stromal interactions. J Biol Chem. 2012;287:39115-39124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 168. | Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, Page GP, Welch DR, Lobo-Ruppert SM, Ruppert JM, Johnson MR. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther. 2006;5:674-683. [PubMed] |
| 169. | Thomas ZI, Gibson W, Sexton JZ, Aird KM, Ingram SM, Aldrich A, Lyerly HK, Devi GR, Williams KP. Targeting GLI1 expression in human inflammatory breast cancer cells enhances apoptosis and attenuates migration. Br J Cancer. 2011;104:1575-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 170. | Das S, Samant RS, Shevde LA. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to Smoothened-targeting Hedgehog inhibition. J Biol Chem. 2013;288:11824-11833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 171. | Bai R, Zhao H, Zhang X, DU S. Characterization of sonic hedgehog inhibition in gastric carcinoma cells. Oncol Lett. 2014;7:1381-1384. [PubMed] |
| 172. | Heiden KB, Williamson AJ, Doscas ME, Ye J, Wang Y, Liu D, Xing M, Prinz RA, Xu X. The sonic hedgehog signaling pathway maintains the cancer stem cell self-renewal of anaplastic thyroid cancer by inducing snail expression. J Clin Endocrinol Metab. 2014;99:E2178-E2187. [PubMed] |
| 173. | Balbous A, Renoux B, Cortes U, Milin S, Guilloteau K, Legigan T, Rivet P, Boissonnade O, Martin S, Tripiana C. Selective release of a cyclopamine glucuronide prodrug toward stem-like cancer cell inhibition in glioblastoma. Mol Cancer Ther. 2014;13:2159-2169. [PubMed] |
| 174. | Chai F, Zhou J, Chen C, Xie S, Chen X, Su P, Shi J. The Hedgehog inhibitor cyclopamine antagonizes chemoresistance of breast cancer cells. Onco Targets Ther. 2013;6:1643-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 175. | Steg AD, Katre AA, Bevis KS, Ziebarth A, Dobbin ZC, Shah MM, Alvarez RD, Landen CN. Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol Cancer Ther. 2012;11:1587-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 176. | Ramaswamy B, Lu Y, Teng KY, Nuovo G, Li X, Shapiro CL, Majumder S. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72:5048-5059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 177. | Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, Allred DC, Lewis MT. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 178. | Okolowsky N, Furth PA, Hamel PA. Oestrogen receptor-alpha regulates non-canonical Hedgehog-signalling in the mammary gland. Dev Biol. 2014;391:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 179. | Kwon YJ, Hurst DR, Steg AD, Yuan K, Vaidya KS, Welch DR, Frost AR. Gli1 enhances migration and invasion via up-regulation of MMP-11 and promotes metastasis in ERα negative breast cancer cell lines. Clin Exp Metastasis. 2011;28:437-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 180. | Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, Seke Etet PF. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20:R1-R17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 181. | Zhou L, Guo X, Jing BA, Zhao L. CD44 is involved in CXCL-12 induced acute myeloid leukemia HL-60 cell polarity. Biocell. 2010;34:91-94. [PubMed] |
| 182. | Razis E, Kalogeras KT, Kotoula V, Eleftheraki AG, Nikitas N, Kronenwett R, Timotheadou E, Christodoulou C, Pectasides D, Gogas H. Improved outcome of high-risk early HER2 positive breast cancer with high CXCL13-CXCR5 messenger RNA expression. Clin Breast Cancer. 2012;12:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 183. | Jaeger S, Min J, Nigsch F, Camargo M, Hutz J, Cornett A, Cleaver S, Buckler A, Jenkins JL. Causal Network Models for Predicting Compound Targets and Driving Pathways in Cancer. J Biomol Screen. 2014;19:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 184. | Konkel MK, Batzer MA. A mobile threat to genome stability: The impact of non-LTR retrotransposons upon the human genome. Semin Cancer Biol. 2010;20:211-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 185. | Kipanyula MJ, Seke Etet PF, Vecchio L, Farahna M, Nukenine EN, Nwabo Kamdje AH. Signaling pathways bridging microbial-triggered inflammation and cancer. Cell Signal. 2013;25:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 186. | Vecchio L, Seke Etet PF, Kipanyula MJ, Krampera M, Nwabo Kamdje AH. Importance of epigenetic changes in cancer etiology, pathogenesis, clinical profiling, and treatment: what can be learned from hematologic malignancies? Biochim Biophys Acta. 2013;1836:90-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 187. | Flanagan JM, Wilhelm-Benartzi CS, Metcalf M, Kaye SB, Brown R. Association of somatic DNA methylation variability with progression-free survival and toxicity in ovarian cancer patients. Ann Oncol. 2013;24:2813-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 188. | Muntané J, De la Rosa AJ, Docobo F, García-Carbonero R, Padillo FJ. Targeting tyrosine kinase receptors in hepatocellular carcinoma. Curr Cancer Drug Targets. 2013;13:300-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 189. | Huang SS, Clarke DC, Gosline SJ, Labadorf A, Chouinard CR, Gordon W, Lauffenburger DA, Fraenkel E. Linking proteomic and transcriptional data through the interactome and epigenome reveals a map of oncogene-induced signaling. PLoS Comput Biol. 2013;9:e1002887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 190. | Zazzu V, Regierer B, Kühn A, Sudbrak R, Lehrach H. IT Future of Medicine: from molecular analysis to clinical diagnosis and improved treatment. N Biotechnol. 2013;30:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 191. | Daniels M, Goh F, Wright CM, Sriram KB, Relan V, Clarke BE, Duhig EE, Bowman RV, Yang IA, Fong KM. Whole genome sequencing for lung cancer. J Thorac Dis. 2012;4:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 192. | Giessrigl B, Schmidt WM, Kalipciyan M, Jeitler M, Bilban M, Gollinger M, Krieger S, Jäger W, Mader RM, Krupitza G. Fulvestrant induces resistance by modulating GPER and CDK6 expression: implication of methyltransferases, deacetylases and the hSWI/SNF chromatin remodelling complex. Br J Cancer. 2013;109:2751-2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 193. | Hohmann AF, Vakoc CR. A rationale to target the SWI/SNF complex for cancer therapy. Trends Genet. 2014;30:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 194. | Kimura A, Arakawa N, Hirano H. Mass Spectrometric Analysis of the Phosphorylation Levels of the SWI/SNF Chromatin Remodeling/Tumor Suppressor Proteins ARID1A and Brg1 in Ovarian Clear Cell Adenocarcinoma Cell Lines. J Proteome Res. 2014;13:4959-4969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 195. | Brglez V, Pucer A, Pungerčar J, Lambeau G, Petan T. Secreted phospholipases A₂are differentially expressed and epigenetically silenced in human breast cancer cells. Biochem Biophys Res Commun. 2014;445:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 196. | Al-Rayyan N, Litchfield LM, Ivanova MM, Radde BN, Cheng A, Elbedewy A, Klinge CM. 5-Aza-2-deoxycytidine and trichostatin A increase COUP-TFII expression in antiestrogen-resistant breast cancer cell lines. Cancer Lett. 2014;347:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 197. | Wilson-Edell KA, Yevtushenko MA, Rothschild DE, Rogers AN, Benz CC. mTORC1/C2 and pan-HDAC inhibitors synergistically impair breast cancer growth by convergent AKT and polysome inhibiting mechanisms. Breast Cancer Res Treat. 2014;144:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 198. | Nickel A, Stadler SC. Role of epigenetic mechanisms in epithelial-to-mesenchymal transition of breast cancer cells. Transl Res. 2014;Apr 12; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 199. | Al-Nakhle H, Smith L, Bell SM, Burns PA, Cummings M, Hanby AM, Lane S, Parker MD, Hughes TA, Speirs V. Regulation of estrogen receptor β1 expression in breast cancer by epigenetic modification of the 5’ regulatory region. Int J Oncol. 2013;43:2039-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 200. | Mohapatra DK, Reddy DS, Ramaiah MJ, Ghosh S, Pothula V, Lunavath S, Thomas S, Valli SN, Bhadra MP, Yadav JS. Rugulactone derivatives act as inhibitors of NF-κB activation and modulates the transcription of NF-κB dependent genes in MDA-MB-231cells. Bioorg Med Chem Lett. 2014;24:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 201. | Liao XH, Li YQ, Wang N, Zheng L, Xing WJ, Zhao DW, Yan TB, Wang Y, Liu LY, Sun XG. Re-expression and epigenetic modification of maspin induced apoptosis in MCF-7 cells mediated by myocardin. Cell Signal. 2014;26:1335-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 202. | Sun S, Han Y, Liu J, Fang Y, Tian Y, Zhou J, Ma D, Wu P. Trichostatin A targets the mitochondrial respiratory chain, increasing mitochondrial reactive oxygen species production to trigger apoptosis in human breast cancer cells. PLoS One. 2014;9:e91610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 203. | Cody JJ, Markert JM, Hurst DR. Histone deacetylase inhibitors improve the replication of oncolytic herpes simplex virus in breast cancer cells. PLoS One. 2014;9:e92919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 204. | Pellon-Maison M, Montanaro MA, Lacunza E, Garcia-Fabiani MB, Soler-Gerino MC, Cattaneo ER, Quiroga IY, Abba MC, Coleman RA, Gonzalez-Baro MR. Glycerol-3-phosphate acyltranferase-2 behaves as a cancer testis gene and promotes growth and tumorigenicity of the breast cancer MDA-MB-231 cell line. PLoS One. 2014;9:e100896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 205. | Katz TA, Vasilatos SN, Harrington E, Oesterreich S, Davidson NE, Huang Y. Inhibition of histone demethylase, LSD2 (KDM1B), attenuates DNA methylation and increases sensitivity to DNMT inhibitor-induced apoptosis in breast cancer cells. Breast Cancer Res Treat. 2014;146:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 206. | Robertson FM, Chu K, Boley KM, Ye Z, Liu H, Wright MC, Moraes R, Zhang X, Green TL, Barsky SH. The class I HDAC inhibitor Romidepsin targets inflammatory breast cancer tumor emboli and synergizes with paclitaxel to inhibit metastasis. J Exp Ther Oncol. 2013;10:219-233. [PubMed] |
| 207. | Deb G, Thakur VS, Limaye AM, Gupta S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol Carcinog. 2014;Jan 31; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 208. | Chiu HW, Yeh YL, Wang YC, Huang WJ, Chen YA, Chiou YS, Ho SY, Lin P, Wang YJ. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, enhances radiosensitivity and suppresses lung metastasis in breast cancer in vitro and in vivo. PLoS One. 2013;8:e76340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 209. | Stark K, Burger A, Wu J, Shelton P, Polin L, Li J. Reactivation of estrogen receptor α by vorinostat sensitizes mesenchymal-like triple-negative breast cancer to aminoflavone, a ligand of the aryl hydrocarbon receptor. PLoS One. 2013;8:e74525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 210. | Tuval-Kochen L, Paglin S, Keshet G, Lerenthal Y, Nakar C, Golani T, Toren A, Yahalom J, Pfeffer R, Lawrence Y. Eukaryotic initiation factor 2α--a downstream effector of mammalian target of rapamycin--modulates DNA repair and cancer response to treatment. PLoS One. 2013;8:e77260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 211. | Salvador MA, Wicinski J, Cabaud O, Toiron Y, Finetti P, Josselin E, Lelièvre H, Kraus-Berthier L, Depil S, Bertucci F. The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin Cancer Res. 2013;19:6520-6531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 212. | Grassadonia A, Cioffi P, Simiele F, Iezzi L, Zilli M, Natoli C. Role of Hydroxamate-Based Histone Deacetylase Inhibitors (Hb-HDACIs) in the Treatment of Solid Malignancies. Cancers (Basel). 2013;5:919-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 213. | Martin M, Bonneterre J, Geyer CE, Ito Y, Ro J, Lang I, Kim SB, Germa C, Vermette J, Wang K. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer. 2013;49:3763-3772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 214. | Curigliano G, Pivot X, Cortés J, Elias A, Cesari R, Khosravan R, Collier M, Huang X, Cataruozolo PE, Kern KA. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. Breast. 2013;22:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 215. | Earl HM, Vallier AL, Hiller L, Fenwick N, Young J, Iddawela M, Abraham J, Hughes-Davies L, Gounaris I, McAdam K. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (Neo-tAnGo): an open-label, 2×2 factorial randomised phase 3 trial. Lancet Oncol. 2014;15:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 216. | Wang J, Song P, Schrieber S, Liu Q, Xu Q, Blumenthal G, Amiri Kordestani L, Cortazar P, Ibrahim A, Justice R. Exposure-response relationship of T-DM1: insight into dose optimization for patients with HER2-positive metastatic breast cancer. Clin Pharmacol Ther. 2014;95:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 217. | Kashiwaba M, Inaba T, Komatsu H, Ishida K, Kawagishi R, Matsui Y, Uesugi N, Sugai T, Wakabayashi G. A phase I study of capecitabine combined with CPT-11 in metastatic breast cancer pretreated with anthracyclines and taxanes. Oncology. 2014;86:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 218. | Reddy JA, Allagadda VM, Leamon CP. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr Pharm Biotechnol. 2005;6:131-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 219. | Harrap KR, Jackman AL, Newell DR, Taylor GA, Hughes LR, Calvert AH. Thymidylate synthase: a target for anticancer drug design. Adv Enzyme Regul. 1989;29:161-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 220. | Kolarevic A, Yancheva D, Kocic G, Smelcerovic A. Deoxyribonuclease inhibitors. Eur J Med Chem. 2014;Jul 15; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 221. | Benzaïd I, Mönkkönen H, Bonnelye E, Mönkkönen J, Clézardin P. In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin Cancer Res. 2012;18:6249-6259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 222. | Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C. Topotecan - A novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology. 1999;56:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 223. | Kumar N. Taxol-induced polymerization of purified tubulin. Mechanism of action. J Biol Chem. 1981;256:10435-10441. [PubMed] |
| 224. | Nicolaou KC, Winssinger N, Pastor J, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P. Synthesis of epothilones A and B in solid and solution phase. Nature. 1997;387:268-272. [PubMed] |
| 225. | Schuurman HJ, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, Tanner M, Zenke G, Schuler W. SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation. 1997;64:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 207] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 226. | McNeil C. Herceptin raises its sights beyond advanced breast cancer. J Natl Cancer Inst. 1998;90:882-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 227. | Sabatier R, Gonçalves A. Pertuzumab (Perjeta®) approval in HER2-positive metastatic breast cancers. Bull Cancer. 2014;101:765-771. [PubMed] |
| 228. | Corrigan PA, Cicci TA, Auten JJ, Lowe DK. Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate. Ann Pharmacother. 2014;48:1484-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 229. | Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255-6263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 495] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 230. | Kangas L, Nieminen AL, Cantell K. Additive and synergistic effects of a novel antiestrogen, toremifene (Fc-1157a), and human interferons on estrogen responsive MCF-7 cells in vitro. Med Biol. 1985;63:187-190. [PubMed] |
| 231. | Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867-3873. [PubMed] |
| 232. | Nicholson RI, Walker KJ, Maynard PV. Anti-tumour potential of a new luteinizing hormone releasing hormone analogue, ICI 118630. Eur J Cancer. 1980;Suppl 1:295-299. [PubMed] |
| 233. | Plourde PV, Dyroff M, Dukes M. Arimidex: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 157] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 234. | Giudici D, Ornati G, Briatico G, Buzzetti F, Lombardi P, di Salle E. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): a new irreversible aromatase inhibitor. J Steroid Biochem. 1988;30:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 235. | Ansfield FJ, Davis HL, Ellerby RA, Ramirez G. A clinical trial of megestrol acetate in advanced breast cancer. Cancer. 1974;33:907-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 140] [Reference Citation Analysis (0)] |