Published online Oct 6, 2025. doi: 10.12998/wjcc.v13.i28.108180
Revised: May 26, 2025
Accepted: July 16, 2025
Published online: October 6, 2025
Processing time: 122 Days and 9.2 Hours
Multiple sclerosis (MS) is known to affect many sensory systems, yet most auditory research in MS has focused on the afferent pathways, with relatively few studies examining efferent function. The brainstem is a common site for MS plaques, and the medial olivocochlear (MOC) system is located in the superior olivary complex (SOC) of the brainstem. The cochlear nuclei are also involved in the MOC reflex arc. Additionally, the temporal cortex can modulate the SOC and cochlear nucleus, so lesions in the brainstem or temporal cortex may affect the MOC reflex in MS.
To investigate efferent auditory system activity in patients with multiple sclerosis via the MOC reflex.
The study included 50 patients with MS and 50 healthy controls. Patients with MS were divided into three subgroups according to cranial magnetic resonance imaging findings: Patients with brainstem lesions (Group 1, n = 20); patients with temporal cortex lesions without brainstem involvement (Group 2, n = 20); and patients without any lesions in the brainstem or temporal cortex (Group 3, n = 10). Tympanometry, acoustic stapedial reflex thresholds, pure-tone audiometry, and transient-evoked otoacoustic emission (TEOAE) tests (with and without contralateral noise) were performed for all participants.
There was no significant difference in pure-tone hearing thresholds or baseline TEOAE amplitudes between the MS and control groups, indicating normal cochlear function in patients with MS; however, MOC reflex suppression was significantly reduced in patients with MS compared to controls (P = 0.021). In particular, Group 1 (MS with brainstem lesions) showed the lowest mean suppression values, which was significantly lower than that of Group 2 and the control group (P = 0.002). By contrast, Group 2 and Group 3 did not significantly differ from controls. Additionally, patients with MS exhibited a sex difference in MOC function: Male patients had significantly lower suppression compared to female patients both within Group 1 and in the MS group as a whole.
The findings indicate that the efferent auditory system (specifically the MOC reflex) is affected by MS. MOC reflex activity was most significantly decreased in patients with MS with brainstem lesions, while temporal cortex lesions alone did not appear to notably impair the MOC reflex. Diminished MOC activity may underlie various auditory difficulties in patients with MS (e.g., hearing in noise), and loss of efferent suppression could contribute to symptoms such as hyperacusis or tinnitus in this population. Further studies are needed to better understand the relationship between MOC dysfunction and auditory symptoms in MS, as well as the potential diagnostic value of MOC testing in MS.
Core Tip: This study provides evidence that the efferent auditory pathway, assessed via the medial olivocochlear (MOC) reflex, is compromised in patients with multiple sclerosis (MS), particularly in those with brainstem lesions. The findings suggest that demyelinating lesions in the brainstem, where the MOC system is anatomically localized, may impair auditory modulation. Temporal cortex involvement appears to have no significant effect. These results support the relevance of efferent auditory evaluation in understanding auditory dysfunctions associated with MS.
- Citation: Gecer IS, Tabaru A, Yilmaz B, Kaya E, Kaya Tutar N, Gumuslu B, Oktay MF. Medial olivocochlear reflex dysfunction in multiple sclerosis: The influence of brainstem lesion localization and its clinical implications. World J Clin Cases 2025; 13(28): 108180
- URL: https://www.wjgnet.com/2307-8960/full/v13/i28/108180.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i28.108180
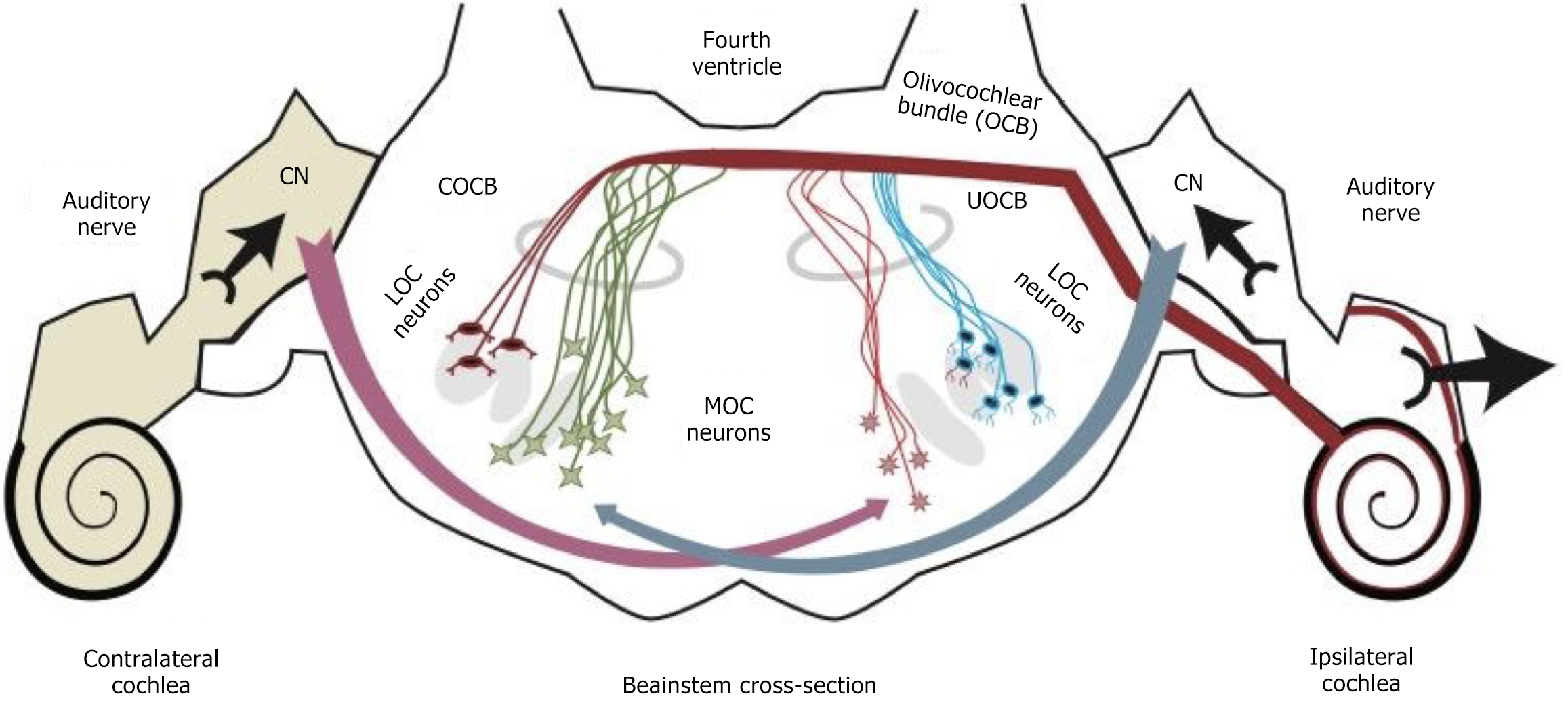
The auditory efferent pathway is a feedback loop that starts from the superior olivary complex (SOC) in the brainstem and ends in the cochlea. It serves critical functions such as protecting the inner ear from acoustic trauma and enhancing hearing in noisy environments by modulating the mechanical vibrations of the outer hair cells. There are two types of olivocochlear efferents: Medial and lateral. Medial olivocochlear (MOC) efferents innervate outer hair cells, whereas lateral olivocochlear efferents innervate the nerve fibers under inner hair cells[1]. The anatomical and physiological differences between the medial and lateral olivocochlear systems suggest they are functionally distinct pathways. Figure 1 illustrates the olivocochlear bundle and the MOC reflex pathway.
Multiple sclerosis (MS) is a chronic, autoimmune, inflammatory disease of the central nervous system[2]. It has been widely researched because it affects many sensory systems. Various auditory studies in MS have documented abnor
Previous case reports have provided clues that MS can indeed impact the efferent auditory system. Several case studies of patients with MS with hearing loss have employed transient evoked otoacoustic emission (TEOAE) measurements to assess cochlear function. In all instances, robust TEOAEs were present, indicating normal outer hair cell function and suggesting that the hearing impairments arose from retrocochlear (central neural) pathology. Changes in TEOAE levels corresponding to alterations in MOC suppression have been observed in individual MS cases. For example, 1 patient with MS and a pontine lesion experienced transient hearing loss; during the acute phase, TEOAE amplitudes increased (indicating a loss of efferent suppression), and then later decreased with prednisolone treatment as hearing recovered. This fluctuation in TEOAE levels—initially elevated and then normalizing—suggests that damage to the MOC system’s inhibitory function can be reversible with disease remission. In another case, a complete loss of bilateral TEOAE suppression was linked to demyelinating lesions in the brainstem affecting the SOC, whereas a different patients with MS with normal hearing showed only minimal contralateral TEOAE suppression (less than 0.5 dB). Such findings indicate that while the efferent pathway can be disrupted by MS, the extent and clinical significance of this disruption across the broader MS population remain unclear.
Given the crucial role of the efferent MOC system in auditory processing, this study systematically examined MOC reflex function in individuals with MS. We specifically stratified patients with MS by lesion location on magnetic resonance imaging (MRI), since we hypothesized that brainstem lesions would have the greatest impact on the MOC reflex. Patients with MS were categorized into three groups based on cranial MRI findings: Those with brainstem lesions, those with temporal lobe lesions (but no brainstem involvement), and those with lesions in other central nervous system (CNS) regions without any brainstem or temporal lobe lesions. By comparing these groups and healthy controls, we sought to determine how MS lesions in different locations affect the MOC reflex, thereby shedding light on the involvement of the efferent auditory system in MS.
This study was approved by the Clinical Research Ethics Committee of Istanbul Training and Research Hospital (Decision No. 2781, March 19, 2021). Participants recruited for the study consisted of patients diagnosed with MS and age- and sex-matched healthy controls. The patients with MS were under the care of the Neurology MS outpatient clinic at Bagcilar Training and Research Hospital (Istanbul, Türkiye) between July 1, 2021 and July 1, 2022. The control group comprised hospital staff, Ear, Nose and Throat clinic visitors, students, and individuals without MS from patients’ families. All participants underwent otoscopic examination of the external ear canal and provided written informed consent.
Inclusion criteria for all subjects were: Age 18-65 years, normal otoscopic findings, normal hearing thresholds, and presence of a valid TEOAE response in at least three of five frequency bands (1 kHz to 4 kHz) with an 80 dB peSPL click stimulus. To be included in the MS group, patients required a confirmed MS diagnosis based on contrast-enhanced cranial MRI and neuroradiologist evaluation. Exclusion criteria (for all participants) included any history of otologic surgery, sudden hearing loss or chronic hearing impairment, exposure to known ototoxic drugs, head and neck cancer, radiation therapy, chemotherapy, cerebrovascular disease, or brain surgery.
The MS group was further stratified into subgroups according to MRI lesion location. Group 1 consisted of patients with MS with one or more demyelinating lesions in the brainstem. Group 2 included patients with MS with lesions in the temporal lobe but no brainstem lesions. Group 3 comprised patients with MS without any lesions in the brainstem or temporal lobes (i.e. lesions confined to other regions of the CNS). This MRI-based classification was performed by reviewing each patient’s cranial MRI reports. Our subgrouping approach is similar to that of Coelho et al[1], who compared patients with MS with brainstem plaques to those without brainstem involvement when evaluating efferent function.
All audiological assessments were conducted by a single audiologist in a sound-attenuated room at Bagcilar Training and Research Hospital. The test battery included tympanometry, acoustic stapes reflex thresholds, pure-tone audiometry (PTA), and TEOAE measurements. PTA was performed to confirm normal hearing (defined as hearing thresholds ≤ 20 dB HL across 250-8000 Hz). Tympanometry confirmed normal middle ear function (Type A tympanograms) in all ears, and acoustic reflex testing ensured the middle ear reflexes were present at expected levels, ruling out middle ear pathology.
TEOAE measurements were obtained using the Madsen Capella 2 OAE system. Click stimuli at 80 ± 3 dB SPL were presented and responses were recorded over the 1000-4000 Hz range. Frequencies with a signal-to-noise ratio (SNR) of at least 3 dB were considered to have a valid OAE response. An SNR criterion of 3 dB was selected to ensure detection of even low-amplitude emissions; although some protocols recommend a stricter 6 dB SNR threshold for OAE presence[5,6], a 3 dB cutoff increases the sensitivity to subtle changes. We required each subject to have TEOAEs present in at least 3 of 5 frequency bands meeting this criterion, which ensured that all included ears had overall reproducible emissions above the noise floor. Using the 3 dB criterion allowed us to include participants with borderline emissions who might otherwise be excluded by a 6 dB cutoff, while maintaining consistent inclusion criteria for both MS and control groups. The overall TEOAE response amplitude (dB SPL) and SNR at each frequency band were recorded for analysis.
To assess the medial olivocochlear reflex (contralateral suppression of TEOAEs), TEOAE testing was performed under two conditions: (1) No contralateral stimulus (baseline TEOAE); and (2) With contralateral acoustic stimulation. For the suppression condition, a continuous white noise at 60 dB HL was presented to the contralateral ear. The choice of a broadband white noise suppressor was based on its effectiveness in eliciting maximal efferent activation across the cochlear frequency range[7] (broadband noise produces greater OAE suppression than other sound types, such as narrowband noise or speech babble, which generally yield less suppression. Therefore, white noise was used to robustly engage the MOC reflex). We recorded TEOAEs simultaneously in the ipsilateral ear with and without the contralateral noise. The amount of contralateral suppression was quantified as the difference in TEOAE amplitude (or SNR) between the no-noise and noise conditions for each ear. Positive suppression values indicate a reduction in OAE amplitude due to the contralateral noise (reflecting MOC activation).
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 17.0 for Windows (IBM SPSS Statistics, Armonk, NY, United States). Descriptive statistics (mean, standard deviation, count, percentage) were used to summarize the data. The χ2 test (or Fisher’s exact test, when appropriate) was used to compare categorical variables (e.g., sex distribution between groups). Normality of continuous data was evaluated with both visual inspection (histograms, Q-Q plots) and analytical tests (Kolmogorov-Smirnov and Shapiro-Wilk). For comparisons of continuous variables between two groups, the independent samples t-test was used (for normally distributed data). One-way analysis of variance (ANOVA) was employed for comparisons across more than two groups; when the ANOVA indicated a significant difference, Bonferroni post hoc tests were conducted to determine which pairwise differences were significant. A significance level of α = 0.05 was adopted for all statistical tests.
A total of 100 individuals participated, including 50 patients with MS (34 females, 16 males) and 50 healthy controls (34 females, 16 males). The MS and control groups were matched in age (mean age 34.8 ± 9.7 years for MS vs 33.5 ± 9.2 years for controls) and had a similar sex distribution. The average disease duration in the MS cohort was 6.62 ± 5.66 years. Based on MRI findings, the patients with MS were divided into three subgroups as described: Group 1 (brainstem lesions, n = 20), Group 2 (temporal cortex lesions without brainstem involvement, n = 20), and Group 3 (no brainstem or temporal lesions, n = 10).
All subjects had normal middle-ear function on tympanometry, and acoustic reflexes were present at expected levels, confirming no significant middle ear pathology. PTA results showed no significant differences between patients with MS and controls; hearing thresholds were within normal limits for both groups, and mean PTA values did not differ statistically (P > 0.05). Similarly, the overall TEOAE response amplitudes did not differ significantly between the MS group and the control group, indicating that outer hair cell function was generally intact in the patients with MS (P > 0.05). These findings suggest that any auditory differences observed are likely due to central (retrocochlear) factors rather than peripheral cochlear damage.
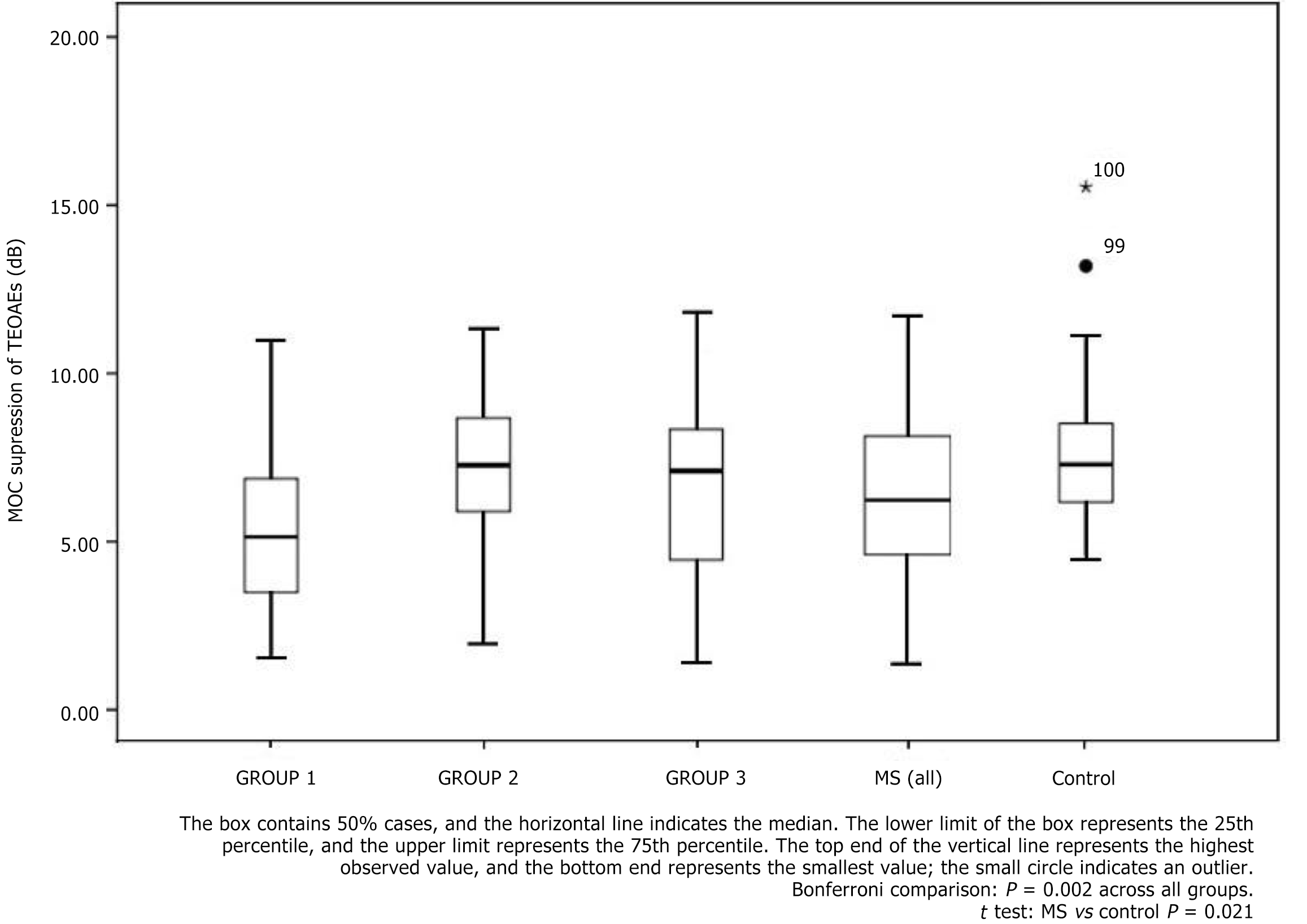
The primary outcome of interest was the contralateral suppression of TEOAEs (MOC reflex strength). Analysis of suppression magnitudes revealed significantly lower MOC-mediated suppression in the patients with MS compared to controls (mean suppression for MS vs control: 6.43 dB vs 7.67 dB; P = 0.021). In other words, on average the presence of contralateral noise reduced TEOAE amplitudes less in patients with MS than in healthy listeners, reflecting an attenuated efferent reflex in the MS group.
When examining the MS subgroups, the effect of lesion location became evident. Group 1 (MS with brainstem lesions) had the smallest mean suppression values among all groups. Group 1’s mean suppression was significantly lower than that of Group 2 (MS with only temporal lesions) and also significantly lower than that of the control group (post hoc Bonferroni comparisons, P < 0.01). By contrast, Group 2 and Group 3 did not show significant suppression differences relative to the control group (P > 0.05 for both comparisons). There was also no significant difference between Group 2 and Group 3 suppression values (P > 0.05). Thus, the only subgroup showing a marked efferent dysfunction was the one with brainstem involvement, highlighting the importance of brainstem lesions in MOC reflex impairment.
Table 1 summarizes the MOC suppression results (expressed as mean ± SD of TEOAE suppression in dB) across groups, further broken down by age subgroup and sex. Figure 2 provides a visual comparison of the suppression magnitudes for each group. As shown, the patients with MS, particularly those in Group 1, tended to have lower suppression values than controls.
| Group 1 | Group 2 | Group 3 | MS (all) | Control | |
| Age, years | |||||
| 18-35 | n = 10 | n = 9 | n = 4 | n = 23 | n = 37 |
| 4.61 ± 2.91 | 6.82 ± 2.64 | 6.89 ± 3.76 | 5.87 ± 3.03 | 7.41 ± 1.96 | |
| 36-65 | n = 10 | n = 11 | n = 6 | n = 27 | n = 13 |
| 5.52 ± 1.71 | 7.66 ± 1.71 | 7.43 ± 3.94 | 6.82 ± 2.48 | 7.98 ± 2.88 | |
| Sex | |||||
| Female | n = 13 | n = 14 | n = 8 | n = 35 | n = 34 |
| 5.83 ± 2.43 | 7.88 ± 1.86 | 7.19 ± 4.11 | 6.96 ± 2.78 | 7.45 ± 2.37 | |
| Male | n = 7 | n = 6 | n = 2 | n = 15 | n = 16 |
| 3.65 ± 1.54 | 5.89 ± 2.33 | 7.32 ± 1.49 | 5.04 ± 2.27 | 7.78 ± 1.92 | |
In addition to group differences, we observed an interesting sex effect on MOC suppression within the MS cohort. Sex-based analysis revealed that male patients with MS had significantly lower mean suppression values than female patients with MS. This trend was evident both in Group 1 (brainstem lesion subgroup) and when considering all patients with MS together. For example, in Group 1, males showed a mean suppression of approximately 3.65 dB vs approximately 5.83 dB in females (as noted in Table 1), a statistically significant difference. Similarly, across the entire MS sample, the average suppression in males was approximately 5.04 dB compared to approximately 6.96 dB in females. No such sex difference was observed in the control group (where males and females had comparable suppression values). It should be noted that the number of male patients in our study was relatively small (n = 15), so this finding, while significant, should be interpreted with caution.
In summary, the results indicate that: (1) Baseline cochlear function (TEOAE presence and amplitude) was normal in patients with MS; (2) MOC reflex suppression was attenuated in patients with MS relative to healthy controls, with the effect most pronounced in patients with brainstem lesions; (3) Patients with MS with only temporal lobe lesions did not show significant efferent deficits, suggesting that cortical lesions alone may not markedly affect the MOC reflex; and (4) Within the MS group, male patients exhibited lower efferent suppression than females, a finding that warrants further investigation.
The efferent auditory system has garnered increasing attention since its initial characterization. Comprising the olivocochlear bundle—specifically, MOC neurons originating in the superior olivary complex—and their connections to the cochlea, this system exerts influence over outer hair cell activity, thereby modulating cochlear responses to sound[8]. Through these effects, the efferent system contributes to sound discrimination in challenging listening environments and helps protect the inner ear from acoustic injury[9]. Medial olivocochlear fibers are heavily myelinated, which enables fast signaling; however, this dependence on myelin also makes them susceptible to demyelinating conditions such as MS[10].
In the present study, we investigated the function of the efferent auditory system (via the MOC reflex) in patients with MS and found clear evidence of efferent dysfunction, especially in those with brainstem lesions. Before discussing the implications of these findings, it is important to note that basic audiometric function was largely preserved in our MS cohort. PTA comparisons showed that patients with MS had, on average, slightly higher hearing thresholds than controls, but still within normal limits. This suggests that significant cochlear (afferent) hearing loss is uncommon in MS, consistent with the literature that hearing loss in MS is relatively rare and often transient[11]. When hearing loss does occur, it is typically due to acute demyelinating lesions in regions such as the pontomedullary junction or the cochlear nucleus in the brainstem[12]. Lesions above the cochlear nucleus (in the lateral lemniscus, midbrain, or cortex) are less likely to cause peripheral hearing loss because the bilateral redundancy of ascending pathways can compensate for unilateral damage[13]. Indeed, our finding of normal TEOAEs and no persistent hearing loss in the MS group aligns with previous reports that cochlear outer hair cell function remains intact in MS.
Despite normal cochlear function, our patients with MS—particularly those with brainstem involvement—demonstrated significantly reduced contralateral suppression of TEOAEs. In other words, the MOC reflex, which normally attenuates cochlear responses when noise is presented to the opposite ear, was blunted. This result is in agreement with earlier work by Coelho and colleagues, who found that a majority of patients with MS had abnormal MOC suppression, especially those with brainstem plaques[1]. Notably, Coelho et al[1] reported that even patients with MS without MRI-visible brainstem lesions showed efferent deficits in about half of cases, suggesting that subclinical or MRI-invisible brainstem damage might affect the efferent pathway. Our study extends these findings by examining a distinct “temporal lobe lesion” group: We observed that patients with MS with lesions confined to the temporal cortex (and none in the brainstem) did not have a significant reduction in MOC suppression compared to controls. This indicates that temporal lobe involvement alone has minimal impact on the efferent reflex, at least in the context of MS. The temporal cortex is known to have a modulatory influence on auditory processing through corticofugal pathways[14], but it appears that such cortical influences are not sufficient to produce a measurable deficit in the peripheral MOC reflex unless the brainstem efferent nuclei or pathways are directly compromised[15]. Our Group 2 vs control results support this: Despite potential cortical disruption, their MOC function was essentially normal, underscoring that the integrity of the brainstem SOC and associated pathways is the critical factor for MOC reflex strength.
All MS subgroups (even those without brainstem lesions) had, on average, slightly lower suppression magnitudes than controls (Table 1), though the differences for Group 2 and Group 3 were not statistically significant. This non-significant trend could hint at subtle efferent effects of MS outside the brainstem, or perhaps a diffuse slight reduction in efferent function in MS due to factors like inflammatory activity or medications. However, given the small sample sizes in these subgroups, firm conclusions cannot be drawn. The clearest pattern is the significant reduction in the brainstem lesion group, reinforcing a direct link between brainstem demyelination and efferent impairment. This finding is consistent with the mechanism of the MOC reflex: The reflex arc is primarily mediated by the brainstem (cochlear nucleus, trapezoid body, SOC, and the olivocochlear fibers)[16]. A demyelinating plaque interrupting this reflex arc at the brainstem level (e.g., in the SOC or its connections) would logically attenuate or abolish the reflex. Indeed, complete absence of contralateral suppression in MS has been documented in cases of SOC lesions[17].
One interesting observation in our data was the sex difference in MOC reflex strength among patients with MS (males < females). In our MS group, males showed roughly 2 dB less suppression on average than females. A similar sex effect has been noted in some studies of efferent function in other populations. For instance, Burguetti et al[18] found that in children with auditory processing disorders, boys had lower mean OAE suppression than girls. The literature on sex differences in the MOC reflex is mixed: While some have reported that females have stronger OAE suppression (possibly related to higher baseline OAE amplitudes in females), other studies, such as Stuart and Kerls[19] and Jedrzejczak et al[20], found no significant sex differences in contralateral suppression. In healthy adults, the consensus is not clear-cut. In the context of MS, our results raise the question of whether male patients might be more susceptible to efferent pathway damage or if this finding was influenced by the relatively small number of male subjects. With only 15 male patients with MS in our sample, caution is warranted. This observation could be a statistical anomaly or related to other factors (e.g., differences in disease duration or lesion load between sexes in our sample). Further research with larger MS cohorts is needed to determine if males with MS consistently show greater efferent dysfunction than females.
Our findings carry implications for understanding auditory symptoms in MS. The MOC reflex is thought to contribute to enhanced frequency selectivity and improved hearing in noise by suppressing masking noise and sharpening cochlear tuning[21]. A reduction or loss of MOC function, as observed in our patients with MS (especially those with brainstem lesions), may therefore translate into real-world listening challenges. Patients with MS with efferent dysfunction might experience difficulty understanding speech in noisy environments, reduced ability to focus on specific sounds in background noise, and overall degraded auditory processing. Indeed, some patients with MS report central auditory processing deficits, such as trouble with speech discrimination or auditory attention, even when their basic hearing thresholds are normal[1,10]. Our results support a physiological basis for such complaints: Without normal efferent suppression, the auditory system may struggle to suppress irrelevant noise and modulate dynamic range, leading to auditory confusion or fatigue in complex listening situations. Furthermore, an overactive cochlear amplifier (due to lack of efferent dampening) can increase internal noise and amplify sounds excessively, potentially contributing to symptoms like hyperacusis (sound sensitivity), aural fullness, or tinnitus[22]. These audiovestibular symptoms have been reported as part of the MS symptom spectrum, and our study suggests efferent dysfunction could be one underlying factor[23].
It is also worth discussing the potential clinical applications of our findings. The significant difference in MOC suppression between patients with MS (particularly those with brainstem lesions) and controls indicates that the contralateral suppression test of TEOAEs might serve as a useful adjunct diagnostic tool. Coelho et al[1] proposed that an abnormal MOC test could help identify brainstem involvement in MS, sometimes even when MRI does not detect a lesion. In our study, none of the control subjects showed absent suppression, whereas a subset of patients with MS did (especially in Group 1). This suggests that if a patient with MS has a markedly reduced or absent OAE suppression, it should prompt careful evaluation for brainstem lesions. However, given that some patients with MS without MRI-evident brainstem lesions can also have reduced suppression (as Coelho’s work showed), the OAE suppression test might detect functional effects of demyelination that imaging misses. Thus, OAE suppression could potentially be used in conjunction with auditory brainstem response (ABR) and MRI as part of an audiological test battery to assess brainstem function in MS.
Several limitations of this study should be acknowledged. First, the sample size—especially when broken into subgroups—was modest, which may limit the generalizability of the sex effect and could mean we lacked power to detect small differences in Groups 2 and 3. Future studies with larger cohorts are needed to confirm subgroup differences and investigate other factors (such as disease subtype or treatment status) on efferent function. Second, our study was cross-sectional and focused on a relatively stable MS population; we did not examine changes over time or during acute relapses. The dynamic fluctuations in MOC function suggested by case reports (i.e. temporary loss of suppression during an MS relapse that recovers after treatment) were not captured in our one-time measurements. Longitudinal studies could explore how MOC reflex measures change with disease activity or treatment (for instance, whether effective MS therapies can improve efferent function or prevent its decline). Third, while we ensured that all participants had present TEOAEs at baseline, our choice of a 3 dB SNR criterion for OAE presence, as opposed to the more stringent 6 dB used in some protocols, could influence the interpretation of “borderline” emissions. We justified this choice on the grounds of sensitivity, and required multiple frequencies to pass at 3 dB SNR to maintain reliability. Nonetheless, using a 3 dB cutoff means some low-level emissions were counted as present; if we had used 6 dB, a few individuals might have been classified differently or had fewer measurable suppression values. This could slightly affect the magnitude of measured suppression (for example, very small suppressions might fall within the noise floor). Encouragingly, our main findings were robust and in line with expectations (significant differences where anticipated), suggesting that the 3 dB criterion did not introduce a systematic bias between groups. Future work could compare results using different OAE pass criteria to confirm that the observed effects are consistent. Finally, we did not include neurophysiological tests such as the ABR in our protocol. ABR is a well-established method to detect auditory pathway demyelination in patients with MS and has a high sensitivity for identifying brainstem involvement[24]. The absence of ABR data in our study means we cannot directly correlate efferent dysfunction with afferent conduction delays or abnormalities. Including ABR (and perhaps other central auditory tests) in future studies would provide a more comprehensive picture of auditory system invo
Despite these limitations, our study clearly demonstrates involvement of the efferent auditory system in MS. The results underscore that MS-related demyelination can impair the medial olivocochlear reflex, particularly when lesions affect the brainstem auditory centers. This efferent dysfunction might be an underrecognized contributor to the auditory complaints reported by some patients with MS, such as difficulty in noisy environments or unusual sound sensitivity. It also highlights a potential role for audiological tests in MS: While MRI remains the gold standard for diagnosing and monitoring MS, functional auditory tests like OAE suppression and ABR could serve as noninvasive probes of brainstem integrity and help in the early detection of brainstem involvement.
In conclusion, this study provides evidence that the efferent auditory system (specifically the medial olivocochlear reflex) is affected in multiple sclerosis. Patients with MS, especially those with brainstem lesions on MRI, exhibit reduced contralateral suppression of otoacoustic emissions, indicating MOC reflex dysfunction. Temporal lobe lesions alone did not significantly alter MOC activity, suggesting that direct brainstem involvement is the key factor in efferent auditory impairment. These findings support the notion that demyelination in MS can disrupt not only afferent auditory pathways but also efferent feedback mechanisms. Efferent dysfunction in MS may contribute to patients’ difficulties in complex listening situations and other auditory symptoms. From a clinical perspective, testing of the MOC reflex (e.g., measuring TEOAE suppression with contralateral noise) may have diagnostic value as an objective indicator of brainstem involvement in MS, potentially detecting functional changes that are not obvious on imaging. Further research is warranted to explore the utility of efferent auditory assessments in MS, to investigate how MOC reflex measures fluctuate with disease activity and treatment, and to better understand the relationship between efferent function and auditory performance in this population. Ultimately, a deeper insight into efferent auditory system involvement in MS could improve our understanding of the disease’s impact on sensory function and inform more comprehensive management of hearing and communication challenges in patients with MS.
| 1. | Coelho A, Ceranić B, Prasher D, Miller DH, Luxon LM. Auditory efferent function is affected in multiple sclerosis. Ear Hear. 2007;28:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Correale J, Gaitán MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017;140:527-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 3. | Silva JDD, Leal Gouveia MC, Hora LCDD, Venancio LGÂ, Muniz LF. Effect of suppression of otoacoustic emissions in individuals with and without central auditory processing disorder: a systematic review. Braz J Otorhinolaryngol. 2025;91:101485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Munoz F, Vicencio-Jimenez S, Jorratt P, Delano PH, Terreros G. Corticofugal and Brainstem Functions Associated With Medial Olivocochlear Cholinergic Transmission. Front Neurosci. 2022;16:866161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cañete OM, El-Haj-Ali M, Fereczkowski M. Comparison of Two Clinical Devices for the Measurement of Distortion Product Otoacoustic Emissions in Normal-Hearing Adults. J Audiol Otol. 2024;28:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Young A, Ng M. Otoacoustic Emissions. 2023 Apr 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 7. | Zevenster S, Naudé A. Contralateral suppression of transient evoked otoacoustic emissions in adults: A normative study. S Afr J Commun Disord. 2022;69:e1-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Guinan JJ Jr. Olivocochlear efferents: Their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hear Res. 2018;362:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Hiel H, Vincent PFY, Wood MB, Elgoyhen AB, Chien W, Lauer A, Fuchs PA. Engineering olivocochlear inhibition to reduce acoustic trauma. Mol Ther Methods Clin Dev. 2023;29:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Küçüköner A, Küçüköner Ö, Özgür A, Terzi M. Effect of Medial Olivocochlear Efferents on Speech Discrimination in Noise in Multiple Sclerosis. Noise Health. 2024;26:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Fernández-Menéndez S, Redondo-Robles L, García-Santiago R, García-González MÁ, Arés-Luque A. Isolated deafness in multiple sclerosis patients. Am J Otolaryngol. 2014;35:810-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Cruz RA, Varkey T, Flavia A, Samways APA, Garza A, Greenlee G, Friess M, Sconzert J, Aijaz A, Arruda W, Khouri J, Ellington K, Frohman TC, Frohman EM. Hearing abnormalities in multiple sclerosis: clinical semiology and pathophysiologic mechanisms. J Neurol. 2022;269:2792-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Eker A, Kaymakamzade B, Kurne AT, Temuçin ÇM, Karabudak R. Sudden Sensoryneural Hearing Loss As a Rare Attack Type in Multiple Sclerosis. Noro Psikiyatr Ars. 2018;55:380-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Di Guilmi MN, Boero LE, Castagna VC, Rodríguez-Contreras A, Wedemeyer C, Gómez-Casati ME, Elgoyhen AB. Strengthening of the Efferent Olivocochlear System Leads to Synaptic Dysfunction and Tonotopy Disruption of a Central Auditory Nucleus. J Neurosci. 2019;39:7037-7048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Ishizaka Y, Otsuka S, Nakagawa S. Relationships between the expectations based on the regularity of preceding sound sequences and the medial olivocochlear reflex. PLoS One. 2024;19:e0304027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Torres Cadenas L, Fischl MJ, Weisz CJC. Synaptic Inhibition of Medial Olivocochlear Efferent Neurons by Neurons of the Medial Nucleus of the Trapezoid Body. J Neurosci. 2020;40:509-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Srinivasan VS, Krishna R, Munirathinam BR. The Interaural Time Difference for High-Pass Filtered Noise and Its Relationship With Brainstem Dysfunction and Disability in Multiple Sclerosis. Am J Audiol. 2023;32:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Burguetti FAR, Carvallo RMM. Efferent auditory system: its effect on auditory processing. Braz J Otorhinolaryngol. 2008;74:737-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Stuart A, Kerls AN. Does Contralateral Inhibition of Transient Evoked Otoacoustic Emissions Suggest Sex or Ear Laterality Effects? Am J Audiol. 2018;27:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Jedrzejczak WW, Pilka E, Skarzynski PH, Skarzynski H. Contralateral suppression of otoacoustic emissions in pre-school children. Int J Pediatr Otorhinolaryngol. 2020;132:109915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Mertes IB. Associations between the medial olivocochlear reflex, middle-ear muscle reflex, and sentence-in-noise recognition using steady and pulsed noise elicitors. Hear Res. 2024;453:109108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Knudson IM, Shera CA, Melcher JR. Increased contralateral suppression of otoacoustic emissions indicates a hyperresponsive medial olivocochlear system in humans with tinnitus and hyperacusis. J Neurophysiol. 2014;112:3197-3208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Peyvandi A, Naghibzadeh B, Ahmady Roozbahany N. Neuro-otologic manifestations of multiple sclerosis. Arch Iran Med. 2010;13:188-192. [PubMed] |
| 24. | Rishiq D, Harkrider A, Springer C, Hedrick M. Click-evoked and speech-evoked auditory brainstem responses from individuals with multiple sclerosis. Neurosci Lett. 2021;740:135460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |