Published online Sep 26, 2025. doi: 10.12998/wjcc.v13.i27.108868
Revised: May 19, 2025
Accepted: June 18, 2025
Published online: September 26, 2025
Processing time: 103 Days and 0.1 Hours
Simultanagnosia is a neurological disorder that impairs an individual's ability to perceive more than one object at a time visually. While the individual may acknowledge the presence of multiple objects in his field of view, he cannot generally summarize the overall percept.
We describe a case of simultanagnosia in Posterior Cortical Atrophy, evidenced by the Ishihara color test. A 54-year-old woman complained of reading problems despite normal visual acuity and a structural eye exam. The patient failed to identify any of the Ishihara color plates in either eye despite adequate naming of colors. Automated visual field testing showed a homonymous hemianopia. Structural and functional neuroimaging and cerebrospinal fluid analysis were consistent with posterior cortical atrophy.
Simultanagnosia can be tested with the Ishihara pseudoisochromatic plates because the recognition of embedded number patterns in the test requires appreciation of a collection of individual stimuli.
Core Tip: The Isihara pseudoisochromatic plate is a commonly available non-invasive, subjective test for color vision. The diagnosis of simultanagnosia relies on the subjective responses of the likely sufferer to visual stimuli. Therefore, the Isihara test plates offer a novel way of diagnosing this condition. A knowledge of the response of simultanagnostic patients can potentially guide diagnosis when performing standard color vision testing using Isihara test plates.
- Citation: Pellegrini F, Cuna A, Musa M, D’Esposito F, Giglio R, Tognetto D, Gagliano C, Zeppieri M. Ishihara color plates utilized as an assessment for simultanagnosia in posterior cortical atrophy: A case report. World J Clin Cases 2025; 13(27): 108868
- URL: https://www.wjgnet.com/2307-8960/full/v13/i27/108868.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i27.108868
The Ishihara test plates is a color-based test to assess color vision status and deficiencies, mostly congenital red-green colorblindness. The subject’s ability to correctly identify numbers on the plates is linked to the ability to visually resolve circles of like hues into a numeral or lines that contrasts with the background, made of different colors. Thus, the test not only requires an intact hue discrimination and color vision but also requires the patient to be able to combine the dots into a distinct figure. In this study, we describe a patient referred for unexplained reading difficulty who failed the Ishihara color test. These results suggested simultanagnosia and the final diagnosis of Posterior Cortical Atrophy (PCA).
Ambiguous near vision complaints without evident ocular abnormalities may be the initial and most significant indicator necessitating further examination for underlying cortical pathology (e.g., Posterior Cortical Atrophy).
A 54-year-old woman was referred for unexplained reading problems for 2 years.
The patient was not taking any medications. She denied smoking and had modest alcohol consumption with meals.
The patient stated that she had no history of past illness.
Previous ophthalmic and neurologic consultations were unremarkable.
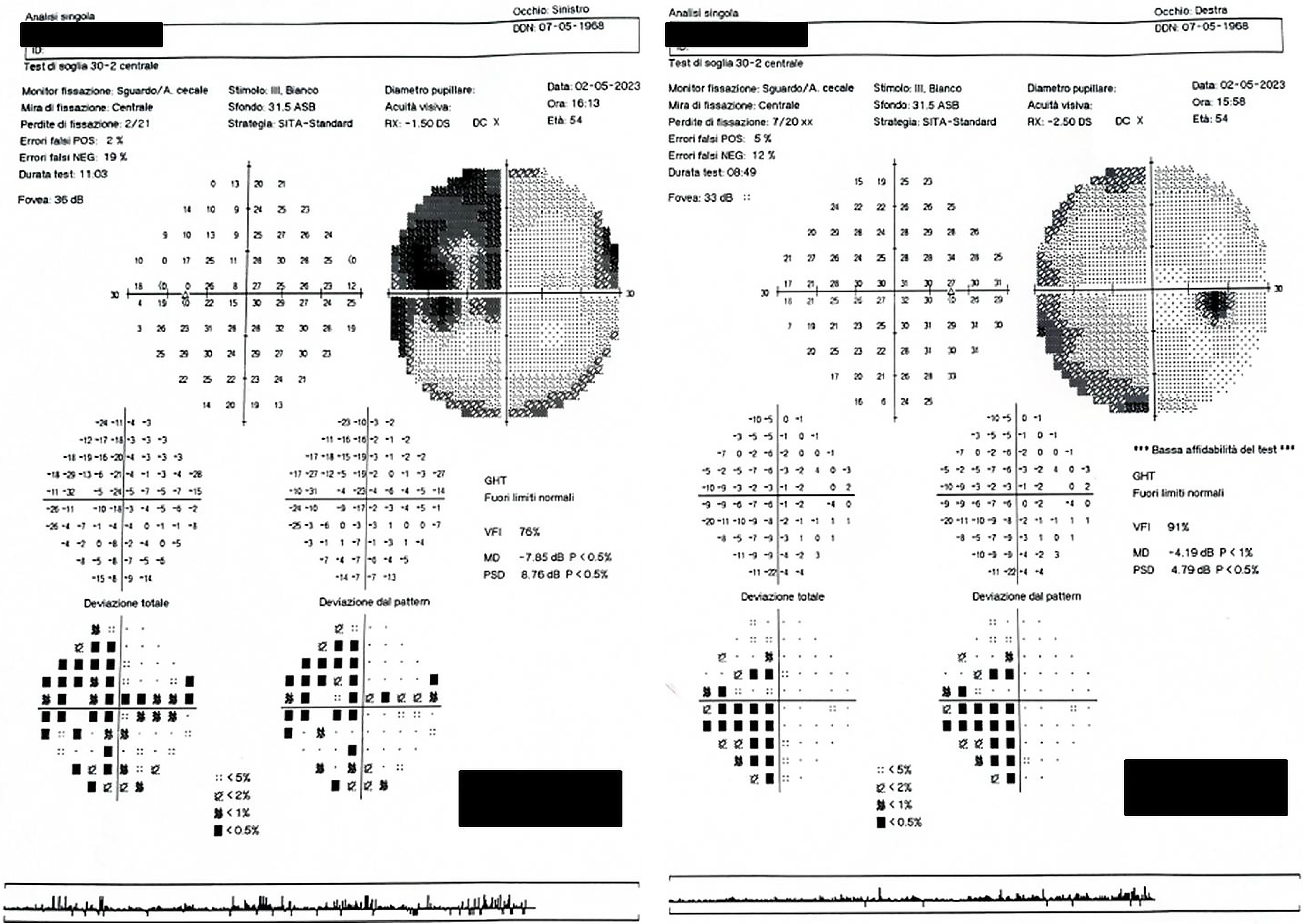
The patient’s visual acuity after refraction was 6/6 in both the right and left eye (OU). Extraocular mobility was smooth and full. The pupils were equal in size, reacted to light and showed no relative afferent pupillary defect. Dilation was performed and ocular health assessed using the slit lamp was normal OU. Reading was difficult with many pauses. She described “getting lost while reading a text”, particularly when moving from line to line. However, comprehension of the text was unaffected. Standard automated Humphrey 30-2 perimetry showed a left homonymous hemianopia denser inferiorly (Figure 1). When color vision was tested, the patient failed to correctly name any numbers on Ishihara color plates, even on the test plate.
Lumbar puncture was positive for increased total tau protein levels of 1549 pg/mL (normal titer: < 340 pg/mL), tau phosphorylated threonine 181 (p-Tau181) of 256.6 pg/mL (normal titer: < 55 pg/mL) with a reduced amyloid Aβ 42 level of 541 pg/mL (normal titer: 660-1075 pg/mL). A diagnosis of PCA was made and the patient was treated with memantine.
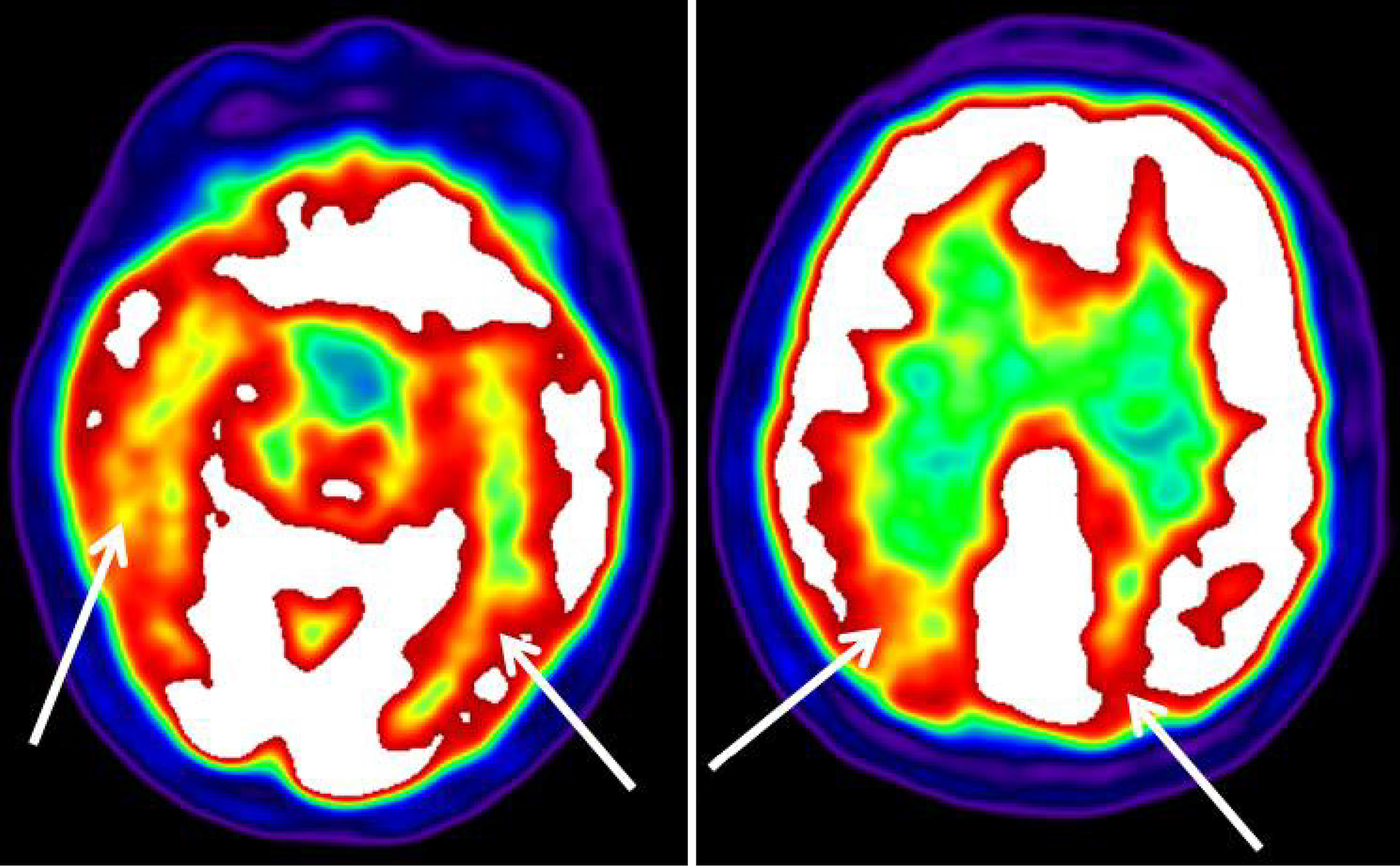
Standard automated 30-2 perimetry showed left homonymous hemianopia (Figure 1). However, the patient was able to correctly name individual circle colors, thus excluding color vision deficiency and anomia for colors. The simultanagnosia was also confirmed with the Boston cookie theft picture test. Although brain computerized tomography and magnetic resonance imaging (MRI) were unremarkable, positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) revealed a bilateral hypometabolism of the occipital, temporal, and posterior parietal cortex (Figure 2). This was more severe on the right.
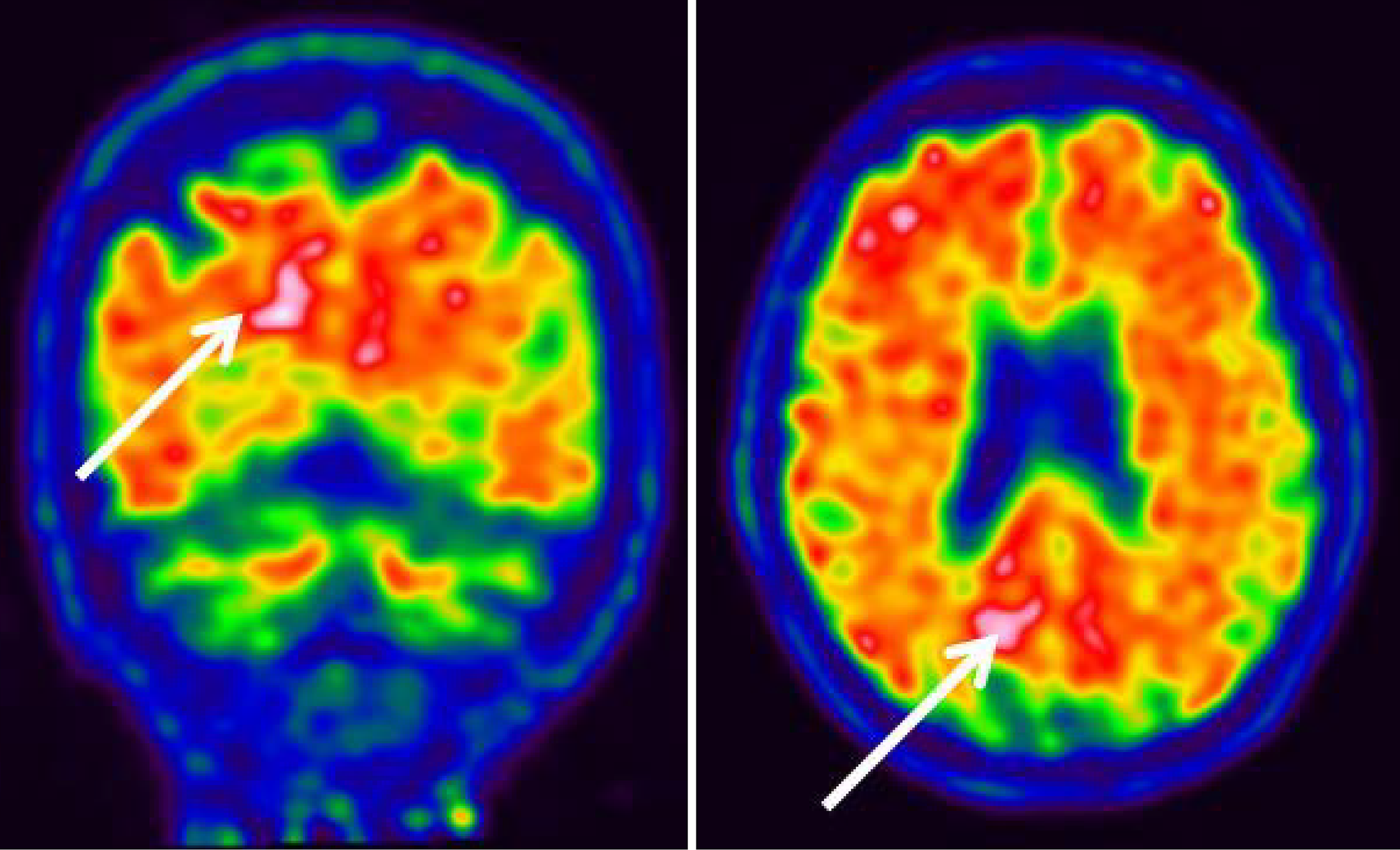
Analysis of amyloid PET using 18F-flutemetamol showed abnormal uptake in the posterior (occipital) cortex, which was more severe on the right (Figure 3).
Simultanagnosia.
The patient was scheduled for routine annual multidisciplinary follow-up appointments with neurology and ophthalmology to assess cognitive status, visual function, and the course of posterior cortical atrophy. During these visits, standardized evaluations—comprising neuropsychological examinations, perimetric analysis, and functional imaging—will be conducted to identify small alterations in visual and cognitive performance. The pharmacological treatment with memantine was sustained, accompanied by regular assessments of therapeutic effectiveness and tolerability. As of now, the patient's condition has remained stable, with no documented ill effects or notable decline in visual fields or everyday functioning.
In 1988, Benson et al[1] published a report on five patients who presented with significant oculovisual problems with no accompanying evident ophthalmic diagnoses. However, all five showed signs of Balint syndrome (e.g., optic ataxia, simultanagnosia, oculomotor apraxia) and Gerstmann syndrome (acalculia, agraphia, finger agnosia, right-left confusion). They also coined the term PCA because of the prominent posterior cerebral involvement discovered after MRI scans.
Brain tissue analyses later showed that Alzheimer’s disease (AD) was the most common underlying pathology. Following this, other diseases/syndromes such as visual variant of AD and Benson syndrome were suggested.
The presentation and characterization of PCA has been expanded to include progressive dementing conditions characterized by higher visual disorders[2]. PCA is characterized by a chronic reduction in visual processing skills and similar functions served by parietal, occipital, and occipito-temporal regions in various combinations. Although PCA has been recognized for decades, the condition remains difficult to diagnose. The vague nature of the complaint may lead to multiple pairs of ineffective reading spectacles, as was the case for our patient. She presented with typical complaints of PCA, i.e., a progressive, pain-free, reading problem associated with a left homonymous hemianopsia without a corresponding lesion on neuroimaging. While the application of Ishihara pseudoisochromatic color plates to reveal simultanagnosia has already been documented in the literature[1,2]. In this instance, the patient's ambiguous report of near vision impairment without discernible ocular pathology served as the initial clinical indicator necessitating a comprehensive diagnostic evaluation. This underscores the significance of evaluating CNS etiologies in similar clinical situations, such as retrochiasmal visual pathway disease. Consequently, the non-specific yet prevalent ophthalmologic issue in this case (near vision impairment) was instrumental for the final diagnosis.
PCA usually occurs in patients younger than those affected with typical AD. As PCA predominantly affects the parieto-occipital cortex, it is likely that the retrochiasmal visual pathway is involved, leading to a homonymous hemianopic visual field defect in up to 78% of cases[3,4]. Data suggest that the condition more strongly affects the right hemisphere and the parietal cortex more severely than the temporal one, thus possibly explaining the frequent occurrence of left homonymous hemianopic visual field defect, denser inferiorly[5]. Because the dorsal (occipito-parietal) system (the “where” pathway) is most frequently involved, PCA is a cause of complete or incomplete Balint syndrome, Gerstmann syndrome, dressing apraxia, and aphasia.
A complete presentation of Balint or Gerstmann syndrome is rare, yet the most common features are simultanagnosia and acalculia[6]. Simultagnosia refers to the failure to simultaneously perceive multiple visual locations[7]. It is the most frequent deficit in PCA, occurring in 9 out of 10 patients[8]. Tasks relying on visual integration, such as the Boston cookie-theft picture, are used to test for simultanagnosia. Moreover, failure to read the Ishihara pseudoisochromatic plates despite preserved color perception is a conspicuous feature in many patients with simultanagnosia[9,10]. An abnormal Ishihara test result may be the only consistent anomaly in routine visual assessments of PCA patients. It is usually misinterpreted as a color deficit, but it should be noted that the ability to interpret the first (control) plate is not dependent on color vision. Patients with simultanagnosia have problems not in seeing the individual-colored circles in each plate, but in combining them into a complex figure (i.e., the number “hidden” in the plate).
Other color vision impairments in PCA may involve compromised color discrimination, resulting from defective visual integration rather than primary retinal cone failure. These anomalies are generally a consequence of higher-order cortical dysfunction. They are not commonly identified by traditional cone function assessments, like the Farnsworth-Munsell 100 Hue test, unless visual crowding or simultanagnosia impairs task execution. Nonetheless, color nomenclature and basic hue recognition are frequently maintained, as exemplified in our case.
Optical coherence tomography (OCT) of the macula and optic nerve revealed a normal foveal contour and intact retinal nerve fiber layer, thereby excluding maculopathy and optic neuropathy as potential causes of the visual complaints. These data reinforced the suspicion of retrochiasmal pathway involvement. This example highlights the need to evaluate retrochiasmal or cortical etiologies in patients experiencing unexplained near vision difficulties, especially when conventional ophthalmologic assessments (e.g., slit-lamp examination, OCT, and refraction) yield no conclusive results. Near vision impairment, often linked to anterior segment disorders, may occasionally indicate a cortical pathology.
During the initial ophthalmologic examination, the patient had difficulties with reading and close vision skills. Considering the lack of anterior segment or retinal disease and the normal OCT results, perimetry was conducted, which indicated a right homonymous hemianopia. This prompted the evaluation of a central nervous system lesion. Ishihara pseudoisochromatic plates were utilized to investigate potential visual processing abnormalities, during which simultanagnosia was hypothesized due to the patient's failure to detect the complete pattern despite having normal color vision. This observation resulted in the implementation of the Boston Naming Test, followed by a neuropsychological assessment and neuroimaging. Differential diagnosis at that stage included neurodegenerative disorders such as PCA or other CNS disorders, such as cerebrovascular accident, space-occupying lesions affecting the parieto-occipital cortex, etc.
Neuroimaging is paramount in evaluating patients with a potential neurodegenerative disorder, such as PCA[11].
Although structural imaging like MRI, may seem inconspicuous in the initial phases of PCA, functional neuroimaging methods such as FDG-PET and amyloid PET have greater sensitivity[12]. These modalities have demonstrated characteristic metabolic alterations, notably hypometabolism in the parieto-occipital cortex. Recent studies have found emerging structural markers, including cortical thinning and atrophy of the posterior corpus callosum, observed by advanced volumetric MRI; however, these may not be detectable with typical imaging procedures.
Ophthalmologists should remember that an abnormal Ishihara test may suggest simultanagnosia and can be mistaken for achromatopsia/dyschromatopsia. In the setting of painless and progressive memory loss, visuospatial abnormalities, or features of the Balint or Gerstmann syndromes, differential diagnosis includes a primary, progressive neurodegenerative disorder. Moreover, difficulty in near vision, a common problem for which the patient presents to the ophthalmologist, may be the first symptom of a progressive neurodegenerative disorder.
| 1. | Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 439] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Pellegrini F, Lee AG, Zucchetta P. Homonymous Hemianopsia Due to Posterior Cortical Atrophy. Neuroophthalmology. 2017;41:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Pelak VS, Smyth SF, Boyer PJ, Filley CM. Computerized visual field defects in posterior cortical atrophy. Neurology. 2011;77:2119-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Formaglio M, Krolak-Salmon P, Tilikete C, Bernard M, Croisile B, Vighetto A. [Homonymous hemianopia and posterior cortical atrophy]. Rev Neurol (Paris). 2009;165:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Maia da Silva MN, Millington RS, Bridge H, James-Galton M, Plant GT. Visual Dysfunction in Posterior Cortical Atrophy. Front Neurol. 2017;8:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Yerstein O, Parand L, Liang LJ, Isaac A, Mendez MF. Benson's Disease or Posterior Cortical Atrophy, Revisited. J Alzheimers Dis. 2021;82:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | McIntosh RD, Schenk T. Two visual streams for perception and action: current trends. Neuropsychologia. 2009;47:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 442] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Beh SC, Muthusamy B, Calabresi P, Hart J, Zee D, Patel V, Frohman E. Hiding in plain sight: a closer look at posterior cortical atrophy. Pract Neurol. 2015;15:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Brazis PW, Graff-Radford NR, Newman NJ, Lee AG. Ishihara color plates as a test for simultanagnosia. Am J Ophthalmol. 1998;126:850-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Ziegler GC, Haarmann A, Daniels C, Herr A. The Difficult Diagnosis of Posterior Cortical Atrophy in a 62-Year-Old Woman. J Geriatr Psychiatry Neurol. 2020;33:59-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Neitzel J, Ortner M, Haupt M, Redel P, Grimmer T, Yakushev I, Drzezga A, Bublak P, Preul C, Sorg C, Finke K. Neuro-cognitive mechanisms of simultanagnosia in patients with posterior cortical atrophy. Brain. 2016;139:3267-3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |