Published online Oct 6, 2025. doi: 10.12998/wjcc.v13.i28.107759
Revised: April 22, 2025
Accepted: July 4, 2025
Published online: October 6, 2025
Processing time: 132 Days and 1.3 Hours
Acute hemorrhagic leukoencephalitis (AHLE), also known as Weston-Hurst syndrome, is a very rare and fulminant form of demyelinating disorder. It is considered a hyperacute and severe variant of acute disseminated encephalomyelitis. Clinically, patients present with fever, headache, seizures, and altered sensorium, which can rapidly progress to coma or death. Magnetic resonance imaging (MRI) is the investigation of choice and plays a pivotal role in diagnosing AHLE. The purpose of this article is to make readers familiar with the typical MRI features of AHLE and to discuss differentials.
This case series reports the clinical presentation and typical neuroimaging findings in four patients diagnosed with AHLE. All patients presented with acute neurological symptoms, such as severe headaches, seizures, and altered con
AHLE is associated with high morbidity and mortality rates, emphasizing the need for early recognition, prompt intervention, and multidisciplinary management. Further research is needed to explain the pathophysiological mechanisms underlying AHLE, identify potential biomarkers for early diagnosis, and develop targeted therapies to improve patient outcomes.
Core Tip: Acute hemorrhagic leukoencephalitis (AHLE) is a rapidly progressive demyelinating disorder often triggered by a viral infection. It involves widespread inflammation and bleeding in the brain, leading to neurological deterioration. Early recognition of symptoms such as confusion, seizures, or motor deficits is essential. Imaging studies, particularly magnetic resonance imaging (MRI), plays a vital role in diagnosis and monitoring treatment response. Timely administration of corticosteroids, immunosuppressive therapy, and supportive care can help manage the inflammation, reduce brain damage, and potentially improve prognosis.
- Citation: Shukla A, Nayyar N, Kumari P, Kumar A, Takkar P. Magnetic resonance imaging spectrum of acute hemorrhagic leukoencephalitis: Four case reports. World J Clin Cases 2025; 13(28): 107759
- URL: https://www.wjgnet.com/2307-8960/full/v13/i28/107759.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i28.107759
Acute hemorrhagic leukoencephalitis (AHLE), also known as Weston-Hurst syndrome, is a very rare and fulminant form of demyelinating disease. Edward Weston Hurst, a British neurologist, first described the disease in 1941[1]. AHLE is considered the variant and most severe form of acute disseminated encephalomyelitis (ADEM), with a rapidly progressive lethal course. It occurs in approximately 2% of ADEM patients, with characteristic multifocal demyelinating lesions within the white matter of the central nervous system (CNS)[2]. AHLE inflammatory lesions, unlike those in ADEM, are associated with hemorrhagic necrosis and extensive white matter edematous changes. The neurological manifestations of AHLE are more severe, with higher mortality and morbidity than other demyelinating disorders[3]. AHLE mainly affects children and young adults with no gender predilection. The exact etiological factor for AHLE is unknown; however like ADEM, it occurs after a viral prodrome or vaccination against viral diseases[3,4]. It is considered an acute autoimmune-mediated condition in which the probability of cross-reactivity between myelin basic protein moieties and various infectious antigens is proposed. In pathology, AHLE is characterized by demyelination with perivascular neutrophilic infiltrate and small vessel necrotizing vasculitis, whereas, on the other hand, in ADEM, perivascular infiltrate consists of a predominance of lymphocytes with no evidence of vascular necrosis or hemorrhage[5]. Clinically, the patient presents with history of fever, headache, lethargy, vomiting, seizure, and altered sensorium, which can lead to death. Laboratory investigations reveal polymorphonuclear peripheral blood leukocytosis and cerebrospinal fluid (CSF) pleocytosis, with predominant polymorphonuclear cells along with elevated protein[2,3]. Magnetic resonance imaging (MRI) is the investigation of choice and reveals multifocal, scattered as well as confluent, mainly white matter lesions with internal micro- or macro-hemorrhagic areas and surrounding edema. In addition, involvement of brainstem structures, corpus callosum, cerebellum, spinal cord, and rarely the basal ganglia can be seen, but cortical gray matter is usually spared[6,7]. Since AHLE is associated with a very poor prognosis and high mortality, prompt diagnosis is required for timely management. Treatment with glucocorticoids may help suppress the immune response, followed by plasmapheresis and intravenous immunoglobulins, depending on the patient's response to steroids[8-10].
Case 1: A 19-year-old female presented to the emergency department with chief complaints of fever, chills, rigors, and altered sensorium.
Case 2: A 40-year-old male presented with fever, severe headache, vomiting, generalized weakness, and subsequent altered sensorium.
Case 3: An 18-year-old female presented with fever, vomiting, cough and difficulty in breathing.
Case 4: A 45-year-old female patient presented to the emergency department with fever, severe headache, abnormal body movements, and altered mental state.
Case 1: The patient presented with history of fever for the last 2 days documented up to 102o F associated with chills, rigors, and altered sensorium in the form of decreased response to verbal commands. There was also history of loose stools and two episodes of abnormal body movements.
Case 2: The patient was referred case from a peripheral hospital to our institute with history of fever for 8 days documented up to 102o F associated with severe headache, vomiting, generalized weakness, and subsequent altered sensorium.
Case 3: The patient presented with history of high grade fever for last 2 days with episodes of vomiting, cough and difficulty in breathing.
Case 4: The patient presented to the emergency department with fever for last 3 days documented up to 103o F associated with severe headache, abnormal body movements, and altered mental state.
All patients were previously healthy with no relevant past medical history. There were no known comorbidities or chronic illnesses reported.
There was no reported personal or family history of neurological or autoimmune disorders in any of the cases.
Case 1: On examination, the patient was febrile (temperature 102° F) with stable vital signs. She was drowsy and exhibited altered sensorium, responding inconsistently to verbal stimuli. There were no signs of meningeal irritation. Neurological assessment revealed decreased level of consciousness without focal deficits. Deep tendon reflexes were preserved, and plantar responses were flexor bilaterally.
Case 2: At the time of admission, the patient was febrile but conscious with the Glasgow coma scale (GCS) 15/15, E4V5M6. However, on the second day of hospitalization, the patient’s condition deteriorated rapidly, marked by the onset of seizures and loss of consciousness, with GCS dropping to 3/15, E1V1M1. The patient was immediately intubated and shifted to the intensive care unit (ICU) for further management.
Case 3: On clinical examination, the patient was tachypneic and hypotensive with evidence of hepatosplenomegaly. Neurologically, she was drowsy with altered mental status and later developed generalized seizures. Deep tendon reflexes were brisk, and there were no signs of meningeal irritation. Cardiovascular and respiratory examinations revealed no additional focal abnormalities.
Case 4: At presentation, the patient was febrile with stable hemodynamic parameters. She was disoriented, with a GCS score of 8/15 (E3V2M3), and exhibited generalized tonic movements. Neurological examination revealed altered mental status and bilateral extensor plantar responses. There were no signs of meningeal irritation. Other systemic examinations were unremarkable.
Case 1: At admission, blood investigations revealed anemia (Hemoglobin-9.0 gm), leukocytosis, and raised erythrocyte sedimentation rate. CSF analysis revealed increased protein and neutrophil levels with normal glucose levels.
Case 2: Routine blood investigations revealed anemia and leukocytosis with increased neutrophils. CSF analysis revealed increased protein, increased neutrophils, and normal glucose levels.
Case 3: Laboratory investigations revealed neutrophilia, increased ferritin, and alkaline phosphatase levels with transaminitis. Viral markers were negative, but the patient was positive for scrub typhus IgM antibodies by enzyme-linked immunosorbent assay test.
Case 4: Blood investigation revealed mild elevation in C-reactive protein levels along with leucocytosis. CSF analysis revealed pleocytosis, increased levels of protein, and normal glucose levels. Detailed laboratory parameters for all cases are summarized in Table 1.
| Investigation | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Complete blood count | Hb-9.0 gm, Leukocytosis with neutrophilia | Hb-12 gm, Leukocytosis | Hb-13.2 gm, Leukocytosis with neutrophilia | Hb-12 gm, Leukocytosis with neutrophilia |
| C-reactive protein | Elevated (14.43 mg/L) | Elevated (19.67 mg/L) | Elevated (15.24 mg/L) | Elevated (13.73 mg/L) |
| Erythrocyte sedimentation rate | Elevated (47 mm/hour) | Elevated (56 mm/hour) | Elevated (52 mm/hour) | Elevated (45 mm/hour) |
| Liver function test | Raised AST, ALT. Normal ALP and bilirubin | Raised AST, ALT. Normal ALP and bilirubin | Raised AST, ALT, ALP and bilirubin (1.1 mg/dL) | Normal AST, ALT and bilirubin level |
| Renal function test | Normal | Normal | Normal | Normal |
| Viral markers | Negative | Negative | Negative | Negative |
| Cerebrospinal fluid analysis | Elevated protein levels, neutrophilic pleocytosis, elevated glucose, no organism detected, normal ADA levels (8.2 U/L) | Decreased glucose level, increased. Proteins and neutrophilic pleocytosis. Normal ADA levels (9.7 U/L). No organism detected on Gram stain | Elevated protein, normal glucose. Neutrophilic pleocytosis. Normal ADA level (7.6 U/L) | Elevated protein, normal glucose. Neutrophilic pleocytosis. Normal ADA level (5.6 U/L) |
| Autoimmune markers | Negative | NA | Negative | Negative |
| Blood culture | Negative | Negative | NA | Negative |
| MRI brain | Multiple flocculent predominant white matter hyperintensties on T2/FLAIR weighted images with internal micro and macrohemorrhages. Corpus callosum, brainstem, capsular region, hypothalamus also involved | Multiple hyperintense areas were seen on T2/FLAIR images involving subcortical/deep white matter of the bilateral cerebral hemisphere with internal hemorrhagic changes with involvement of hypothalamus, brainstem, cerebellum and scanned spinal cord | Multiple white matter hyperintensties on T2/FLAIR weighted images with involvement of corpus callosum, capsular region and brainstem. Internal hemorrhagic foci were detected in few lesions | Multifocal white matter hyperintensities both in subcortical/deep white matter on T2/FLAIR weighted images with internal hemorrhagic areas |
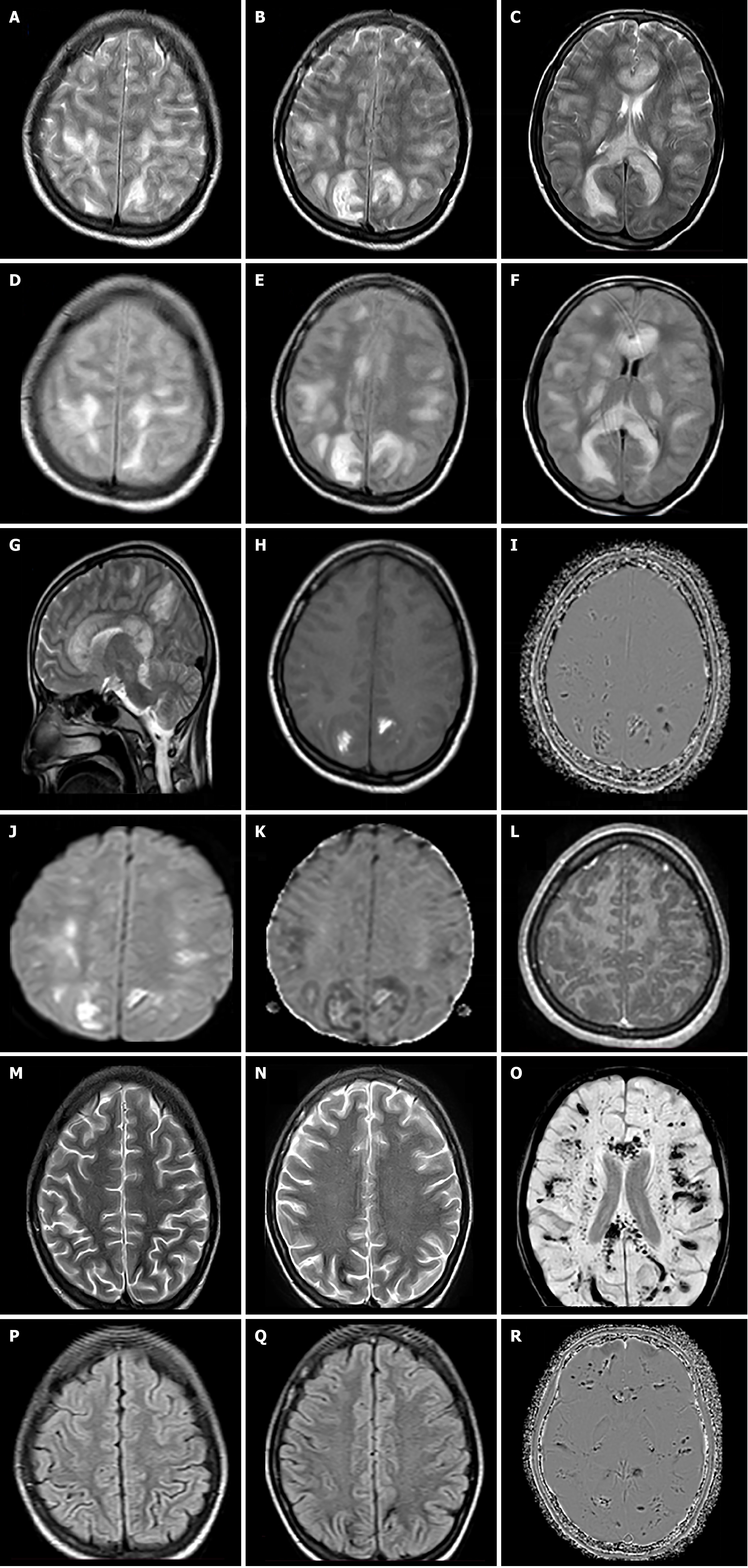
Case 1: Initial non-contrast computed tomography images suggested patchy white matter hypodensities with no evidence of hydrocephalus or intracranial bleed. The patient underwent MRI for further evaluation, which revealed multiple flocculent white matter hyperintensities on T2/Fluid attenuated inversion recovery (FLAIR)-weighted images involving the bilateral centrum semiovale region, subcortical/periventricular white matter, internal capsular region, and corpus callosum. There was also involvement of the bilateral ventrolateral thalami, hypothalamus, midbrain, and cerebellum (Figure 1A-G). T1-weighted images showed multiple hyperintense areas in these lesions and on susceptibility weighted imaging (SWI) micro/macro-hemorrhages were detected within most of these lesions (Figure 1H and I). Diffusion-weighted imaging (DWI) showed patchy areas of restriction; however, no obvious enhancement seen on T1-weighted post contrast images (Figure 1J-L).
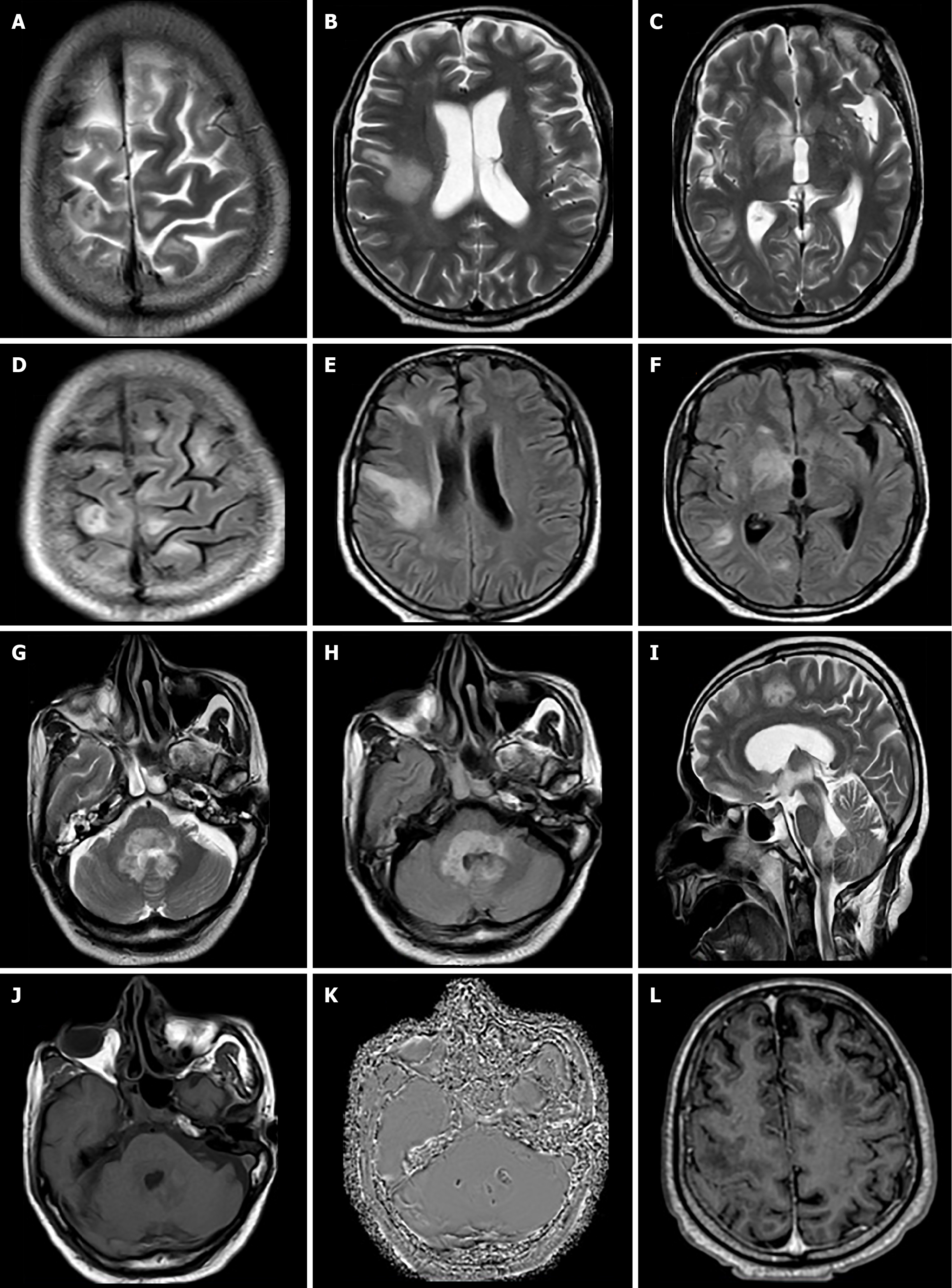
Case 2: On MRI, multiple white matter hyperintense areas were seen on T2/FLAIR images involving subcortical/deep white matter of the bilateral cerebral hemisphere with involvement of the right hypothalamus, right half of the midbrain, pons, medulla, bilateral middle cerebellar peduncles, cerebellum, and scanned part of the cervical spinal cord (Figure 2A-I). DWI sequence showed restriction within few of the lesions. On T1-weighted images, the involved areas were hypointense, with a few lesions showing internal hyperintense areas and negative phase on SWI suggestive of hemorrhages (Figure 2J and K). T1-weighted post contrast images showed no obvious enhancement (Figure 2L).
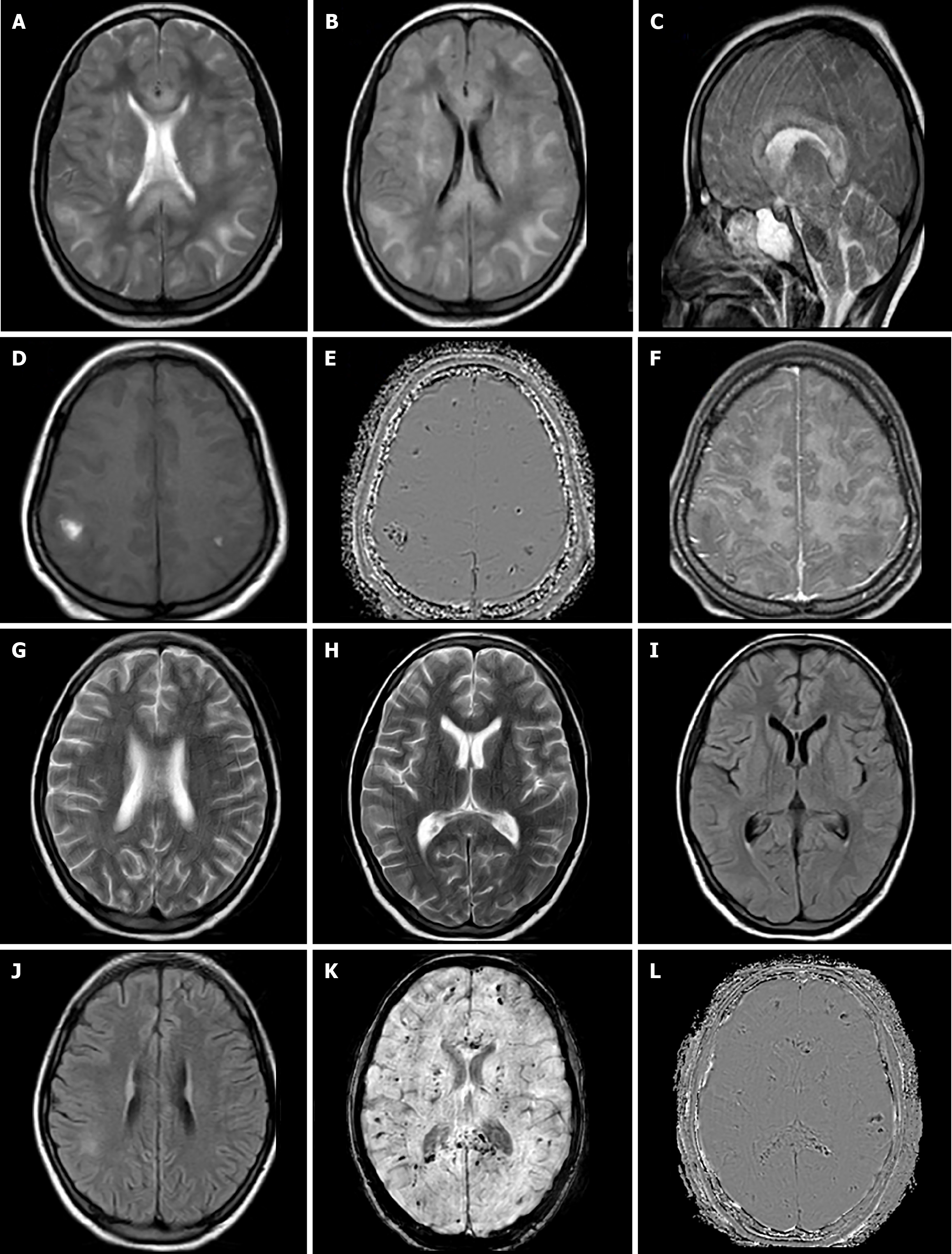
Case 3: MRI brain was done, which revealed white matter hyperintensities on T2/FLAIR-weighted images also involving the corpus callosum, internal/external capsular region, and brainstem structures (Figure 3A-C). On T1-weighted images, white matter lesions were hypointense with evidence of few hyperintensities in the lesions, which were showing negative phase effect on SWI images suggesting hemorrhages (Figure 3D and E). On post contrast images, these lesions were non-enhancing (Figure 3F).
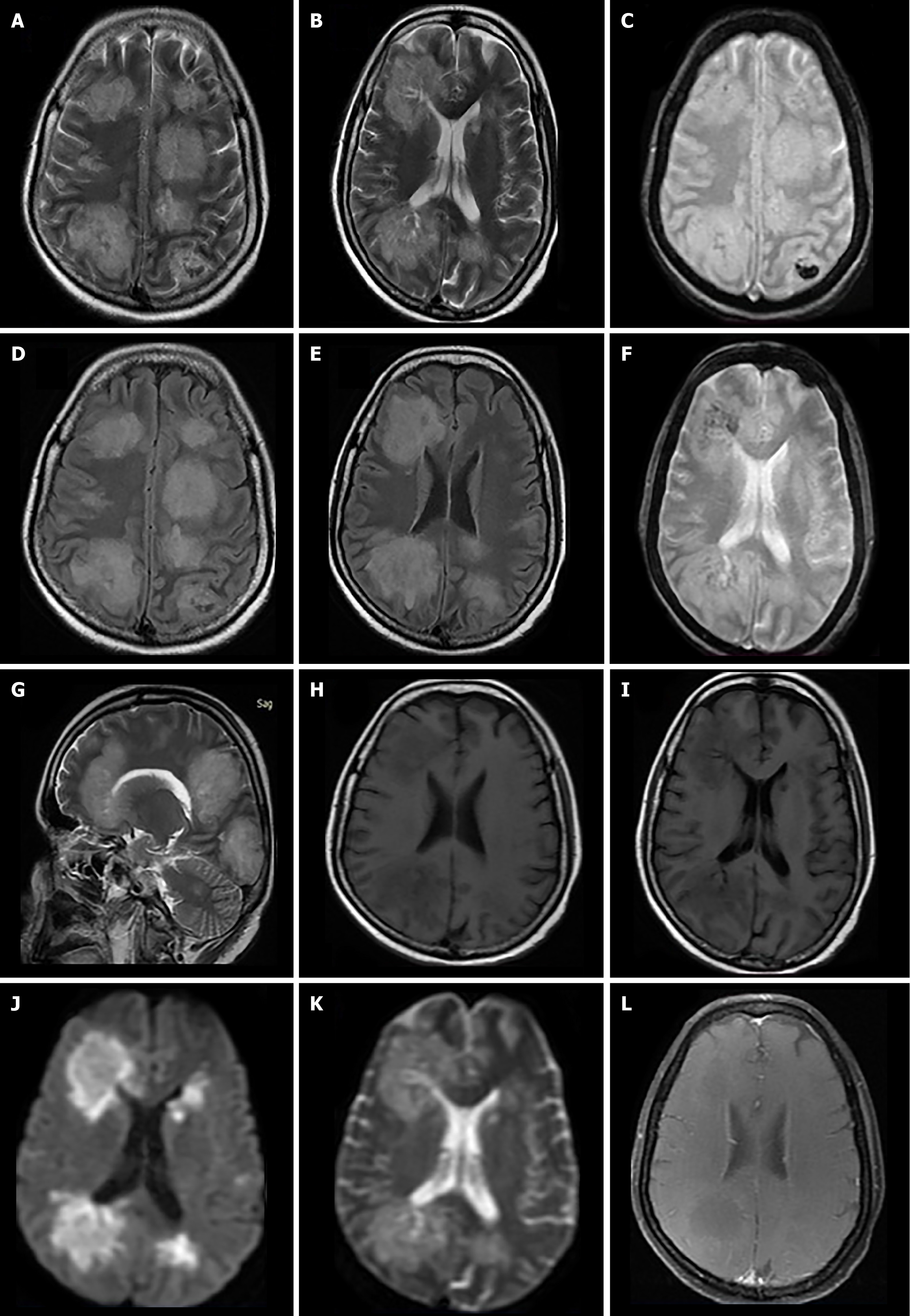
Case 4: A Non contrast computed tomography scan of brain was done, which suggested multiple hypodensities in the cerebral white matter bilaterally (Figure 4). MRI brain was done for further evaluation, which revealed multifocal white matter hyperintensities both in subcortical/deep white matter on T2/FLAIR-weighted images (Figure 4A, B, D and E). On T1-weighted images, involved areas were hypointense with subtle internal hyperintensities, which showed blooming on gradient sequence (GRE) suggesting haemorrhages (Figure 4C, F, H and I). DWI showed patchy restriction areas within the lesions (Figure 4J and K). On post contrast T1-weighted images, few of the lesions showed peripheral subtle enhancement (Figure 4L). Imaging findings for all the cases are summarized in Table 1.
The combination of clinical presentation and imaging findings suggested the diagnosis of AHLE in all the cases.
Case 1: The patient was admitted to the ICU and treated with a pulse dose of intravenous methylprednisolone (30 mg/kg/day) for five days, followed by a gradual tapering of the dose.
Case 2: He was admitted to the ICU and treated with high-dose intravenous dexamethasone as part of aggressive management; however, despite initial improvement, his condition deteriorated after a few days, and he ultimately succumbed to the illness.
Case 3: The patient was started on empirical treatment for scrub typhus, but during the course of management, she developed altered mental status and seizures accompanied by brisk deep tendon reflexes. Anticonvulsant therapy and steroids were initiated alongside supportive care.
Case 4: High-dose steroid (1 g/day) treatment started, but the patient’s condition deteriorated with the need for mechanical ventilation and intensive care. Despite aggressive treatment, she remained in a coma for 2 weeks.
Case 1: The patient demonstrated gradual clinical improvement and was discharged after one month of hospitalization. At the time of discharge, mild neurological sequelae were noted, including limb weakness with impaired coordination and cognitive slowing. At follow-up two months later, the patient exhibited subtle motor incoordination and residual weakness in lower limbs. Follow-up MRI, showed resolution of the previously noted white matter hyperintensities; however, there was an increase in the number of hemorrhagic foci/residues (Figure 1M–R).
Case 2: Although the patient initially showed improvement with treatment and supportive care in the ICU, his condition deteriorated a few days later, and he ultimately succumbed to the illness.
Case 3: The patient showed gradual improvement in general condition, however, mild neurological deficits with slowed response persisted. Follow-up MRI was done after 1 month, which showed resolution of white matter lesions; however, there was an increase in the number of hemorrhagic foci/residues (Figure 3G-L), reflecting evolving micro-hemorrhagic changes.
Case 4: Despite aggressive treatment, she remained in coma for 2 weeks before succumbing to the illness. This reflects the severe and rapidly progressive nature of the disease.
AHLE is a severe neurological disorder characterized by hemorrhagic demyelinating brain parenchyma lesions mainly involving white matter[2,3]. Diagnosing AHLE can be challenging because of its rarity and nonspecific clinical presentation[5-7]. Differential diagnosis includes other acute neurological conditions like ADEM, infectious encephalitis, hemorrhagic ischemic stroke, and primary CNS vasculitis (PCNSV). However, characteristic findings on neuroimaging, coupled with CSF analysis showing elevated protein levels and pleocytosis, can aid in distinguishing AHLE from other conditions. AHLE is considered a severe form of ADEM. Both conditions involve inflammation of the brain and spinal cord, but AHLE is distinguished by the presence of hemorrhagic lesions within the white matter on imaging studies. ADEM lesions typically lack hemorrhage and may show more diffuse involvement in the brain parenchyma[2,3]. Differential diagnosis should also include consideration of acute infectious etiologies such as viral encephalitis or bacterial meningoencephalitis, which can present with acute neurological conditions like AHLE. Differentiation is based on clinical history, epidemiological factors, and CSF analysis, including polymerase chain reaction testing for specific pathogens. Unlike AHLE, infectious encephalitis may show meningeal enhancement. Ischemic stroke can present with acute neurological deficits similar to AHLE. However, in ischemic stroke, imaging studies typically reveal focal infarctions in the territory of affected blood vessels, often with associated vascular occlusions on angiography. Unlike AHLE, hemorrhagic transformation in ischemic stroke occurs within the infarcted area rather than diffusely throughout the white matter. PCNSV is a rare inflammatory disorder characterized by the inflammation of blood vessels within the CNS. While both AHLE and PCNSV can present with acute neurological symptoms, PCNSV often shows a pattern of vascular involvement on imaging studies, such as vessel wall enhancement or stenosis. Biopsy of the affected vessels may reveal inflammatory changes in PCNSV, which are not typically seen in AHLE.
Imaging studies, particularly MRI, play a crucial role in the diagnosis of AHLE, demonstrating diffuse white matter abnormalities with areas of hemorrhage and edema[2,4,11]. Treatment strategies for AHLE primarily focus on sup
We described the clinical profiles and characteristic imaging findings of AHLE in four patients across different age groups. The reported cases reflect the wide clinical and radiological spectrum of AHLE and are consistent with earlier case reports and systematic reviews in terms of presentation, imaging features, and final outcomes. The clinical onset in all cases included fever, altered mental status, seizures, and focal neurological deficits, aligning with prior reports by Grzonka et al[3], Kuperan et al[6], and also seen in recent reports by Alsaid et al[12] and Kalafatakis et al[13], where rapid neurological deterioration occurred shortly after systemic symptoms.. The duration from symptom onset to neurological deterioration was short, reinforcing the hyperacute nature of the disease. CSF analysis in all patients revealed elevated protein and neutrophilic pleocytosis (Table 1), findings commonly observed in AHLE but nonspecific, as also noted by Tan et al[14] in their systematic review. Similar CSF findings were reported in the cases described by Alsaid et al[12] and Pujari et al[15], further supporting their diagnostic relevance despite lack of specificity.
Radiologically, three of our patients demonstrated classic imaging features typical for AHLE, with multifocal white matter hyperintensities on T2/FLAIR MRI sequences and hemorrhagic foci on SWI/GRE sequences (Table 1). These findings are consistent with previous studies done by Pinto et al[2]; Tenembaum et al[5] and Meilof et al[9], where radiological differentiation from ADEM was noted by the presence of hemorrhagic components. These radiological findings are also observed in studies done by Pujari et al[15], Alsaid et al[12] and Kalafatakis et al[13]. Case 2 showed involvement of the scanned cervical spine, a finding that, although rare, had been reported earlier by Pinto et al[2] and Grzonka et al[3] (Table 2).
| Ref. | Study type | Patient profile | Clinical features | Neuroimaging/pathology findings | Treatment | Final outcome |
| Hurst[1], 1941 | Case report (1st ever reported case) | Young male | Post infectious fever, seizures, rapid coma | Not available, hemorrhagic demyelination was seen on autopsy | Not described | Death |
| Meilof et al[9], 2001 | Case report | Adult male | Fever, headache, altered consciousness, and motor deficits | MRI findings-extensive white matter lesions with haemorrhages | High-dose IV methylprednisolone | Full recovery with early steroids |
| Tenembaum et al[5], 2002 | Prospective cohort study | 84 pediatric patients (age range: 5 months to 14 years) diagnosed with ADEM | ADEM cases; some with hemorrhagic features suggestive of AHLE | MRI findings: Widespread T2 hyperintense lesions in white matter; some with basal ganglia/thalami involvement | High-dose intravenous methylprednisolone followed by oral tapering. IVIG or plasmapheresis in some refractory cases | Good recovery in most patients. Poorer outcomes were seen in cases with brainstem or hemorrhagic involvement |
| An et al[7], 2002 | Retrospective case series and molecular pathology study | 6 Post-mortem confirmed AHLE adult patients | Fever, headache after viral prodrome with rapid and fulminant neurological decline leading to death in all patients | Diffuse white matter changes with haemorrhages detected on imaging. Detected HSV, CMV, EBV and other viruses in AHLE brain sample which suggested infection triggered immune pathology hypothesis | IV corticosteroids, Supportive ICU care | All patient died despite aggressive interventions |
| Kuperan et al[6], 2003 | Comparative case study | 1 patient with ADEM and 1 with AHLE | Clinical presentation was similar with rapid onset of symptoms in AHLE while subacute onset in ADEM | MRI findings- Extensive white matter lesions with central necrosis, peripheral edema, and hemorrhagic foci in AHLE patient while ADEM patient showed multifocal, non-hemorrhagic, symmetric lesions without necrosis | High dose intravenous corticosteroids were given in both patients | AHLE patient showed rapid neurological deterioration ultimately leading to coma and death. Favorable outcome seen in ADEM patient |
| Ryan et al[10], 2007 | Case report with review of literature | Young female | Headache, confusion and rapid progression to coma | Diffuse bilateral white matter hyperintensities with hemorrhagic foci on MRI | High-dose IV methylprednisolone with supportive care followed by therapeutic plasma exchange 5 times | Survived with good functional recovery |
| Pinto et al[2], 2011 | Case report | Adult male | Fever, headache, progressive altered consciousness | On MRI: Diffuse white matter hyperintensities on T2/FLAIR weightedimages with hemorrhage. Brainstem and spinal cord also involved. Diagnosis confirmed on pathology with findings of perivascular demyelination with fibrinoid and hemorrhagic necrosis of small vessels associated with neutrophilic infiltration | High-dose IV methylprednisolone | Death |
| Grzonka et al[3], 2020 | Case report and systematic review | Adult male | Rapid progression of neurological decline | T2-weighted FLAIR images showed increasing bilateral confluent widespread hyperintensities of the supratentorial white matter predominantly on the left side. SWI images demonstrated microbleeds in corpus callosum and pedunculus cerebelli | Intensive immunosuppressive therapy including intravenously administered immunoglobulins, high dose IV methylprednisolone for 3 days, cyclophosphamide with supportive care | Death |
A distinctive feature in Case 3 was scrub typhus positivity, suggesting a potential infectious autoimmune trigger, in line with theories positing post-infectious autoimmune mechanisms by An et al[7]. Similarly, more recently studies by Alqahtani et al[16], Alsaid et al[12] and Tan et al[14] in their review emphasized infectious triggers including influenza and severe acute respiratory syndrome coronavirus 2. This supports the growing view that AHLE may represent a hyperacute autoimmune response to various infectious antigens.
Despite early recognition and treatment with high-dose corticosteroids, two patients succumbed to the illness, highlighting the high mortality reported in earlier studies by Ryan et al[10], Grzonka et al[3], also highlighted in recent studies by Alsaid et al[12] and Kalafatakis et al[13]. On the other hand, two young female patients showed partial recovery, with follow-up MRI showing resolution of white matter lesions but persistence or increase of hemorrhagic residues, highlighting that even in survivors, sequelae may persist. The finding in these cases were more pronounced than in some studies like Duggal et al[17] and Pujari et al[15]. The high mortality rate and partial recovery with residual sequelae observed in our study are consistent with findings from previous reports. These mixed outcomes highlight the unpredictable course of AHLE and the critical role of early diagnosis and immunosuppressive treatment. This compa
However, our study has methodological limitations, including small sample size and a relatively short follow-up period, challenges that are commonly encountered in similar studies of this rare condition. Future studies should aim to include multicenter collaboration, standardized diagnostic criteria, and longer follow-up durations to better characterize disease progression, therapeutic response, and long-term outcomes.
In conclusion, AHLE is a rare but very harmful neurological condition characterized by widespread inflammation and hemorrhages within the white matter of brain parenchyma. Despite advances in diagnostic imaging and treatment modalities, AHLE remains associated with high morbidity and mortality rates, emphasizing the need for early recognition, prompt intervention, and multidisciplinary management involving neurologists, critical care specialists, and supportive care teams. Further research is needed to explain the pathophysiological mechanisms underlying AHLE, identify potential biomarkers for early diagnosis, and develop targeted therapies to improve patient outcomes.
| 1. | Hurst EW. Acute hemorrhagic leukoencephalitis: a previously undefined entity. Med J Aust. 1941;2:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Pinto PS, Taipa R, Moreira B, Correia C, Melo-Pires M. Acute hemorrhagic leukoencephalitis with severe brainstem and spinal cord involvement: MRI features with neuropathological confirmation. J Magn Reson Imaging. 2011;33:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Grzonka P, Scholz MC, De Marchis GM, Tisljar K, Rüegg S, Marsch S, Fladt J, Sutter R. Acute Hemorrhagic Leukoencephalitis: A Case and Systematic Review of the Literature. Front Neurol. 2020;11:899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Palace J. Acute disseminated encephalomyelitis and its place amongst other acute inflammatory demyelinating CNS disorders. J Neurol Sci. 2011;306:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Kuperan S, Ostrow P, Landi MK, Bakshi R. Acute hemorrhagic leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology. 2003;60:721-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | An SF, Groves M, Martinian L, Kuo LT, Scaravilli F. Detection of infectious agents in brain of patients with acute hemorrhagic leukoencephalitis. J Neurovirol. 2002;8:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Berzero G, Cortese A, Ravaglia S, Marchioni E. Diagnosis and therapy of acute disseminated encephalomyelitis and its variants. Expert Rev Neurother. 2016;16:83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Meilof JF, Hijdra A, Vermeulen M. Successful recovery after high-dose intravenous methylprednisolone in acute hemorrhagic leukoencephalitis. J Neurol. 2001;248:898-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ryan LJ, Bowman R, Zantek ND, Sherr G, Maxwell R, Clark HB, Mair DC. Use of therapeutic plasma exchange in the management of acute hemorrhagic leukoencephalitis: a case report and review of the literature. Transfusion. 2007;47:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Koelman DL, Chahin S, Mar SS, Venkatesan A, Hoganson GM, Yeshokumar AK, Barreras P, Majmudar B, Klein JP, Chitnis T, Benkeser DC, Carone M, Mateen FJ. Acute disseminated encephalomyelitis in 228 patients: A retrospective, multicenter US study. Neurology. 2016;86:2085-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Alsaid HM, Atawneh MAA, Abukhalaf S, Daoud A, Hamadah A, Gharaibeh K. Acute Hemorrhagic Leukoencephalitis - A Rare but Fatal Form of Acute Disseminated Encephalomyelitis - Complicated by Brain Herniation: A Case Report and Literature Review. Am J Case Rep. 2022;23:e935636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Kalafatakis K, Margoni A, Liakou ME, Stenos C, Toulas P, Korkolopoulou P, Lakiotaki E, Lafazanos SA, Zekiou K, Kardara P, Terentiou A, Nikolaou G, Stouraitis G. Acute hemorrhagic leukoencephalitis following the first dose of BNT162b2 vaccine against SARS-CoV-2: A case report. Heliyon. 2024;10:e25545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Tan Z, Lin SZZ, Foong WD, Yong MH. Acute hemorrhagic leukoencephalitis: a case report and systematic review of factors associated with severe disability and death. Neurol Sci. 2024;45:5859-5874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Pujari SS, Kulkarni RV, Ojha P, Gursahani R, Nadgir D, Patil S, Soni G, Bangar S, Harshe A, Mandolkar M, Joshi A, Kadam S, Goyal A. Acute haemorrhagic leukoencephalitis (AHLE) - our experience and a short review. J Neuroimmunol. 2021;361:577751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Alqahtani A, Alaklabi A, Kristjansson S, Alharthi H, Aldhilan S, Alam H. Acute necrotic hemorrhagic leukoencephalitis related to COVID-19: a report of 2 cases. Radiol Case Rep. 2021;16:2393-2398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Duggal N, Ahmed I, Duggal N. Acute hemorrhagic leukoencephalitis associated with autoimmune myopathy. J Vasc Interv Neurol. 2014;7:19-22. [PubMed] |