Published online Aug 26, 2025. doi: 10.12998/wjcc.v13.i24.107825
Revised: April 21, 2025
Accepted: May 13, 2025
Published online: August 26, 2025
Processing time: 79 Days and 10.3 Hours
Segmental atrophy (SA) of the liver is a rare, often underrecognized, benign condition that presents as a mass lesion, mimicking a neoplasm, which poses a significant diagnostic challenge. Given its rarity, only a limited number of case reports and series have been published, resulting in sparse literature on the entity. This review aims to summarize the clinicopathologic and diagnostic features of SA and thus improve its recognition.
Core Tip: Segmental atrophy (SA) of the liver is a rare pseudotumor that typically presents as a liver mass mimicking a neoplasm. It is usually asymptomatic and most often discovered incidentally. SA is more commonly seen in female patients, with an average age of 59.6 years. The exact pathogenesis remains poorly understood, though it is strongly associated with vascular injury. Histopathologically, the lesion progresses through several stages, ranging from parenchymal collapse to nodular elastosis and eventually nodular fibrosis. Recognizing these distinct morphologic stages is essential for accurate diagnosis.
- Citation: Younus A, Liu Y, Connor EE, Wu ZY, Lee H, Fu ZY. Segmental atrophy of the liver: Review of a rare pseudotumor. World J Clin Cases 2025; 13(24): 107825
- URL: https://www.wjgnet.com/2307-8960/full/v13/i24/107825.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i24.107825
Segmental atrophy (SA) of the liver is a rare and underrecognized pseudotumor. It was first described by Singhi et al[1] in 2011. SA typically presents as a mass-like lesion clinically and radiographically, leading to potential misinterpretation as a neoplastic process[1]. Diagnostic challenges are further complicated by its variable morphology and the limited literature available, resulting in a lack of comprehensive understanding of this entity.
Given the limited awareness of SA and its potential for misdiagnosis, accurate diagnosis requires careful clinicopathologic correlation. This review aims to consolidate our current knowledge of SA, including its proposed pathogenesis, clinical presentation, imaging characteristics, histopathologic features, differential diagnosis, and treatment strategies. Increasing awareness and understanding of this rare entity will enhance its recognition, which is essential for accurate diagnosis and appropriate management.
The precise pathogenesis of SA is not well understood but is postulated to develop as a response to vascular injury[1]. Abnormal blood vessels are consistently observed in this lesion, including thick-walled arterioles and venules that often show signs of prior vascular injury—such as luminal thrombosis, fibrosis of the vessel wall, and recanalization, indicating a reparative response to previous vascular occlusion[1]. The associated vascular findings suggest that underlying ischemic injury may play a role in hepatic SA by inducing the production of elastic fibers leading to the development of nodular elastosis[1]. In normal liver tissue, elastic fibers are found in the walls of the portal and centrilobular venous branches, as well as in the walls of the major vessels. Hepatic injury can trigger a sequence of reactions that lead to cell death, which in turn, causes portal myofibroblasts to produce an excess of elastic fibers that are deposited in a disorganized pattern, which is the characteristic finding in SA[2]. These findings are supported by similar results reported by Hobbs et al[3] in colonic elastotic polyps. The authors note that the elastotic lesions within the submucosa of the gastrointestinal tract appear to be centered around blood vessels, suggesting a possible shared vascular etiology with nodular elastosis in the liver. Furthermore, a recent retrospective study involving 45 patients found that 78% had a history of hypertension or cardiovascular disease. This demonstrates a potential link between SA and remote vascular injury due to chronic cardiovascular conditions, especially hypertension[4].
Although both fibrosis and elastosis represent stromal responses to tissue injury, they appear to separate from the very beginning stage due to their different underlying mechanisms. Classic hepatic fibrosis typically arises from hepatocellular injury and chronic inflammation, promoting collagen deposition via activation of hepatic stellate cells. In conditions such as nonalcoholic steatohepatitis, elastic fibers tend to accumulate only in advanced diseases, with a diffuse distribution generally proportional to the extent of fibrosis[5]. By contrast, elastosis in SA is a dominant and early feature with focally prominent, well-demarcated, and disproportionately abundant, especially in the nodular elastosis stage[1]. The accumulation of elastic fibers is always accompanied by a slower and more gradual increase in fibrotic septa.
While the precise signaling pathways underlying elastosis in SA have not been elucidated, several regulatory mechanisms involved in elastosis and remodeling, which have been previously described in chronic liver diseases, are likely relevant. Transforming growth factor-β plays a central role as a profibrotic cytokine and is stored in association with elastic fibers; upon release during tissue remodeling, it can modulate fibroblast activity and promote extracellular matrix deposition[6]. In addition, elastin-derived peptides generated through partial degradation have been shown to influence fibroblast activation, inflammation, and angiogenesis, suggesting a role beyond structural support[6]. Other potential contributors include hypoxia-induced signaling pathways [e.g., hypoxia-inducible factor-1alpha (HIF-1alpha)][7] and platelet-derived growth factor[8] may be mediated by ischemia-induced activation of portal myofibroblasts. These molecular events could contribute to early and nodular stage of elastosis observed in SA[1,5].
Studies show that the lesion is usually asymptomatic[1]. However, when symptomatic, the most common presentation is right upper quadrant abdominal pain[1,9-11] (Table 1), followed by ascites. The lesion can also become apparent as an incidental finding on imaging[1,12-15].
| Ref. | Gender | Age | Presentation | Coexisting conditions | Radiology findings | Procedure/treatment | Pathology findings | Follow-up |
| Singhi et al[1], N = 18 | F = 13; M = 5 | Mean age: 60.5 years (14-91) | Right upper abdominal pain (78%), ascites (11%), incidental mass (11%) | NA | Subcapsular mass in 83% of cases; size 18-10.0 cm (mean 5.2 cm) | Segmental resection (n = 3), wedge resection | Ranges from parenchymal collapse with hepatocyte islets/ductular proliferation to nodular elastosis with dense fibrosis | NA |
| Thomas et al[9] | M | 54 | Right-sided abdominal pain, progressive abdominal distention, and lower extremity edema for 3 weeks. History of alcoholic cirrhosis | NA | CT (triple phase scan): 1.7 cm × 2 cm intensely enhancing lesion with arterial attenuation but no washout on delayed phase images | Ultrasound-guided biopsy | Active alcoholic hepatitis with cirrhosis and segmental atrophy | NA |
| BedadaI et al[10] | F | 4 | 2-year history of abdominal pain and a palpable mass | None | CT: 9 cm × 10 cm liver lesion with central hypodense and peripheral isodense areas in the left lobe | Complete lesion excision | Areas of elastosis to fibrosis, small islets of hepatocytes, liver parenchyma with fibrous tracts and mild bile duct proliferation. Thin-walled veins were present in the fibrous septa, with a few prominent vessels | No recurrence at 6 months |
| Li et al[11] | M | 3 | 2-month history of progressively worsening right upper abdominal pain | None | CT: A large (9.2 cm × 7.4 cm × 13.3 cm) heterogeneous hypodense mass in the right liver lobe with cystic and solid components; the solid parts mildly enhanced post-contrast | Right hemi-hepatectomy | Hepatocyte atrophy fibrous tissue proliferation with collagenization, visible vasculature, and occasional dilated bile ducts | No recurrence at 12 months |
| Spolverato et al[12] | M | 73 | Incidental mass during laparoscopy for gastric conduit revision after esophageal cancer | None | NA | Segmental resection | Loss of parenchyma, mild inflammation, ductular proliferation, biliary retention cysts, early fibrosis/elastosis | NA |
| Spolverato et al[12] | M | 74 | Suspected cholangiocarcinoma | None | Left liver atrophy; possible mass in left hemi-liver | Left hemi-hepatectomy | Atrophic liver with marked inflammation, large bile ducts, and rare reactive epithelium (consistent with segmental atrophy) | NA |
| Spolverato et al[12] | M | 73 | Questionable lesion in pancreatic adenocarcinoma context | Pancreatic adenocarcinoma | NA | Wedge resection | Dense chronic inflammation with scarring and bile duct proliferation | NA |
| Ishizaki et al[13] | F | 45 | Asymptomatic, lesion found on routine ultrasound | None | CT: Low-density mass (10.5 cm × 7.5 cm) in anterior/medial segments, no enhancement | Central hepatectomy | Parenchyma replaced by fibrous tissue with elastic fibers, anomalous vessels, intimal thickening, thrombi, and recanalization | No recurrence at 5 years |
| Garg et al[14], N = 6 | F = 6 | Mean age: 58.3 years (37-80) | Incidental findings were noted in 5 cases during workup or surgery for nodules or tumors elsewhere in the body. One additional case presented with right upper abdominal pain | 4/6: Lung adenocarcinoma | Single lesion in each patient; mean size 18 mm (range 3-36 mm) | NA | NA | NA |
| Findeis-Hosey et al[15], N = 10 | F = 6; M = 4 | Median age: 68 years (44-80) | All asymptomatic. Identified incidentally during whole-body imaging, surgery for carcinoma history, or autopsy | 6/10: Colorectal adenocarcinoma | 56% of cases had a single liver lesion, mean tumor size 16 mm | 12 cases from 10 patients; segmental resections (n = 7); needle core biopsies (n = 5) | 92% of cases showed elastic material and thick-walled vessels; 50% of cases have ductular proliferation; 17% of cases have biliary cysts and residual hepatocytes | NA |
An analysis of underlying conditions (Table 1) in 41 reported cases reveals that 11 (26.8%) have a coexisting malignancy, including colorectal adenocarcinoma (n = 3), pancreatic adenocarcinoma (n = 2), lung adenocarcinoma (n = 1), pancreatic neuroendocrine tumor (n = 1), ovarian granulosa cell tumor (n=1), breast carcinoma (n = 1), hepatocellular carcinoma (n = 1), and carcinoma of unknown primary (n = 1) (Table 1). Other common conditions, such as coagulopathy, infections, rheumatologic diseases, or generalized inflammatory conditions (e.g., alcoholism, obesity), are not described in the reported cases.
There is no specific laboratory test or biomarker for SA. Abnormal laboratory values may be present though and can include elevated cholestatic markers, such as alkaline phosphatase and gamma-glutamyl transferase, as well as increased liver transaminases, including alanine aminotransferase and aspartate aminotransferase[2]. Total and direct bilirubin levels are within normal ranges in the limited cases reported[10], as SA does not appear to impair biliary drainage in a clinically significant manner unless associated with liver malignancy or other underlying hepatic conditions.
Most studies indicate that SA is more common in females, with a female to male ratio of 2:1 (Table 1). The average age at diagnosis is 59.6 with a range of 3-91 years. It is rare in children, with only a few cases reported[10,11].
Although SA is considered a rare condition, its true prevalence may be underestimated due to asymptomatic cases, misdiagnosis, or underreporting in medical literature. Most published studies are case reports or small case series (Table 1), making it difficult to establish definitive epidemiological patterns.
SA is an uncommon benign lesion and can mimic primary malignancy or metastases due to variable imaging features. Most lesions are subcapsular, and their size ranges from 0.3 to 13.3 cm with a mean of 4.9 cm (Table 1). Garg et al[14] summarized the imaging features of SA. On ultrasound, the atrophied liver can appear hypoechoic or isoechoic compared to uninvolved hepatic parenchyma with ill-define margins and no Doppler flow signal, suggesting reduced vascularity[2]. On contrast-enhanced single-phase (portal venous) computed tomography (CT), most lesions are well-circumscribed and hypodense with occasional focal calcifications. However, in cases with background fatty liver, the lesion may appear hyperdense. In magnetic resonance imaging, the lesion appears hypointense on T1-weighted images, iso- to hyperintense on T2-weighted images, and hyperintense on diffusion-weighted images. The lesion remains hypointense on arterial, portal venous, and delayed phases, except in cases with background fatty liver where it may appear hyperintense in all phases. In a fluorodeoxyglucose-positron emission tomography, atrophic areas are isometabolic compared to the background hepatic parenchyma with no fluorodeoxyglucose uptake, which serves as a useful clue to its benign nature.
A well-defined intrahepatic atrophic area with a distinct transition between healthy and affected liver tissue should raise suspicion for SA. Given its imaging variability and potential to mimic malignant conditions, radiologic correlation with clinical and histopathological findings is essential for accurate diagnosis.
Singhi et al[1] and Spolverato et al[12] describe a stepwise progression of histopathologic changes in SA, with lesions transitioning from parenchymal collapse and minimal elastosis to nodular elastosis and dense fibrosis. Microscopically, progressive elastosis is a defining feature present in all stages except in the final stage, in which the parenchyma is completely replaced by dense fibrosis. The sequential nature of this histologic progression suggests an evolution from early parenchymal collapse to advanced fibrosis with elastosis playing a central role.
Early lesions are composed of collapsed hepatic parenchyma with occasional trapped islands of residual hepatocytes, chronic inflammation, and brisk bile duct proliferation[1]. This bile duct proliferation is typically observed at the interface between the atrophic parenchyma and surrounding liver tissue, with small, irregular bile ducts[10]. This pattern is consistent with a type 1 ductular reaction, commonly associated with parenchymal loss and stromal remodeling. A finely granular, gray-tinged amphophilic extracellular matrix is present and stains positive with Verhoeff-van Gieson elastic stain. The extent of elastosis is mild (1%-10%).
As the lesion progresses to the second stage, chronic inflammation decreases along with the degree of ductular proliferation such that it may no longer be evident. Whereas elastosis significantly increases to involve 11%-80% of the affected tissue.
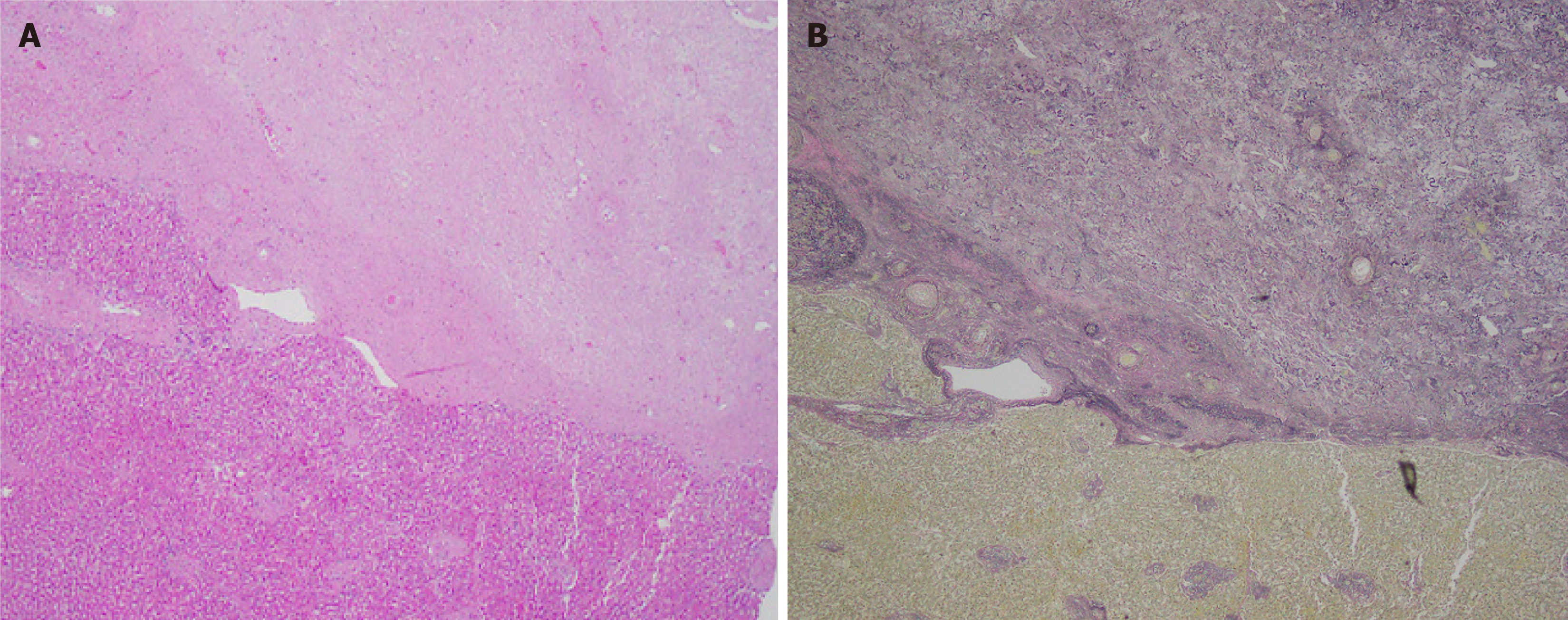
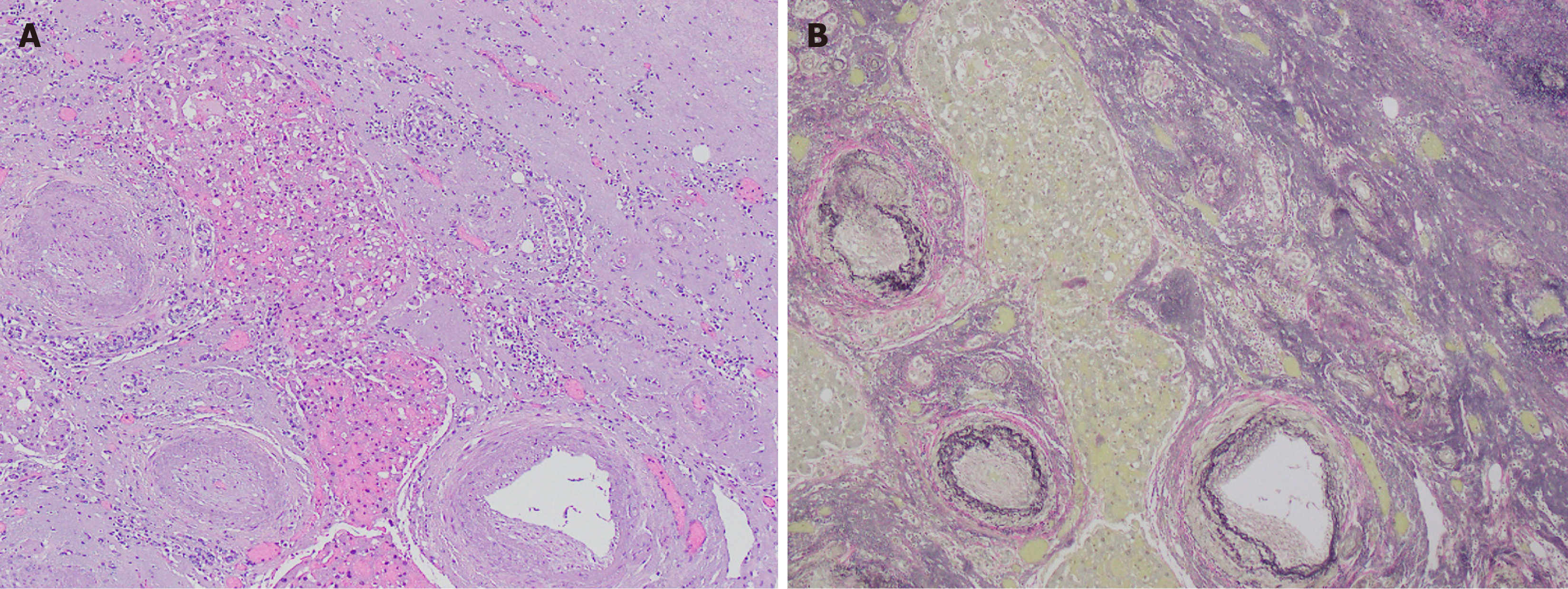
At this stage, the lesion forms a nodule that is well-delineated from the background parenchyma and is composed almost entirely of elastotic fibers (> 90%). The entity is more distinct at this point with an abrupt interface between lesional tissue and background liver (Figure 1A). The lesional tissue consists of an elastic-rich matrix with rare, scattered islands of hepatocytes (Figure 2A) and occasional entrapped portal tracts and central veins. An abrupt transition is also observed between the elastic-rich matrix and the residual hepatocyte islands. The matrix demonstrates mild cellularity with small, bland-appearing spindle cells that are devoid of atypia or mitoses.
In the final stage, referred to as nodular fibrosis, the lesion exhibits dense fibrosis with small, scattered islands of hepatocytes. Residual portal tracts, small biliary cysts, and abnormal vascular structures are also present, but fibrosis is the key element as it replaces most of the hepatic parenchyma.
A striking histologic feature that is common to all reported cases is the presence of abnormally thick-walled vessels (Figure 2A). This wall thickening occurs in both arteries and veins, many of which show thrombosis, fibrosis, and recanalization. Notably, the vascular change is limited to the lesional tissue as thick-walled vessels are not observed in the surrounding uninvolved hepatic tissue.
Another feature of SA are biliary cysts of variable size that tend to be located at the periphery of the lesion. They range from large, grossly evident cysts to small cysts that are only microscopically evident as dilated preexisting ducts within portal tracts. The presence of large biliary cysts can increase clinical concern for a neoplastic process, such as mucinous cystic neoplasm or intraductal papillary neoplasm[16]. Some large biliary cysts may show rupture, epithelial denudation, fibrosis, and granulomatous inflammation. Of note, background liver cirrhosis has been reported in one case[12].
Singhi et al[1] were the first and, to date, the only researchers to describe the role of immunohistochemistry in SA. They describe several special histochemical stains useful for confirming the diagnosis and distinguishing SA from other hepatic conditions.
Verhoeff-van Gieson elastic stain highlights elastic fibers and reveals the extensive elastotic deposition characteristic of SA (Figure 1B and 2B). Reticulin staining shows numerous reticulin fibers within the matrix, while Masson trichrome stain helps differentiate collagen deposition, which is notably absent in nodular elastosis. Movat pentachrome stain further supports the diagnosis, showing diffuse black staining of elastic fibers with fine yellow strands of reticulin dissecting through the matrix, and reinforces the presence of significant elastosis. Additionally, vimentin immunohistochemical staining shows positivity in the benign spindle cells within the matrix, which exhibit a dendritic appearance that is not easily visible on standard hematoxylin and eosin staining.
Although SA demonstrates a well-defined histological evolution, it is important to consider a broad differential diagnosis, as various other diseases can present with similar findings.
Sclerosing cavernous hemangiomas (SCH) are benign tumors that can mimic the nodular elastosis stage of SA[15] with similar residual entrapped hepatocytes and at least mild elastic fibrosis. However, thick-walled blood vessels and ductular reaction are significantly more common in SA than in SCH. Additionally, elastic staining is more diffuse in SA[15].
SA needs to be differentiated from secondary types of atrophy. Areas of focal liver atrophy are associated with various intrahepatic neoplastic lesions, most commonly cholangiocarcinoma and metastatic adenocarcinoma[2,17,18]. Takayasu et al[17] report hepatic atrophy in six patients with bile duct obstruction caused by cholangiocarcinoma, all of whom had portal vein occlusion. Similar findings are reported by Han et al., noting that both primary and metastatic liver malignancies can lead to hepatic atrophy in the setting of combined biliary obstruction and portal vein occlusion[19]. In cases of cancer-associated atrophy, the malignant tumor is typically apparent and conversely is absent in cases of SA[1]. Thus, the differentiation of these entities relies on comprehensive evaluation of laboratory tests, radiologic imaging, and histology.
Amyloid refers to the extracellular deposition of misfolded, insoluble proteins in tissues. Histologically, it shows amorphous eosinophilic/pale, glassy, hyaline material. In contrast, the elastosis of SA appears as finely granular and/or fibrillar pale, eosinophilic to gray-tinged amphophilic material, and occasionally with a fibrous component. Distinguishing elastosis in SA from amyloid on hematoxylin and eosin staining can be challenging due to overlapping features. However, Congo red staining is positive in amyloid but negative in elastosis, while elastin stain can confirm the presence of elastosis.
Multiple small cysts can occur in SA and may mimic multi-cystic diseases of the liver. However, these entities can be distinguished by imaging characteristics and histology examination. Radiologically, diffuse cystic changes in the liver or cysts within the kidneys support cystic liver disease. Whereas, SA is diagnosed histologically by identifying areas of parenchymal collapse, thrombosed vessels, or the ductular reactions that are typical of early lesions[1,16].
A simple hepatic cyst or a solitary bile duct cyst, potentially resulting from a limited form of ductal plate malformation, is also a consideration in the differential diagnosis due to their morphologic similarity to the retention-type biliary cyst seen in SA[16]. The presence of thick-walled vessels with elastosis adjacent to the cyst supports the diagnosis of SA.
A recently described pseudotumor in association with localized vascular thrombi can be differentiated from SA based on their distinct histology findings[20]. In regenerative hepatic pseudotumor, the vascular changes, such as central vein thrombi and portal vein thrombi, are associated with reactive parenchymal changes, including sinusoidal dilation, patchy bile ductular proliferation, and portal vein abnormalities, but elastosis is absent or only focally present[16]. This is notable given that progressive elastosis is a defining feature of SA.
SA shares some features with the atrophy-hypertrophy complex but differs in its localized involvement[14]. The atrophy-hypertrophy complex is a compensatory process where part of the liver atrophies while another part hypertrophies, usually in response to biliary obstruction or vascular injury[21,22]. In contrast to SA, which is typically subsegmental or segmental and subcapsular, the atrophy-hypertrophy complex usually affects entire lobes. The underlying causes of hepatic atrophy-hypertrophy are broad, and include cirrhosis, malignancies (hilar cholangiocarcinoma, hepatocellular carcinoma, hepatic metastases), iatrogenic factors (post-cholecystectomy changes, portal vein embolization), parasitic biliary infections, and trauma[22-24]. Additional distinguishing features are that the atrophic region in SA shows elastosis and fibrosis, and the atrophy-hypertrophy complex is characterized by hyperplasia of the unaffected liver[25].
To date, only a few cases of SA have been reported in the literature, all confirmed through histological examination of needle core biopsies or surgical specimens (wedge or segmental resections) (Table 1). Surgical resection is reserved for when the atrophy is suspected to be secondary to another condition[2]. There are no standardized treatment protocols for SA, and there is no evidence to support routine follow-up given that no studies to date suggest an association between SA and the transformation of malignancy.
SA is a benign pseudotumor that presents diagnostic challenges due to its diverse histologic presentation and limited characterization in the literature. When diagnosing SA, it is crucial to rule out other potentially serious underlying diseases, such as carcinomas, which can cause atrophy. SA lesions exhibit a sequential histologic progression, with elastotic changes playing a key role. Treatment is typically limited to surgical resection, and further research is needed to improve diagnostic accuracy and management strategies for this underrecognized entity.
| 1. | Singhi AD, Maklouf HR, Mehrotra AK, Goodman ZD, Drebber U, Dienes HP, Torbenson M. Segmental atrophy of the liver: a distinctive pseudotumor of the liver with variable histologic appearances. Am J Surg Pathol. 2011;35:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Ferraina F, Fogliati A, Scotti MA, Romano F, Garancini M, Ciulli C. Lobar and Segmental Atrophy of the Liver: Differential Diagnoses and Treatments. Livers. 2024;4:320-332. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Hobbs CM, Burch DM, Sobin LH. Elastosis and elastofibromatous change in the gastrointestinal tract: a clinicopathologic study of 13 cases and a review of the literature. Am J Clin Pathol. 2004;122:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Farsi N, Sanchez-Avila M, Duarte M, Requena DO, Alkathery T, Ronquillo NR, Garcia-Buitrago M, Stoll LM, Montgomery EA, Byrnes K. Hepatic segmental atrophy: a diagnostic challenge with variable clinicopathologic features and an association with cardiovascular disease. Virchows Arch. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Nakayama H, Itoh H, Kunita S, Kuroda N, Hiroi M, Matsuura H, Yasui W, Enzan H. Presence of perivenular elastic fibers in nonalcoholic steatohepatitis Fibrosis Stage III. Histol Histopathol. 2008;23:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Kanta J. Elastin in the Liver. Front Physiol. 2016;7:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Pagani A, Aitzetmüller MM, Brett EA, König V, Wenny R, Thor D, Radtke C, Huemer GM, Machens HG, Duscher D. Skin Rejuvenation through HIF-1α Modulation. Plast Reconstr Surg. 2018;141:600e-607e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ying HZ, Chen Q, Zhang WY, Zhang HH, Ma Y, Zhang SZ, Fang J, Yu CH. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (Review). Mol Med Rep. 2017;16:7879-7889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 9. | 9 Thomas T, Cezar C, Laiyemo A. Segmental Atrophy: A Rare Pseudotumor of the Liver. American Journal of Gastroenterology. 2018;113:S1337-S1338. [DOI] [Full Text] |
| 10. | BedadaI AG, Sreekumaran MI, Azzie G. Segmental atrophy of the liver in a child: Case report and review of the literature. S Afr J Child Health. 2019;13:100-101. [DOI] [Full Text] |
| 11. | Li X, Chen W, Yue Q, Gong Q. Segmental atrophy of the liver in a child: a case description and literature analysis. Quant Imaging Med Surg. 2025;15:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Spolverato G, Anders R, Kamel I, Pawlik TM. Segmental atrophy of the liver: an uncommon and often unrecognized pseudotumor. Dig Dis Sci. 2014;59:3122-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ishizaki Y, Mizuno T, Hara K, Kawasaki S. Advanced segmental atrophy of the liver with marked elastosis. Surgery. 2015;157:826-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Garg I, Graham RP, VanBuren WM, Goenka AH, Torbenson MS, Venkatesh SK. Hepatic segmental atrophy and nodular elastosis: imaging features. Abdom Radiol (NY). 2017;42:2447-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Findeis-Hosey JJ, Zhou Z, Gonzalez RS. Hepatic sclerosing cavernous haemangioma can mimic the nodular elastosis stage of segmental atrophy. Histopathology. 2019;75:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Assarzadegan N, Montgomery E. Uncommon Benign Neoplasms and Pseudotumors of the Liver. Arch Pathol Lab Med. 2023;147:390-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Takayasu K, Muramatsu Y, Shima Y, Moriyama N, Yamada T, Makuuchi M. Hepatic lobar atrophy following obstruction of the ipsilateral portal vein from hilar cholangiocarcinoma. Radiology. 1986;160:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Friesen BR, Gibson RN, Speer T, Vincent JM, Stella D, Collier NA. Lobar and segmental liver atrophy associated with hilar cholangiocarcinoma and the impact of hilar biliary anatomical variants: a pictorial essay. Insights Imaging. 2011;2:525-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Hann LE, Getrajdman GI, Brown KT, Bach AM, Teitcher JB, Fong Y, Blumgart LH. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. AJR Am J Roentgenol. 1996;167:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Torbenson M, Yasir S, Anders R, Guy CD, Lee HE, Venkatesh SK, Wu TT, Chen ZE. Regenerative hepatic pseudotumor: a new pseudotumor of the liver. Hum Pathol. 2020;99:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Lory J, Schweizer W, Blumgart LH, Zimmermann A. The pathology of the atrophy/hypertrophy complex (AHC) of the liver. A light microscopic and immunohistochemical study. Histol Histopathol. 1994;9:541-554. [PubMed] |
| 22. | Kim RD, Kim JS, Watanabe G, Mohuczy D, Behrns KE. Liver regeneration and the atrophy-hypertrophy complex. Semin Intervent Radiol. 2008;25:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Karabulut K, Ozden I, Poyanli A, Bilge O, Tekant Y, Acarli K, Alper A, Emre A, Arioğul O. Hepatic atrophy-hypertrophy complex due to Echinococcus granulosus. J Gastrointest Surg. 2006;10:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Vilgrain V, Condat B, Bureau C, Hakimé A, Plessier A, Cazals-Hatem D, Valla DC. Atrophy-hypertrophy complex in patients with cavernous transformation of the portal vein: CT evaluation. Radiology. 2006;241:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Lorigan JG, Charnsangavej C, Carrasco CH, Richli WR, Wallace S. Atrophy with compensatory hypertrophy of the liver in hepatic neoplasms: radiographic findings. AJR Am J Roentgenol. 1988;150:1291-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |