INTRODUCTION
Malignant degeneration of post-burned lesions and scars is an inevitable eventuality, afflicting at least 0.77%-2% of the deep burns that had been allowed to heal by secondary intention, those which never healed completely and the unstable post-burned scars that frequently ulcerate on trivial traumatic insults of daily life activities[1-3]. Celsus AC deserves acknowledgment for his earliest recognition of this phenomenon in the first century AD[4]. Later on in 1828, the French physician Marjolin JN etiologically classified ulcers as those due to “local” causes and those secondary to “internal” causes, however he couldn’t specifically recognize the malignant potential of these lesions[5,6]. Dupuytren[7] in 1839 provided full description of a case of amputation for a cancer in a patient who had suffered a sulfuric acid burn injury. Da Costa[8] in 1903 was the first to coin the term Marjolin’s ulcer (MU) to describe malignant degeneration of skin scars particularly the post-burned scars.
Not surprisingly, MUs can emanate from any chronic wound or unhealed scar, however the neglected burn wounds constitute their commonest seats of origin[9-11]. The following review focuses on the epidemiological and clinical details of MU emanating in the aftermath of burn injuries with a view to provide a comprehensive summary of the key conceptual issues as well as recent updates on management for those who happen to be the frontline care providers for the patients with MU.
EPIDEMIOLOGIC CONSIDERATIONS
Whereas 0.77%-2% of the post-burned wounds and scars are reported to undergo malignant degeneration[3], overall the post-burned wounds and scars contribute to 2% of all squamous cell carcinomas (SCCs) and 0.03% of all basal cell carcinomas (BCCs) of the skin[4].
MU is relatively commoner among males than females[12-15]. The exact explanation for this is not yet known, however more frequent initial burn trauma among males as well as their more prolonged exposure to sunlight are some of the possible contributors to this higher frequency of MU among males. No age is immune to MU with individuals from almost all age groups including children being afflicted worldwide[1-5]. MU has been reported among individuals of all races[16-20].
There is usually a prolonged latency period between sustaining initial burn insult and developing MU in the post-burned wounds and scars. There is considerable variation in this lag period reported in the published literature[1,2,13,14], ranging from as short as 6 wk[15] to as prolonged as 70 years[18]. The average latency period to malignant transformation is 35 years[1,21,22]. Based on the latency period, the MUs are subdivided into acute and chronic subtypes. The former type refers to the scar carcinoma that evolves within a year of sustaining burn injury, while the later type refers to those that develop from then on[22]. The acute MU usually develops in association with more superficial burn scars and is often a basal cell carcinoma on histology[23]. The latency period of MU inversely relates to the patient’s age at the time of sustaining initial burn insult[24]. The younger the patient is at the time of initial burn insult, the longer the time it takes to undergo the malignant transformation. Contrary to this, the older the patient at the time of burn injury the shorter the lag period and more is the chance of acute MU. Understandably, a newly acquired burn injury in an adult of advancing age is more likely to evolve an acute MU, hence a biopsy of all such lesions (of a duration of > 6 wk) is imperative.
Although all underlying mechanisms of burn injury pose an equal risk for subsequent malignant transformation, MU has been reported more frequently among those who had sustained flame burns as compared to the other burn injury mechanisms such as scalds, electric burn injuries, chemical burns, and contact burns. Except for the BCC where contact is the most frequent underlying burn injury mechanism, the other histological types of MU occur with equal frequency amongst flame, scalds and contact burn injuries[2].
Given the global statistics, the burden of burn injuries is disproportionately shared across the globe with most of its brunt being taken by the developing nations such as India, Bangladesh and Pakistan[25]. These countries together with other south Asian countries like Sri Lanka, Bhutan, Nepal, Maldives, and Afghanistan collectively constitute 20% of the world’s population, however they contributed only 1.1% of the total PubMed publications during the 25 years period from 1985-2009[26]. One can easily imagine the magnitude of MU that certainly exists in these burn injury endemic countries but is under-reported. These developing nations have recognized limitations of their health care systems where the ideal treatment for acute burn injuries is often not instituted[27]. Also many of the patients in these developing countries present late, when the MU is not amenable to curative resection.
PATHOLOGIC CONSIDERATIONS
Etiopathogenesis of MU
MUs originating from post-burned scars possess certain peculiarities that make them distinct from other cutaneous malignancies. The exact mechanism of how the malignant transformation supervenes the post-burned scars continues to be explored. Many theories have been proposed to provide possible explanations of the mechanisms involved, however no single theory alone can provide a satisfactory answer to all questions that surround this complex process of malignant degeneration.
As per Ewing J’s postulates[2,28], MU of post-burned scars would meet the criteria such as evidence of a burn scar, tumour within the boundaries of the scar, no previous tumour in that location, tumour histology being compatible with the cell types found in the skin/scar and presence of a lag period between the burn injury and the tumour development. The post-burned scars is certainly a mutogenic focus with continuous mitotic activity of regeneration and repair being in progress. The same represents the key mechanism that eventually triggers the malignant transformation[10,29,30]. A myriad of factors have been postulated as possible contributors toward the process of malignant transformation. Among these include chronic irritation, repeated trauma, impaired immunologic reactivity of the scar tissue to tumour cells, release of toxins from the unhealthy scar, relative avascularity of the scar tissue, lymphatic obstruction within the scar tissue making it an inaccessible site for the body’s natural immunosurveillance[2,10,31,32]. When the full thickness skin loss areas are allowed to heal by secondary intention, there is formation of unstable depigmented substitution tissue which lacks the qualities of normal skin. These unstable depigmented scars have reduced ability to withstand carcinogens[4,32]. Whether genetics or heredity have any contribution to the malignant degeneration of the post-burned scars is not exactly known, however abnormalities in the p53 gene among these patients have been reported[33,34].
The major risk factors for the development of post-burn MU include healing of full thickness skin burns by secondary intention, non-healing burn wounds, and fragile scars that ulcerate and are easily traumatized[1,2]. The post-burned scars is typically less resistant to injuries, heals poorly especially in body areas such as the joints.
Histopathology of MU
In most cases, the MU is an SCC (71%), followed by BCC (12%), melanoma (6%), sarcoma (5%), squamo-basal cell carcinoma (1%), SCC-melanoma (1%) and other rare neoplasms (4%)[2]. A variety of rare tumours may emerge in the post-burned wounds and scars and include fibrosarcoma, liposarcoma, dermatofibrosarcoma protuberans, and mesenchymal tumors[2,3,35,36]. The grade of the MU can be defined as follows: grade I : more than 75% of the cells are differentiated; grade II: 25%-75% of the cells are differentiated; grade III: less than 25% of the cells are differentiated[10]. Grade of the tumour has bearing on the prognosis of MU. In general, the incidence of metastasis increases with increasing grade and so is the worsening of prognosis.
CLINICAL COSIDERATIONS
Clinical presentation
Clinically MU presents in two major morphologic forms[18,37]. The commoner form is the flat, indurated, infiltrative, ulcerative variant while the other less frequent form is the exophytic papillary variety which is generally less severe. The well-differentiated exophytic lesions have a better prognosis than the poorly differentiated, ulcerated and infiltrating forms. Typically the edge of the ulcerated lesion is everted and the floor has poor granulation tissue (Figures 1-9 are representative photographs of some patients with MUs secondary to burn injuries).
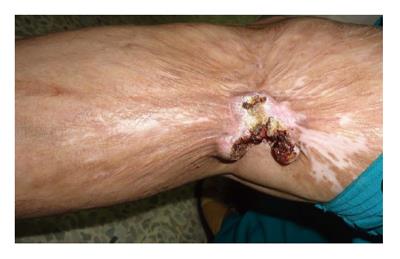
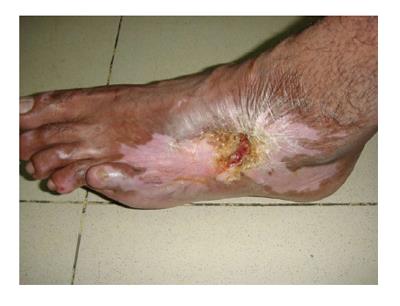
Figure 1 Marjolin's ulcer in the left popliteal fossa region in a 45 years old lady who had sustained flame burn injury at the age of 13.
There is characteristic ulcer with everted edges and poorly granulating floor. The surrounding skin shows post-burned sequel. Histopathology confirmed it to be well differentiated squamous cell carcinom.
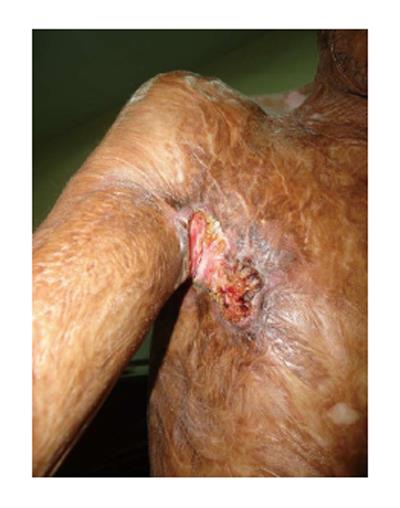
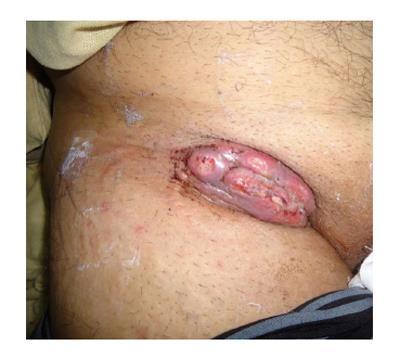
Figure 2 A 46 years male with 3 years history of ulceration and bleeding in right axilla.
He had sustained scald burns at the age of 3. Biopsy confirmed it to be squamous cell carcinoma while computed tomography scan revealed metastasis in the axilla as well as chest.
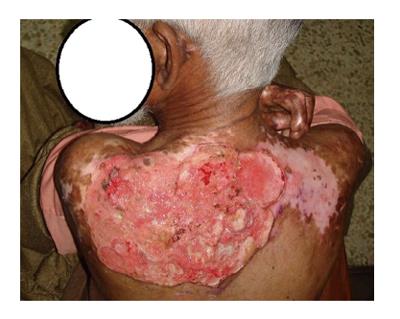
Figure 3 A 63 years old male presented with two years history of slowly progressive ulceration in the post burned white skin on his upper back.
He had childhood scald burns at the age of 3 years, and had received burn injury treatment with months of dressings without skin grafting. Multiple biopsies revealed squamous cell carcinoma, while computed tomography scan revealed axillary nodal invasion without chest metastasis. Culture sensitivity revealed Methicillin resistant staphylococcus and pseudomonas aeruginsa.
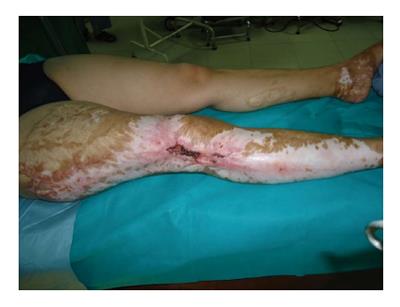
Figure 4 A lady aged 41, had sustained burn injury secondary to lightning 3 years ago.
She had her burn injuries managed with months of dressing without skin grafting. Subsequently she had recurrent ulceration with bleeding from the unhealed wounds around the knee. Multiple biopsies of the lesions revealed well differentiated squamous cell carcinoma. The groin nodal basin was negative clinically as well as radiologically.
Figure 5 A 41 years male who had sustained flame burn injury to his left foot in childhood at the age of 4.
The burn injury was managed with months of dressings and the wound never healed completely. There was history of recurrent bleeding and ulceration on the affected site. Multiple biopsies revealed moderately differentiated squamous cell carcinoma. The groin was clinically node positive.
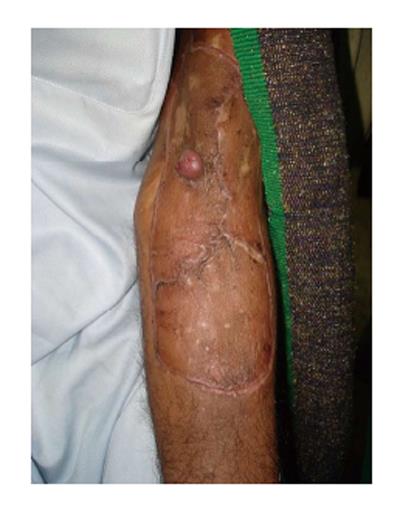
Figure 6 A 36 years male had sustained chemical burn injury to his left cubital fossa 7 years ago.
The initial burn injury was managed with dressings and had never healed completely. The patient had undergone wide local excision and split thickness skin grafting for Marjolin's ulcer three months ago. Later he presented with a recurrent nodule which was confirmed as squamous cell carcinom on histopathology while the axilla was node negative clinically.
Figure 7 Right groin metastasis secondary to Marjolin's ulcer on the right side of ankle in 57 years male.
Metastatic work up revealed ascites and lung metastasis. The patient had sustained flame burn injury to the right ankle at the age of 2 years and was managed with wound dressings without skin grafting.
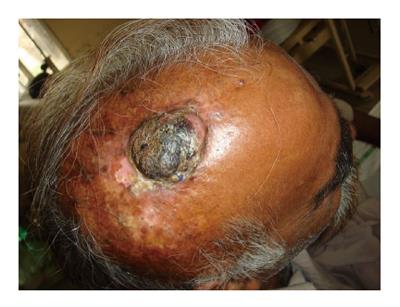
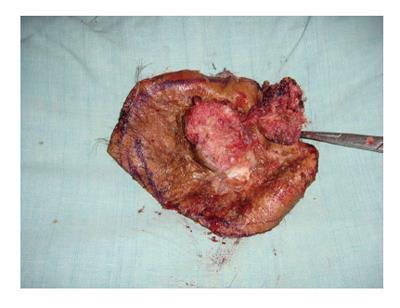
Figure 8 A 47 years male had sustained flame burn injury to his scalp at the age of 3.
The initial burn injury was managed with months of dressings without skin grafting. Histopathology confirmed it as well differentiated squamous cell carcinoma. Computed tomography scan head and neck did not show deep structures invasion.
Figure 9 Same patient (as in Figure 8), the resected Marjolin's ulcer with wide local margins.
A history of a non healing post-burned wound of full thickness skin loss should alert the clinician of the possibility of an MU. It is usually painless. The easy bleeding fragile areas may at times present with unprovoked bleeding, offensive discharge or increasing pain. Superadded infection of the wound may at times be the first clinical presentation[18,38,39].
Anatomic sites affected by MUs
Lower limbs constitute the most frequent site of MUs. The other sites affected in order of reducing frequency include head and neck region (face, scalp, neck), upper limbs and other body parts[2,3,35]. MU has been reported in post burned scars at rare locations such as the nose[40]. Lower limbs are the commonest sites of MU primarily owing to their more frequent involvement in burn injury insults involving full thickness skin loss. Additionally these patients often present with lesions around the knee joints as the joints are frequently moved and recurrent ulcerations commonly ensue and persist without healing.
Diagnosis of MU
The diagnosis of MU is based on the suggestive findings in the patient’s history, detailed examination of the ulcer and its draining nodal basin, and the histology of the lesion.
The classic triad of nodule formation, induration, and ulceration at the post-burned scars should prompt a biopsy to confirm the diagnosis[41]. Other clinical signs suggestive of MU include everted or rolled margins, exophytic granulation tissue formation, increasing size, bleeding and regional lymphadenopathy[1-10].
Once the biopsy confirms the diagnosis of MU, determination of the local extent of the lesion and staging comes to the fore. An magnetic resonance imaging (MRI) or computed tomography (CT scan) is performed to determine the local extent of the lesion and invasion of any underlying structures. MRI is certainly the ideal imaging tool for evaluation of the status of the soft-tissues, infiltration of any underlying bone and the involvement of adjacent neurovascular structures[42-44].
The draining lymphatic basin is staged clinically as well as radiologically with either high resolution ultrasonography, MRI or CT scan. Given the aggressive nature of MU, distant metastasis are ruled out with metastatic work up that includes chest CT scan, abdominal ultrasonography and CT scan brain (for lesions on the scalp and face)[1-10].
Metaststic spread of the MU and the stage of the MU disease
By and large, as long as the MU is confined to the scar it shows typically slow growth and is amenable to curative resection. However when the MU breaks free of the scar it metastasises rapidly via lymphatic spread[31]. Once broken free of the confines of the primary lesion, an SCC of the MU variety is known to possess greater metastatic potential than the SCC occurring de novo[18]. At presentation, regional lymph nodes are involved in 20%-36% of the patients[2,18,37,45]. Aydoğdu et al[18] have reported even higher percentage of patients (66.66%) with involved regional lymph nodes, dura or bone at initial presentation. Distant metastases are reported among 14% of the patients[2]. Although metastatic spread is primarily to the regional lymph nodes, metastasis to organs such as the liver, lung, brain, kidney may also occur[18].
Stage of the MU has implications for the management as well as the prognosis. As is the case with other malignancies, staging is performed by considering the size of the primary lesion (T), lymph node involvement (N), and distant metastasis (M). As yet there is no MU specific TNM classification of the Union for International cancer control, however the TNM classification for SCC is commonly applied to the MU.
TREATMENT OF MU
Role of surgery
Surgery constitutes the mainstay of treatment of the MU. The oncologic clearance entails excision of the primary lesion with a 2-4 cm horizontal clearance margins, and vertical clearance of the un-involved next barrier structure. All the wide local excisions are preferably performed initially with cautery dissection to prevent seeding of the tumor cells and their iatrogenic spread into the blood and lymphatic streams. Additionally a small margin of skin is then excised with a surgical scalpel to ensure good healing[14,20,35]. It is prudent to have the histopathologist on-board when performing such crucial resections to ensure resection of tumour free margins with the help of frozen section studies performed simultaneously with surgery. The defects resulting from MU extirpation are either skin grafted or flap covered. Anecdotally we are now preferring flap coverage of the resultant defects where ever possible and subsequently offer the patients radiotherapy for the tumor bed with the help of radiation oncologist.
As is the established norm of surgical oncology, the clinically or radiologically confirmed involved nodal basins are managed with therapeutic lymph node dissection.
Although there is lack of general consensus regarding management of the clinically negative nodal basins in MU, yet given the aggressive biologic behavior of MU, prophylactic nodal treatment with either elective lymph node dissection or regional nodal irradiation sounds rational[31,37,46-48]. Long term studies are certainly needed to confirm if this aggressive approach offers real benefits in terms of disease free survival or not, as the formal nodal clearance has its own morbidity (particularly lymphedema) attended to the procedure.
The sentinel lymph node dissection (SLND) has been primarily employed for staging the regional nodal basins in malignant melanoma of the limbs, however there is a recent growing recognition of its utility among patients with non-melanoma skin cancers also[49-54]. SLND technique holds the potential to be used more frequently in MU patients as it on one hand will save MU patients from the unnecessary morbidity of formal nodal clearance for negative nodes and on the other hand identify the MU patients who are clinically node negative but have subclinical nodal metastasis.
Role of radiotherapy
Given the aggressive biological behaviour of the MU and the frequent squamous cell histology, radiotherapy finds an important adjunctive role in managing these malignancies. The indications for radiotherapy include: (1) inoperable regional lymph node metastasis; (2) grade 3 lesions with positive lymph nodes after nodal dissection; (3) tumors with a diameter greater than 10 cm and with positive lymph nodes after regional lymph node dissection; (4) grade 3 lesions with a tumor diameter greater than 10 cm and negative lymph nodes after regional lymph dissection; and (5) lesions of the head and neck with positive lymph nodes after regional lymph node dissection[46].
Role of chemotherapy
The exact role of chemotherapy or indications thereof in managing MU are not yet established, however chemotherapy constitutes part of the aggressive multimodal therapy which is often instituted among patients when surgical extirpation of the MU is not possible because of the unfit patient, presence of distant metastasis, recurrent disease, and patients not consenting for surgery.
The chemotherapy is usually based on 5-Fluorouracil with a combination of cisplatin, methotrexate and bleomycin. It may be in the form of adjuvant or neo adjuvant therapy[18].
PROGNOSIS
Generally speaking, the MU tends to be more aggressive and rapidly spreading as compared to other skin carcinomas of similar histotypes[1,55]. The overall mortality rate of MU is reported to be at least 21%[2]. The survival rates of MU are 52%, 34% and 23% at 5, 10 and 20 years[56].
Poor prognostic clinical features in MU include regional nodal spread, local extension of lesion, lower limb lesions (as these have a greater propensity for nodal involvement), infiltrative variety, primary lesions of ≥ 2 cm, latency period of ≥ 5 years, recurrent MU, and the presence of distant metastasis. The poor prognostic indicators on histology include poor differentiation, scarce or absent peritumour T cell infiltration, invasion of reticular dermis or deeper structures, and ≥ 4 mm vertical thickness of the neoplastic lesion[4,37,46,55-60].
PREVENTION
Although MU constitutes a formidable foe for reconstructive and burns surgeons around the globe, it is still surmountable to primary as well as secondary prevention. Early excision and grafting of deep burns adequately averts all the wound problems that otherwise predispose the post-burned scars to malignant transformation[61]. Moreover even if an initially neglected or mismanaged burn wound presents later with ulceration or frequent wounding, before any malignancy has set in, the choice of excision and grafting of these unstable scars should still be availed. So primary prevention is ensured by provision of adequate surgical care in the acute phase of burn injury management, while secondary prevention can be instituted where a patient had an initial mismanagement but seeks medical advice before MU has established.
CONCLUSION
MU is a largely preventable dreadful menace of considerable morbidity and mortality. Although over the years, significant progress has been made in managing MU, the key to successful eradication lies in prevention by ensuring adequate surgical care (with early excision and grafting) of the deep burns in their acute phase.
There is need for randomized controlled trials and high quality evidence on the not yet fully established aspects of the MU management such as the oncologically safe horizontal clearance margins of resection, prophylactic management of the negative nodal basins and MU specific TNM staging system. All these issues need be adequately addressed by future clinical studies.
P- Reviewer: Stanojevic GZ S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ