Published online Aug 26, 2025. doi: 10.12998/wjcc.v13.i24.105923
Revised: April 12, 2025
Accepted: May 10, 2025
Published online: August 26, 2025
Processing time: 125 Days and 16.5 Hours
Leuconostoc garlicum is commonly found in fermented foods and very few infected patients have been reported, who typically present symptoms such as fever and fatigue. Conventional clinical examinations often struggle to identify this ba
We report a patient ultimately diagnosed with Leuconostoc garlicum infection. The primary manifestations included persistent fever, cough and fatigue. These sy
For patients with suspected bacterial infection and experiencing fever, conventional anti-infective treatment can be ineffective in controlling their symptoms, and an infection due to rare bacteria or drug-resistant bacteria should be considered. Next-generation sequencing enables rapid and precise identification of infection-related pathogens in febrile patients.
Core Tip: We report a case of Leuconostoc garlicum infection with persistent fever accompanied by fatigue. For patients with fever of unknown origin as the primary manifestation, comprehensive physical examination and thorough medical history are essential to minimize the risk of a missed or incorrect diagnosis. For most patients with fever caused by infection, conventional anti-infective treatment can lead to recovery. However, special attention should be paid to patients with rare or drug-resistant bacterial infections, whose diagnosis and treatment may be more difficult. Next-generation sequencing technology may provide support for the diagnosis and treatment of these patients.
- Citation: Zang DY, Li LG, Yang SG, Wang YY, Yu XQ. Combination of next-generation sequencing and traditional examinations for identifying Leuconostoc garlicum: A case report. World J Clin Cases 2025; 13(24): 105923
- URL: https://www.wjgnet.com/2307-8960/full/v13/i24/105923.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i24.105923
The bacterium Leuconostoc garlicum belongs to the family Leuconostocaceae[1]. This strain was initially isolated from fermented foods[2]. There have been previous clinical case reports indicating that this bacterium can infect the human body and cause respiratory tract lesions, and is resistant to vancomycin [2]. However, in recent years, there have been relatively few clinical case reports of infection by this bacterium[3-5]. At present, there are no relevant reports on Leuconostoc garlicum causing lesions in other tissues or organs of the human body. Therefore, its detailed pathogenic spectrum remains unclear. Here, we describe a patient who developed a fever due to infection with Leuconostoc garlicum. After treatment, the patient's fever and other clinical symptoms resolved.
The patient experienced intermittent fever for two months and chill and fatigue for two days.
The 52-year-old male patient was admitted to the Respiratory Department of the First Affiliated Hospital of Henan University of CM with fever that progressed over the last two months. The patient had mild respiratory symptoms with fever and cough. During the past two months, the patient initially developed fever with a peak temperature of 39°C. After self-administering ibuprofen for 5 days without improvement, he sought treatment at a community hospital. A complete blood count revealed elevated white blood cell and neutrophil counts, leading to a provisional diagnosis of bacterial infection. He was treated with oral amoxicillin-clavulanate and ibuprofen for one week, but his symptoms persisted. Subsequently, the patient presented to another hospital where a chest X-ray demonstrated bilateral pulmonary infiltrates, suggestive of pneumonia. He was treated with intravenous moxifloxacin and piperacillin-tazobactam. Following one week of this adjusted antimicrobial regimen, his fever completely resolved. Unfortunately, one month later, the patient experienced recurrent fever with a peak temperature of 39°C. Despite reinitiating ibuprofen, his body temperature remained elevated. Therefore, he attended our hospital for diagnosis and treatment.
The patient had no history of past illness.
The patient had no relevant personal or family history.
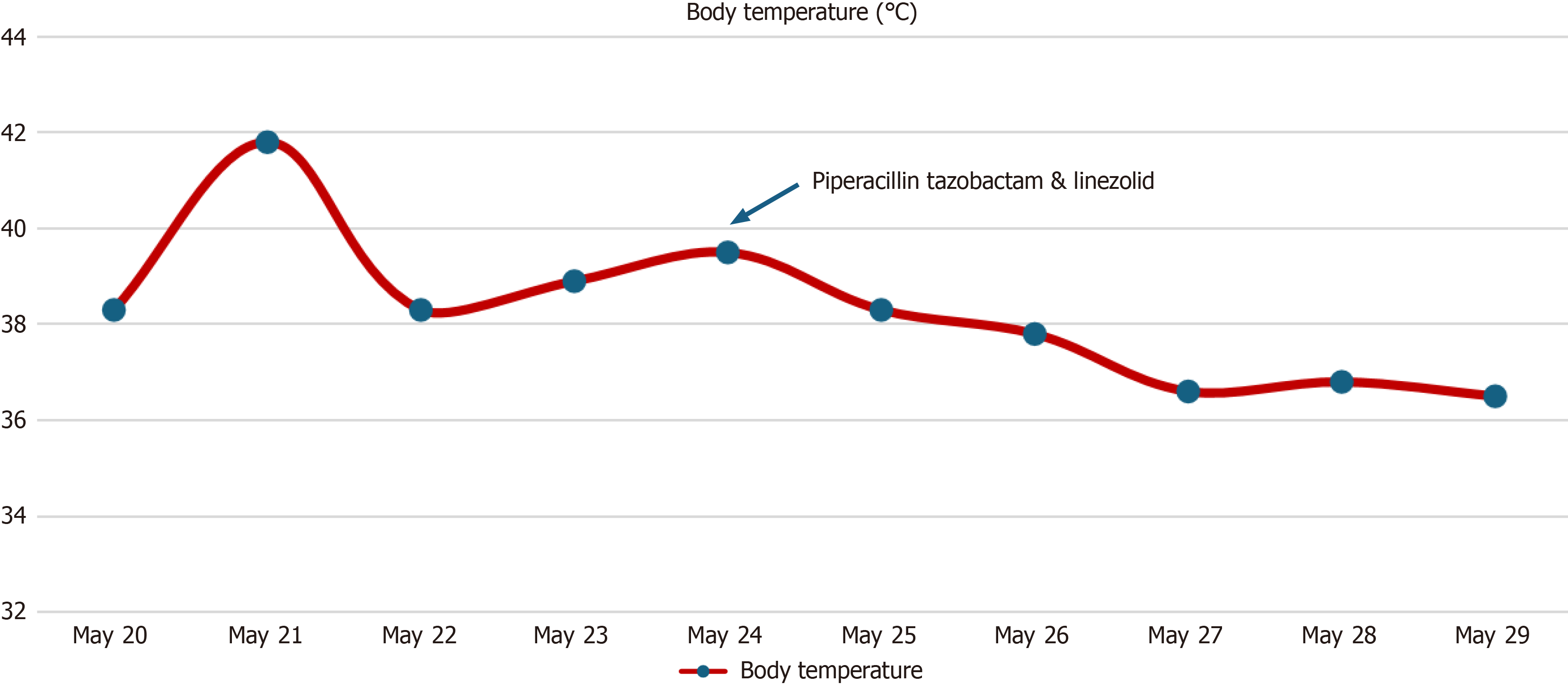
Physical examination showed the following: Body temperature was 38.3°C [maximum body temperature was 41.8°C (107.24°F)], pulse was 84 beats/min, respiratory rate was 22 breaths/min, blood pressure was 130/81 mmHg, body height was 168 cm, and body mass was 78 kg. Alertness and cooperation were good. No rash or bleeding spots were observed on the body. Superficial lymph nodes were not palpable. The conjunctivas were not hyperemic. The lips were not cyanotic, the oral mucosa was smooth, pharyngeal hyperemia was obvious, the tonsils were normal, and no purulent secretions were observed. The neck was soft and without resistance. The patient had no butterfly erythema, joint swelling or pain. On auscultation, heart sounds were normal, and coarse breath sounds were heard in both lungs. Abdominal examination and nervous system examination were normal. The patient’s temperature record is shown in Figure 1.
Clinical and laboratory investigations were performed on admission. Hemoglobin level was 111 g/L, and leukocyte count was 8.8 × 109/L (neutrophils 90.3%, lymphocytes 5.6%, and monocytes 4.0%). The C-reactive protein (CRP) level was 235.9 mg/L, erythrocyte sedimentation rate (ESR) was 67 mm/h, and procalcitonin level was 12.6 ng/mL. The Epstein-Barr virus (EBV) DNA test was negative. The cytomegalovirus test was also negative. Rheumatism-related tests, urinalysis, respiratory pathogen detection, routine stool examination and autoantibody testing showed no abnormalities.
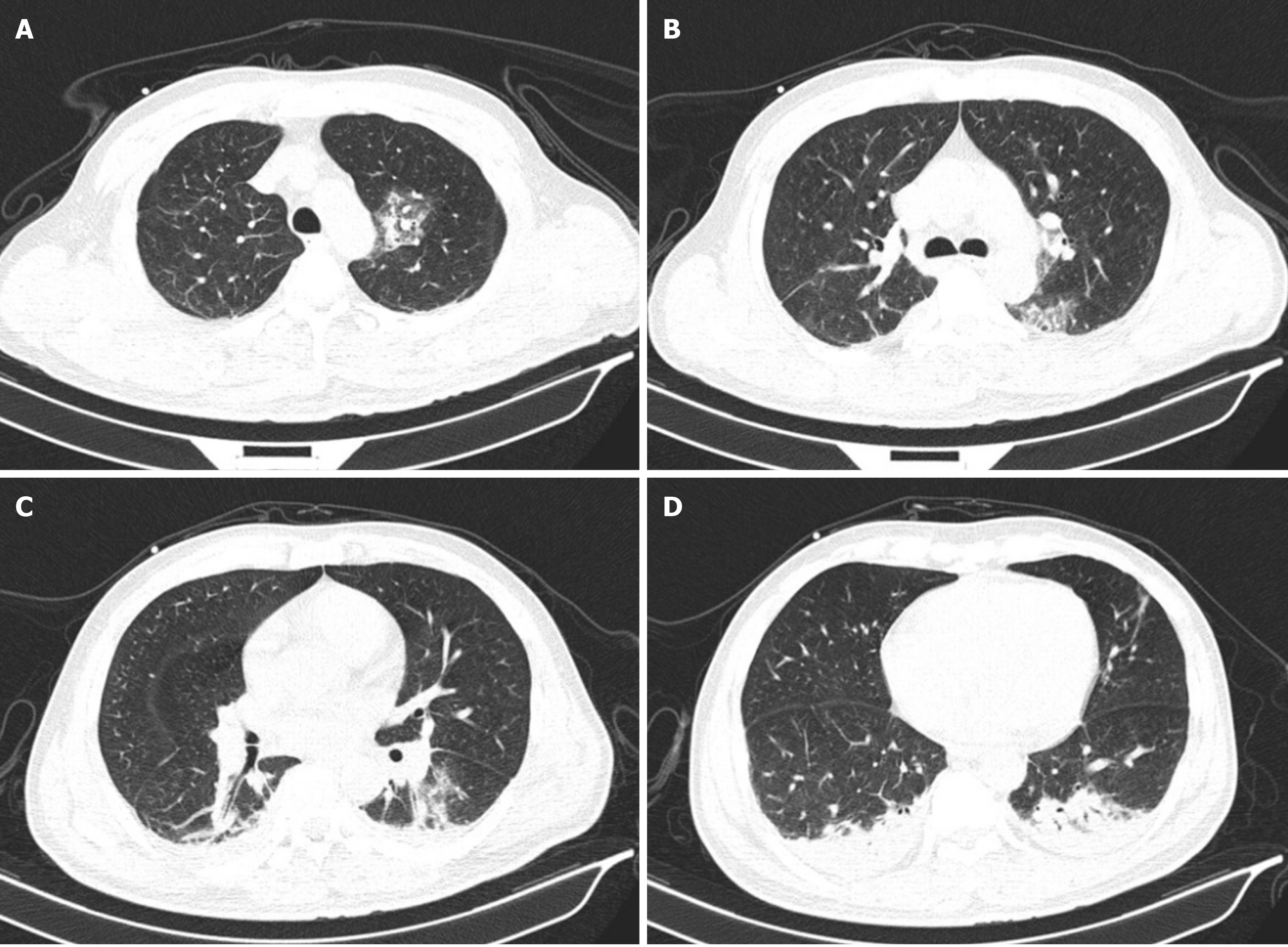
A lung computed tomography (CT) scan suggested severe pulmonary infection in both lungs and a small amount of pleural effusion. Lung CT images are shown in Figure 2.
The patient was finally diagnosed with fever of unknown origin (FUO).
On days 1 and 2, complete blood count tests showed the following: Hemoglobin was 111 g/L, leukocyte count was 8.8 × 109/L (neutrophils 90.3%, lymphocytes 5.6%, and monocytes 4.0%), and CRP level was 235.9 mg/L. The patient was given symptomatic and basic anti-infective treatment. Loxoprofen and Latamoxef were administered.
On days 3 and 4, the patient still had a fever, and the highest body temperature was 41.8°C (107.24°F). Complete blood count tests showed the following: Hemoglobin was 105 g/L, leukocyte count was 10.0 × 109/L (neutrophils 91.3%, lymphocytes 4.2%, and monocytes 3.6%), and CRP level was 227.9 mg/L. The patient showed no significant treatment effect following moxalactam; thus, moxifloxacin was initiated instead.
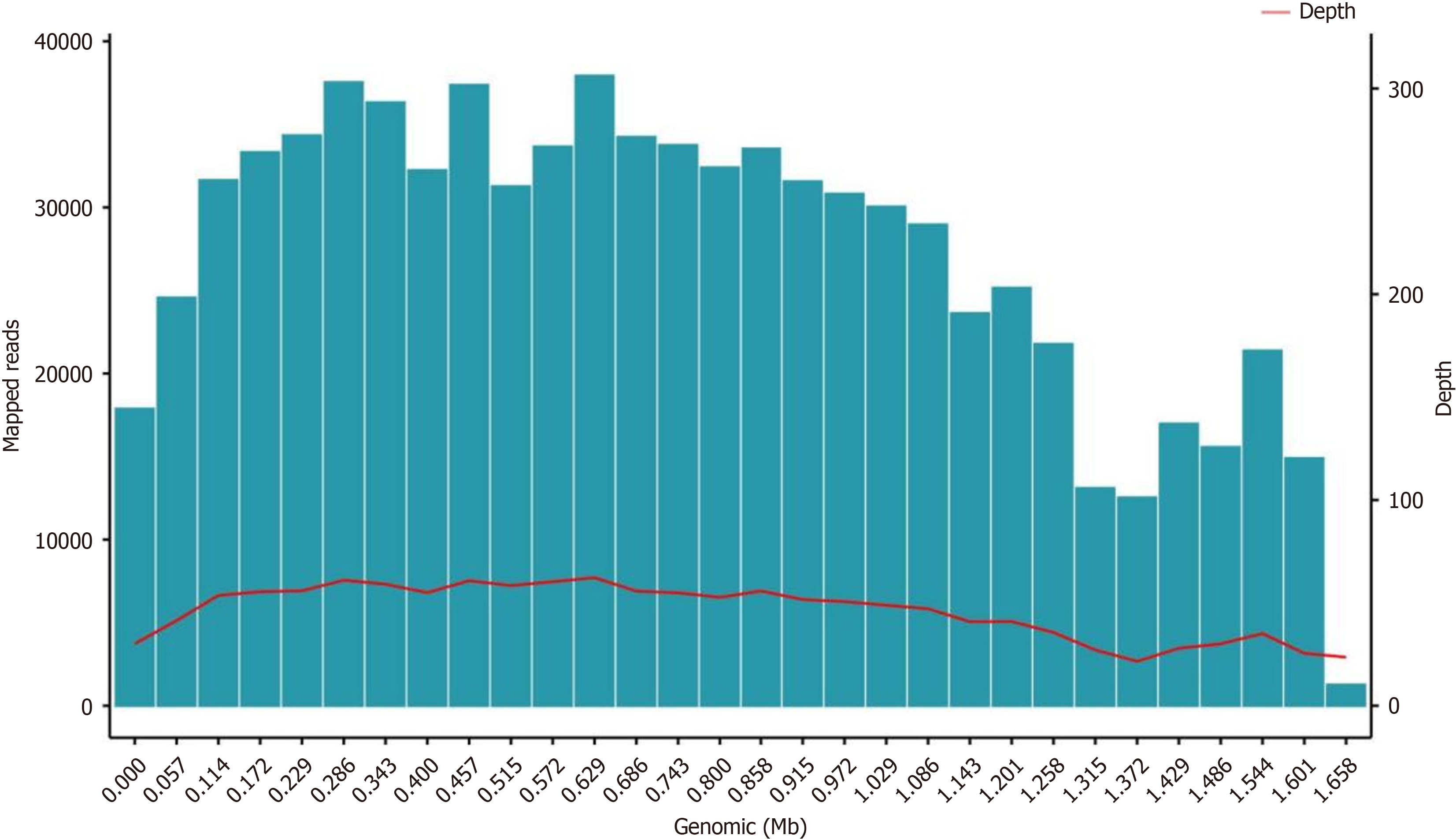
On days 5 and 6, he continued to experience fever, and the highest temperature was 38.5°C. To identify the cause of the fever, the patient underwent bronchoscopy examination and next-generation sequencing (NGS) testing was performed using the patient's bronchoalveolar lavage fluid (BALF). NGS revealed Leuconostoc garlicum: Coverage = 99.99%, Average depth = 47. There is evidence to indicate that Leuconostoc garlicum is resistant to vancomycin; therefore, he was treated with piperacillin tazobactam and linezolid. NGS test results are shown in Figure 3.
On days 7 and 8, his body temperature was in the normal range.
On days 9 and 10, his temperature remained in the normal range.
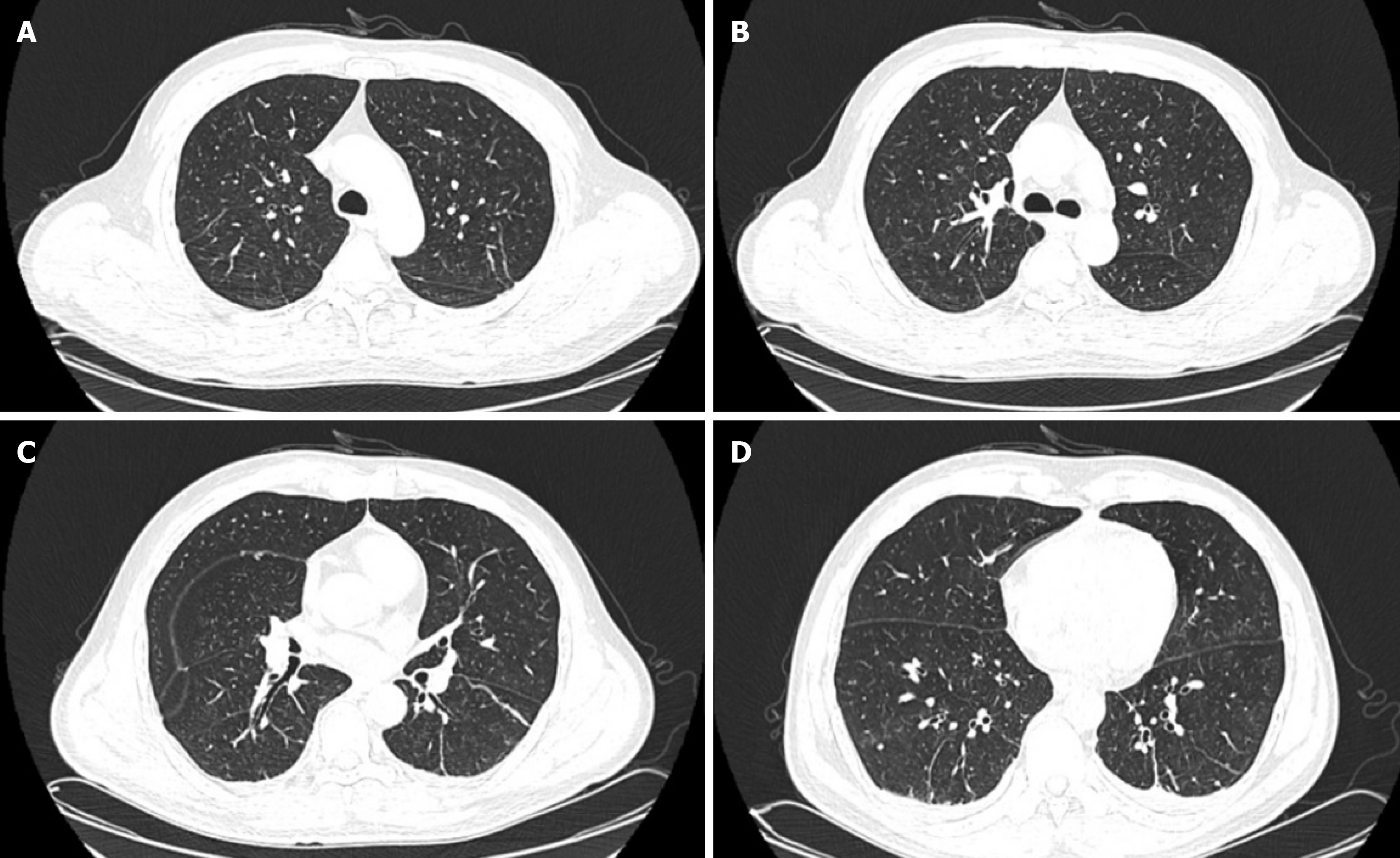
The patient was discharged on day 14. Follow-up complete blood count tests, CRP, and ESR revealed no significant abnormalities. The patient underwent another lung CT scan on June 10th, and the results showed a significant im
Fever of undetermined origin, also known as FUO[6], is a condition characterized by a prolonged fever that does not resolve spontaneously and for which the cause remains unidentified despite thorough diagnostic investigations. The classic definition of FUO, as proposed by Petersdorf and Beeson in 1961, includes the following criteria: (1) A fever greater than 38.3°C (101°F) on multiple occasions; (2) The duration of fever lasts more than three weeks; and (3) No definitive diagnosis after one week of intensive investigation in a hospital setting.
There are many reasons for FUO, such as infection, tumors, autoimmune diseases, endocrine disorders, and medication[7]. We usually determine the cause of a patient's FUO using the following methods, a detailed medical history, physical examinations, laboratory tests, imaging examinations, NGS technology, tissue biopsies, etc. Only when we have identified the cause can we make the correct clinical decisions. For this patient, we needed to identify whether his fever was caused by an infection or a non-infectious cause. When the patient was admitted to hospital, we first found that he may have a bacterial infection through complete blood count tests. We then began to determine the infection site. Neurological examination revealed no abnormalities in the patient, which preliminarily excluded neurological infection. The patient also underwent urine testing to exclude the possibility of a urinary tract infection. The urine test results showed no abnormalities. We then attempted to exclude the possibility of gastrointestinal infection. As the patient did not have symptoms such as abdominal pain, diarrhea, or nausea, and the routine stool examination showed no abnormalities, the possibility of a gastrointestinal infection was excluded. Finally, we shifted our focus to the respiratory system. Auscultation detected rales in the patient's lungs. He was advised to undergo a lung CT scan to confirm the diagnosis. The CT report indicated bilateral pulmonary infections. Therefore, we considered that the patient's fever was caused by pneumonia. Based on our experience, we used latamoxef for anti-infection treatment. However, the patient still had a high white blood cell count, and fever symptoms did not improve. We considered that the patient may be co-infected with other pathogens such as fungi and viruses; thus, he underwent the beta-glucan test, galactomannan test, EBV test, cytomegalovirus test, and respiratory pathogen detection. However, the test results indicated that the patient was not infected with the above-mentioned pathogens. As the patient wished to obtain a timely diagnosis and receive appropriate treatment, we recommended that he undergo bronchoscopy, as it can help exclude the possibility of tumor and BALF can be collected and subjected to NGS to identify the pathogen[8].
NGS is a revolutionary technology in the field of genomics that has significantly improved our ability to analyze DNA and RNA sequences[9]. Traditional sequencing methods such as Sanger sequencing are laborious and time-consuming, with limited throughput[10-12]. NGS has overcome these limitations, enabling the rapid and cost-effective sequencing of large amounts of genetic material. NGS can sequence millions to billions of DNA fragments in a single run, enabling the analysis of large genomes and multiple samples simultaneously[13]. It can detect low-abundance DNA or RNA molecules and identify rare genetic variations[14]. Fortunately, we used NGS technology to quickly identify the bacterium causing the patient's infection. The NGS results showed that the patient was infected with Leuconostoc garlicum.
Leuconostoc garlicum is a species of lactic acid bacteria that belongs to the family Leuconostocaceae. This bacterium is known to have a role in food fermentation, particularly in the production of fermented vegetables, dairy products, and other foods. Leuconostoc garlicum is a Gram-positive, non-spore-forming, and non-motile bacterium[15]. It typically appears as cocci (spherical or ovoid cells) that may form pairs or short chains. This species is commonly associated with plant materials and fermented foods. Some studies suggest that Leuconostoc garlicum may have probiotic properties, potentially benefiting gut health and immune function. However, research in this area is still in the early stages. There are very few research reports available for reference. Only four studies have reported human infection with Leuconostoc. A research report has indicated that Leuconostoc garlicum is resistant to vancomycin; thus, we chose to treat the patient with piperacillin tazobactam and linezolid. The results proved that this was the right decision.
We subsequently reviewed this case to determine why the patient had been infected with this pathogen. This bacterium is not common, and there are very few reported cases related to it. Following investigation, we found that the patient resided in a rural area and worked as a vegetable farmer. He frequently took unsold vegetables home to make pickles, and long-term residence in such an environment may be one of the contributing factors to his infection with Leuconostoc garlicum. We also interviewed the patient's family members. The patient lives with his son, who did not exhibit similar symptoms. Considering the patient's advanced age, his immune system was weaker compared to younger individuals, which may explain why he alone contracted the bacterial infection. While the presence of homemade pickles suggests a plausible source of Leuconostoc garlicum exposure, the absence of microbial analysis on these pickles or the household environment precludes definitive confirmation of the pathogen’s origin. This is regrettable.
This case indicates that FUO patients with suspected bacterial infection who do not improve after empirical treatment, that rapid pathogen identification is critical. From this case, we can learn that environmental exposure to lactic acid bacteria-rich settings (e.g., pickle production) may serve as a risk factor for Leuconostoc garlicum infection, particularly in immunocompromised hosts, warranting early empirical treatment. When conventional diagnostic methods fail to identify the causative pathogen and empirical therapy proves ineffective, NGS can be performed to determine the infectious agent. NGS enables swift and precise pathogen detection, offering significant clinical benefits and guiding effective treatment decisions.
We thank the NGS Technology Research Center of KingMed Diagnostics Group for its technological support.
| 1. | Hong SP, Shin SC, Kang WK, Chin YW, Turner TL, Choi HW, Song KM, Kim HJ. Complete genome sequence of Leuconostoc garlicum KCCM 43211 producing exopolysaccharide. J Biotechnol. 2017;246:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Kumar A, Augustine D, Mehta A, Dinesh KR, Viswam D, Philip R. Leuconostoc garlicum: an unusual pathogen in the era of vancomycin therapy. Indian J Chest Dis Allied Sci. 2012;54:127-130. [PubMed] |
| 3. | Camarasa A, Chiner E, Sancho-Chust JN. [Pulmonary abscess due to Leuconostoc species in an immunocompetent patient]. Arch Bronconeumol. 2009;45:471-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Borer A, Weber G, Avnon LS, Riesenberg K, Alkan M. Pleural empyema caused by Leuconostoc spp. Scand J Infect Dis. 1997;29:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ferrer S, de Miguel G, Domingo P, Pericas R, Prats G. Pulmonary infection due to Leuconostoc species in a patient with AIDS. Clin Infect Dis. 1995;21:225-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Wright WF, Mulders-Manders CM, Auwaerter PG, Bleeker-Rovers CP. Fever of Unknown Origin (FUO) - A Call for New Research Standards and Updated Clinical Management. Am J Med. 2022;135:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Betrains A, Moreel L, Mulders-Manders CM, Auwaerter PG, Torné-Cachot J, Weitzer F, Terasawa T, Ly KH, Schönau V, Blockmans D, Wright WF, Rovers C, Vanderschueren S. Comparison of diagnostic spectrum between inflammation of unknown origin and fever of unknown origin: A systematic review and meta-analysis. Eur J Intern Med. 2024;124:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Li J, Pan D, Guo Y, Zhang B, Lu X, Deng C, Xu F, Lv Z, Chen Q, Zheng Y, Nong S, Su L, Qin R, Jiang F, Gai W, Qin G. Clinical application value of simultaneous plasma and bronchoalveolar lavage fluid metagenomic next generation sequencing in patients with pneumonia-derived sepsis. BMC Infect Dis. 2024;24:1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, Rawool S, Thakare RP, Banday S, Mishra AK, Das G, Malonia SK. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology (Basel). 2023;12:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 346] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 10. | Eren K, Taktakoğlu N, Pirim I. DNA Sequencing Methods: From Past to Present. Eurasian J Med. 2022;54:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Chiodini R, Badr A, Zhang G. The impact of next-generation sequencing on genomics. J Genet Genomics. 2011;38:95-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Slatko BE, Gardner AF, Ausubel FM. Overview of Next-Generation Sequencing Technologies. Curr Protoc Mol Biol. 2018;122:e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 473] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 13. | Lohmann K, Klein C. Next generation sequencing and the future of genetic diagnosis. Neurotherapeutics. 2014;11:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Méndez-Vidal C, Bravo-Gil N, Pérez-Florido J, Marcos-Luque I, Fernández RM, Fernández-Rueda JL, González-Del Pozo M, Martín-Sánchez M, Fernández-Suárez E, Mena M, Carmona R, Dopazo J, Borrego S, Antiñolo G. A genomic strategy for precision medicine in rare diseases: integrating customized algorithms into clinical practice. J Transl Med. 2025;23:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Kumar S, Bansal K, Sethi SK. Comparative genomics analysis of genus Leuconostoc resolves its taxonomy and elucidates its biotechnological importance. Food Microbiol. 2022;106:104039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |