Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.106321
Revised: April 14, 2025
Accepted: April 25, 2025
Published online: August 16, 2025
Processing time: 102 Days and 14 Hours
Type 1 diabetes is an autoimmune disease leading to insulin deficiency, and it is mainly diagnosed in young adults. One of the major acute complications of type 1 diabetes is diabetic ketoacidosis (DKA), which is a metabolic emergency that can be triggered by stress, infection, or poor blood glucose control. The association of DKA with conditions such as acute pancreatitis and malaria is rare and therefore represents a major diagnostic and therapeutic challenge.
A 20-year-old female was admitted to the emergency room for abdominal pelvic pain, fever, asthenia, polyuria, and polydipsia with a progressive deterioration of her state of consciousness. At admission, she was in a mild coma (Glasgow score: 9), had a fever of 38.5 °C, and had hyperglycemia (6 g/dL). The tests revealed severe DKA, hypertriglyceridemia, hyperamylasemia, and hyperlipasemia as well as malaria parasite density. The computed tomography scan confirmed acute stage E pancreatitis. The diagnosis was that of inaugural ketoacidosis of type 1 diabetes unbalanced by pancreatitis and malaria. Treatment included insulin therapy, rehydration, and antimalarial and analgesic treatment. After 10 days, the outcome was favorable with a normalization of the blood sugar, and an endocrine follow-up was recommend.
Rapid and multidisciplinary management of DKA, pancreatitis, and malaria led to a favorable and stable prognosis.
Core Tip: Type 1 diabetes is an autoimmune disease that can lead to serious metabolic disorders, electrolyte imbalances, and acute complications. Malaria impacts inflammation and metabolism and can aggravate diabetic ketoacidosis and electrolyte imbalances associated with type 1 diabetes, complicating clinical management of the patient. Prompt and targeted treatment is essential to avoid serious decompensation of the patient and ensure a favorable outcome. It is therefore essential to consider malaria as an aggravating factor when diagnosing and treating complications of type 1 diabetes.
- Citation: Kouame KI, Mobio PMN, Bouh JK, Konan JK, Coulibaly TK, Toure CW, Diebi LAA, Kouakou JNH, Koffi BE, Yapo PY. Convergence of diabetic ketoacidosis, acute pancreatitis, and malaria: A case report. World J Clin Cases 2025; 13(23): 106321
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/106321.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.106321
Type 1 diabetes is an autoimmune disease characterized by the destruction of β cells in the islets of Langerhans, resulting in absolute insulin deficiency. This form of diabetes, which primarily affects young people, requires rigorous management to prevent acute complications such as diabetic ketoacidosis (DKA)[1]. DKA is a common metabolic emergency for patients with type 1 diabetes. It manifests as hyperglycemia, high ketonemia, and metabolic acidosis and can be life threatening if not diagnosed and treated early[1].
Acute pancreatitis (AP) is an inflammation of the pancreas that can be triggered by multiple factors, such as infections, alcoholism, or severe hypertriglyceridemia[2]. Although rare, cases of AP secondary to hypertriglyceridemia associated with type 1 diabetes have been documented, highlighting the diagnostic and therapeutic complexity of these situations[3]. Hypertriglyceridemia, often exacerbated by poor glycemic control, plays a key role in the development of AP, contributing to severe metabolic alterations[3].
Herein, we report the case of a 20-year-old female admitted to the Resuscitation Department of the University Hospital of Yopougon for the management of an ketoacidosis coma associated with severe AP and malaria. This case illustrates the importance of early diagnosis and appropriate management of metabolic imbalances that may occur when type 1 diabetes is discovered and highlighted the challenges posed by the association of several pathogenic factors in a complex clinical situation.
A 20-year-old female was admitted to the Emergency Department for abdominopelvic pain and fever.
The onset of symptomatology occurred 4 days prior to admission and included diffuse abdominal pain of increasing intensity, unquantified fever, intense asthenia, polyuria, and polydipsia. The parents of the patient brought her to the Emergency Department due to the progressive deterioration of her state of consciousness.
The patient was not taking any medication, had not been exposed to any toxic substances, and had no underlying disease.
The patient had no significant personal medical or family history.
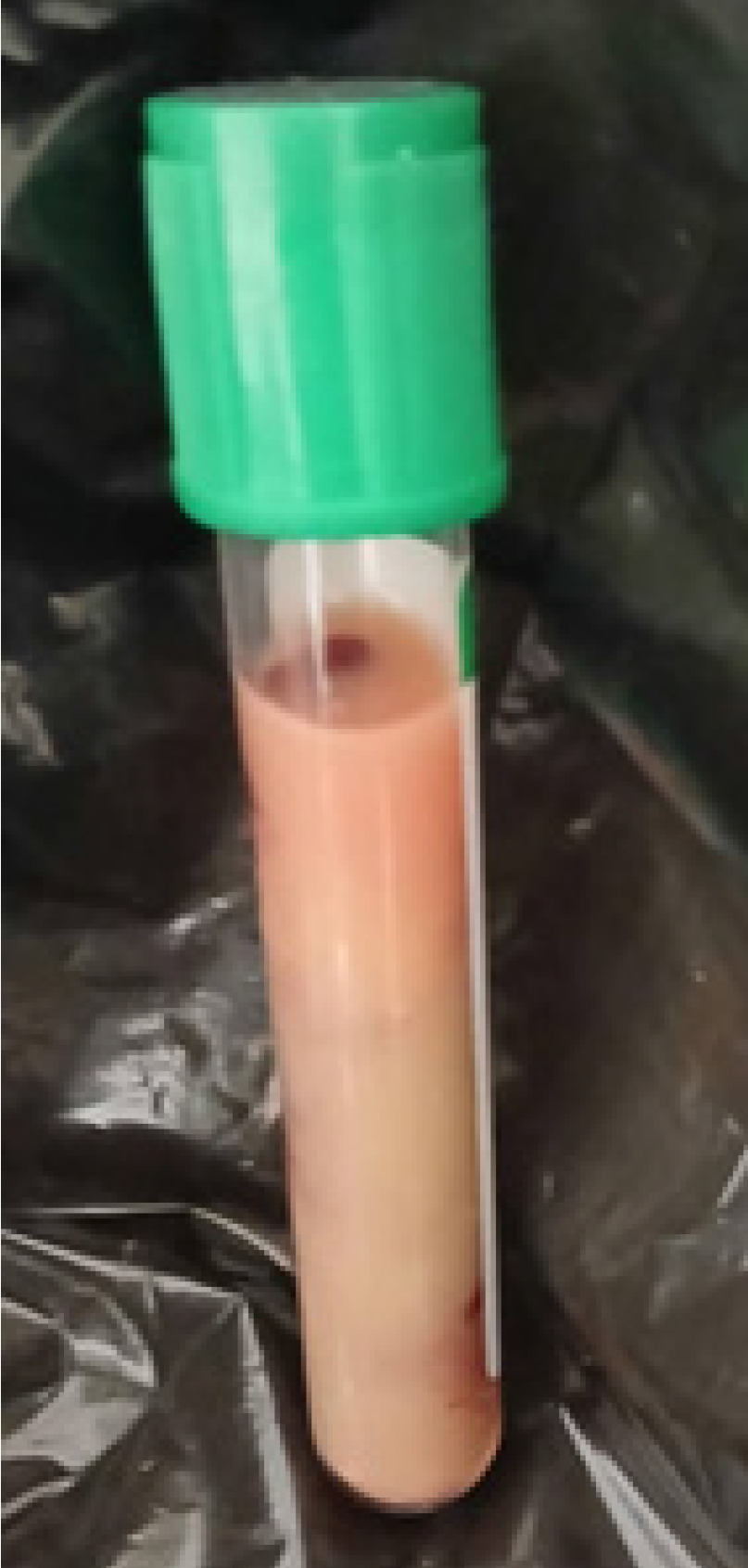
Upon admission to the Emergency Department, the patient was dehydrated and had a fever of 38.5 °C. She had a Glasgow score of 9 without signs of localization or meningeal irritation. In addition, Kussmaul dyspnea was noted. Initial biological examination revealed hyperglycemia (6 g/dL), ketonuria (3 crosses), and glucosuria (4 crosses). She was transferred to the intensive care unit where the clinical examination revealed severe dehydration, a fever of 38.9 °C, Kussmaul dyspnea, and stage 2 coma (Glasgow score: 9, Y2V2M5) with no signs of localization or irritation of the meninges. The venous blood sample had a lactescent appearance (Figure 1).
Biological examinations revealed: (1) Severe metabolic acidosis: Pondus hydrogenii at 6.9; (2) Bicarbonates at 3.9 mmol/L; (3) Anion hole at 30; (4) High blood sugar: 6 g/L (normal range: 0.6-1.10 g/L); (5) Hyperkalemia: 6.1 mmol/L (normal range: 3.5-5 mmol/L); (6) Creatinine: 71 mg/L (normal range: 06-14 mg/L); (7) C-reactive protein: 156 mg/L (normal range: < 6 mg/L); (8) Hyperleukocytosis: 13000 elements/mm3 (normal range: 3500-10000 elements/mm3); (9) Positive thick drop with a parasite density of 2100 trophozoites; (10) Triglycerides: 13.80 mmol/L (normal range: 2.86-8.25 mmol/L); (11) Hyperamylasemia: 131 IU/L (normal range: 00-82 IU/L); (12) Hyperlipasemia: 374.9 IU/L (normal range: 10-170 IU/L); and (13) Glycated hemoglobin: 22.34% (normal range: 04%-6.5%).
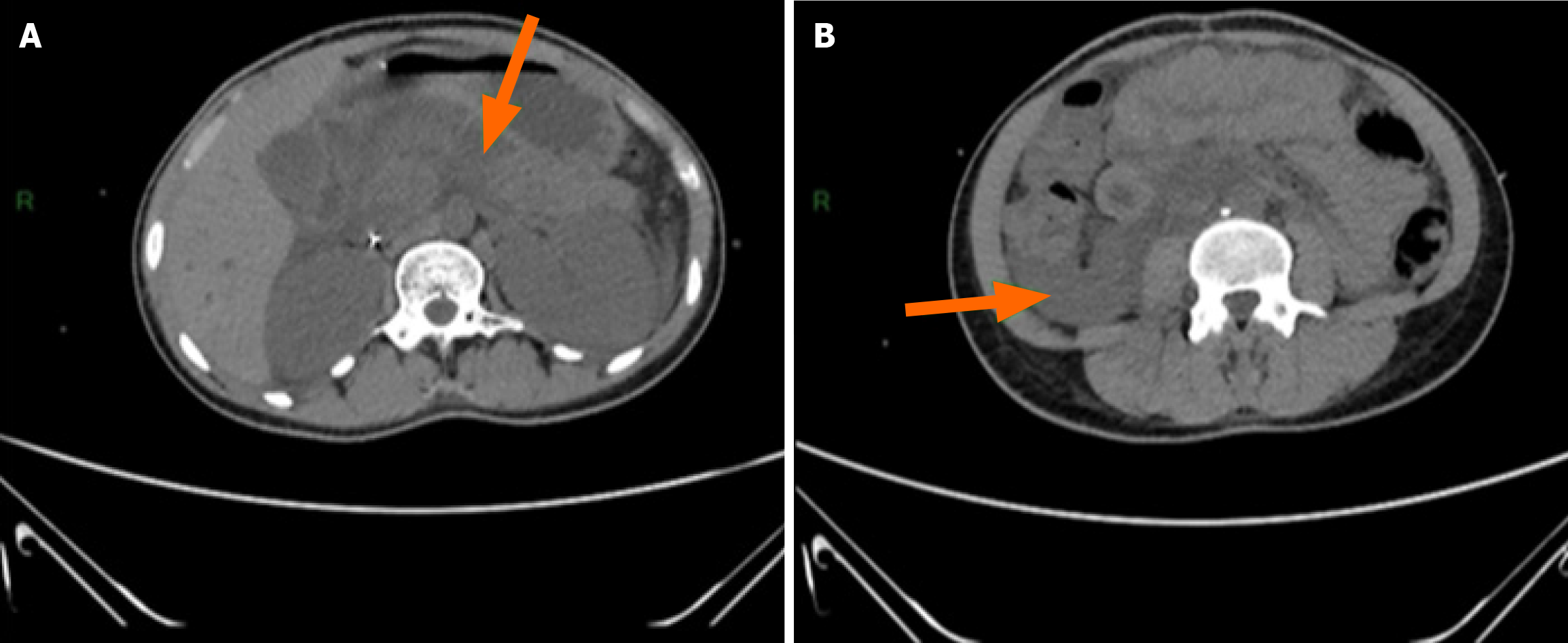
An abdominal computed tomography (CT) scan revealed AP, classified as stage E according to the Balthazar classification, with a CT Severity Index score of 4 (Figure 2).
Initial presentation of type 1 diabetes complicated by DKA, AP, and malaria.
Treatment included insulin therapy with intensive rehydration, antimalarial treatment, and analgesic treatment.
The patient experienced normalization of the blood glucose with a disappearance of the acetone crosses. The patient’s pain was alleviated, and we observed a progressive normalization of biological parameters. After 10 days of hospitalization, the patient was stable and was transferred to internal medicine for follow-up and ongoing management of diabetes.
DKA is an acute metabolic complication of type 1 diabetes. It is often triggered by infection, physiological stress, or non-adherence to insulin treatment[1]. In our case DKA was severe as evidenced by marked metabolic acidosis and the presence of significant hyperglycemia. The very high level of glycated hemoglobin indicated undiagnosed diabetes that likely developed for several weeks or months.
The uniqueness of this case was based on the association of DKA with AP and malaria. This clinical trio is rare but raises interesting questions about the pathophysiological links between these conditions. AP is a rare but well-documented cause of metabolic imbalance in patients with diabetes, especially when it is secondary to severe hypertriglyceridemia[2]. The lactescent appearance of the serum was an easily identifiable visual indicator of severe hypertriglyceridemia. Lactescent serum is an indication of the possibility of AP, especially if other biological and clinical signs (such as severe abdominal pain, elevated levels of pancreatic enzymes, or general inflammation) are present. This justifies the need for additional tests, such as pancreatic enzyme testing (amylase and lipase), abdominal imaging (such as CT or ultrasound), and monitoring of blood triglyceride levels[3].
In our case, hypertriglyceridemia contributed to pancreatic involvement and the worsening of DKA. Triglyceride levels above 11.3 mmol/L are well known to be a major risk factor for AP[3]. Hypertriglyceridemia in DKA is the result from an overproduction of very low density lipoprotein (VLDL) by the liver and to a lesser extent decreased catabolism of VLDL due to reduced activity of lipoprotein lipase.
The synthesis of lipoprotein lipase is normally stimulated by insulin. It is responsible for the hydrolysis of triglycerides transported by VLDLs and chylomicrons and the transfer of the fatty acids to adipose tissue and muscles. The overproduction of VLDLs is due to two factors: (1) The inability of insulin to reduce their hepatic synthesis; and (2) An accelerated flow of fatty acids from the adipose tissue to the liver[4]. In the absence of insulin, VLDL clearance is impaired, thus promoting the accumulation of triglycerides. Hydrolysis of triglycerides by pancreatic lipase generates toxic free fatty acids, which induce pancreatic inflammation and cell necrosis, thus explaining the onset of AP. This intrapancreatic release of free fatty acids results in cellular damage, edema, and pancreatic ischemia caused directly by capillary hyperviscosity due to hyperchylomicronemia[5].
In our patient, imaging confirmed AP classified as stage E according to the Balthazar classification and indicating severe pancreatic involvement. This AP stage is associated with a high risk of local and systemic complications and requires close monitoring[6].
The concomitant presence of malaria with a parasite density of 2100 trophozoites/mm3 may have played an aggravating role in the progressive symptoms of our patient. Malaria is a well-known cause of physiological stress, which may induce transient hyperglycemia and promote systemic inflammation[7]. Some studies suggest a link between malaria and pancreatitis although this is rare. Systemic inflammation induced by Plasmodium activates a cascade of proinflammatory cytokines (tumor necrosis factor alpha and interleukin 6) that could promote pancreatic involvement[7]. In addition, malaria infection often leads to hemolysis, tissue hypoxia, and vascular alterations that can contribute to pancreatic ischemia, thus aggravating underlying pancreatitis[8]. Furthermore, diabetes increases vulnerability to malaria infection due to diabetes-induced immunosuppression[9]. In our case the association of malaria with DKA and pancreatitis likely exacerbated systemic inflammation, delaying the resolution of DKA and requiring prompt and appropriate management.
Management consisted of insulin therapy combined with aggressive rehydration, antimalarial treatment, and correction of hydroelectrolyte imbalances. The use of insulin gradually reduced ketonemia and improved triglyceride clearance by restoring lipoprotein lipase activity[3]. The clinical course was favorable with alleviation of the abdominal pain, acidosis correction, and glycemic stabilization. After 10 days of hospitalization, the patient was transferred to internal medicine for follow-up and therapeutic education on diabetes.
This case highlighted the complexity of managing a patient with type 1 diabetes whose initial presentation was complicated by AP and malaria. The combination of DKA, hypertriglyceridemia, and infections such as malaria was a major diagnostic and therapeutic challenge. The impact of severe hypertriglyceridemia on AP and type 1 diabetes was clearly observed, highlighting the importance of monitoring triglyceride levels and pancreatic enzymes in these complex cases. Intensive insulin therapy was effective in regulation of glucose, reduction of ketonemia, and improvement in triglyceride clearance, leading to a regression of symptoms. This case also highlighted the importance of early and multidisciplinary management, combining metabolic care, malaria treatment, and endocrine monitoring to achieve a favorable outcome. A quick and targeted approach optimized the management of these complications and minimized the risk of serious organ damage. Continuous monitoring is essential to prevent further decompensation and improve the long-term prognosis of patients with complex conditions such as those observed in this case.
| 1. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2131] [Article Influence: 426.2] [Reference Citation Analysis (0)] |
| 2. | Frossard J, Von Laufen A, Dumonceau J, Felley C. Diagnostic et bilan étiologique d’une pancréatite aiguë. Revue Médicale Suisse. 2003;61:229-233. [DOI] [Full Text] |
| 3. | Zaher FZ, Boubagura I, Rafi S, Elmghari G, Elansari N. Diabetic Ketoacidosis Revealing a Severe Hypertriglyceridemia and Acute Pancreatitis in Type 1 Diabetes Mellitus. Case Rep Endocrinol. 2019;2019:8974619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | James RW. Particularités de la dyslipidémie du diabète. Revue Médicale Suisse. 2002;2:545-552. [DOI] [Full Text] |
| 5. | Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 960] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 7. | Popa GL, Popa MI. Recent Advances in Understanding the Inflammatory Response in Malaria: A Review of the Dual Role of Cytokines. J Immunol Res. 2021;2021:7785180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Bush MA, Anstey NM, Yeo TW, Florence SM, Granger DL, Mwaikambo ED, Weinberg JB. Vascular Dysfunction in Malaria: Understanding the Role of the Endothelial Glycocalyx. Front Cell Dev Biol. 2021;9:751251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis. 2010;16:1601-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |